Published online Oct 26, 2023. doi: 10.12998/wjcc.v11.i30.7403
Peer-review started: July 4, 2023
First decision: August 30, 2023
Revised: September 15, 2023
Accepted: October 8, 2023
Article in press: October 8, 2023
Published online: October 26, 2023
Processing time: 113 Days and 4.9 Hours
Congenital infantile fibrosarcoma (CIF) and congenital hemangioma (CH) have similarities on prenatal ultrasound and are rare.
We report 3 cases of fetuses with superficial hypervascular tumors, confirmed by postnatal pathology as CIF (1 case) and CH (2 cases, including 1 in a twin fetus). In Case 1, a mass with a rich blood supply in the fetal axilla was discovered by prenatal ultrasound at 28+0 wk of gestation. The postpartum pathological diagnosis was CIF, the mass was surgically removed, and the prognosis of the child was good. In Case 2, at 23+1 wk of gestation, a mass was discovered at the base of the fetus’s thigh on prenatal ultrasound. The postpartum pathological diagnosis was CH. After conservative treatment, the mass shrank significantly. Case 3 occurred in a twin fetus. At 30+0 wk of gestation, prenatal ultrasound revealed a bulging mass with a rich blood supply on the abdominal wall of one of the fetuses. Three weeks later, the affected fetus died, and the unaffected baby was successfully delivered by emergency cesarean section. The affected fetus was pathologically diagnosed with CH.
Prenatal ultrasound can provide accurate information, such as the location, size and blood supply of a surface mass in a fetus. We found similarities between CIF and CH in prenatal ultrasound findings. Although it is difficult to distinguish these conditions by prenatal ultrasound alone, for superficial hypervascular tumors that rapidly increase in size in a short period, close ultrasound monitoring of the fetus is required to quickly address possible adverse outcomes.
Core Tip: Congenital infantile fibrosarcoma (CIF) and congenital hemangioma (CH) found prenatally are very rare. We report one case of CIF and two cases of CH, one of which involved a twin fetus. The imaging, pathological, treatment and follow-up data in our manuscript are very detailed. These data can provide useful clinical experience for prenatal diagnosis and consultation for such tumors.
- Citation: Liang RN, Jiang J, Zhang J, Liu X, Ma MY, Liu QL, Ma L, Zhou L, Wang Y, Wang J, Zhou Q, Yu SS. Prenatal ultrasound diagnosis of congenital infantile fibrosarcoma and congenital hemangioma: Three case reports. World J Clin Cases 2023; 11(30): 7403-7412
- URL: https://www.wjgnet.com/2307-8960/full/v11/i30/7403.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i30.7403
Congenital infantile fibrosarcoma (CIF) and congenital hemangioma (CH) are two types of hypervascular tumor in newborns and infants that may involve the body surface. The incidence rate of soft tissue sarcomas in the first year of life was reported to be 16.0 per million[1], and one prospective study reported CH in 2/594 newborns (0.3%)[2]. Most current research focuses on infants and children[3,4]. Fetal CH is relatively common in existing related reports, while there are few reports on CIF, another extremely rare fetal tumor. Following a literature review, only 6 cases with both prenatal ultrasonography and confirmation by immunochemistry and specific fusion genes after delivery were reported. Both CIF and CH can manifest as body surface masses with rich blood supplies on prenatal ultrasound, which brings challenges in prenatal diagnosis. Given the rarity of cases detected prenatally and confirmed as CIF or CH postnatally, this report summarizes the cases of fetal CIF and CH discovered by prenatal ultrasound in our hospital. One case of CIF and two cases of CH, including one case in a twin fetus, provide experience in prenatal counseling and perinatal management for such tumors discovered prenatally.
Case 1: A 31-year-old Chinese singleton primigravida (gravida 1 para 0) at 34+5 wk of gestation was admitted to our hospital due to abnormalities shown on fetal imaging examination 6 wk previously.
Case 2: A 29-year-old Chinese singleton primigravida (gravida 1 para 0) at 23+1 wk of gestation presented to our hospital for routine follow-up.
Case 3: A 32-year-old Chinese twin pregnant woman (gravida 2 para 1) at 30+0 wk of gestation was admitted to our hospital due to abnormalities shown on fetal imaging examination 2 wk previously.
Case 1: The patient was admitted to Xijing Hospital for routine follow-up at 28+0 wk of gestation. Fetal imaging examinations revealed a mass in the left axilla and the left chest wall of the fetus. The pregnant woman had been followed up by ultrasound and had not received any treatment.
Case 2: The patient had no history of disease.
Case 3: The patient was admitted to Xijing Hospital for routine follow-up at 28+3 wk of gestation. Fetal imaging examination showed a mass protruding from the abdominal wall of one of the twins. The affected fetus was alive without pleural or peritoneal effusions. No abnormalities were found in the other twin fetus. The pregnant woman had been followed up by ultrasound and had not received any treatment.
Case 1: The woman had no history of past disease.
Case 2: The woman had no history of past disease.
Case 3: The woman delivered her first child vaginally 5 years ago.
Cases 1-3: The personal and family histories of all patients were unremarkable.
Case 1: The pregnant woman’s physical examination was normal. Physical examination of the newborn revealed a mass of approximately 7.0 cm × 5.0 cm from the left axilla to the left chest wall. The surface of the mass was purplish red and hyperemic, without ulceration or hemorrhage. The mass was tough and unsmooth, and the fetus had poor mobility and tenderness.
Cases 2: The pregnant woman’s physical examination was normal. Physical examination of the newborn revealed a purplish red mass of approximately 7.0 cm × 6.0 cm at the root of the right thigh, with unclear borders, tough quality, and no rupture on the surface. The mobility of the right lower limb was poor.
Case 3: The pregnant woman’s physical examination was normal. The affected fetus was stillborn with an enormous mass in the thoracic and abdominal wall. The vital signs of the other fetus were stable.
Case 1: Laboratory examinations of the pregnant woman showed no abnormalities. The newborn’s postpartum laboratory examination results for platelet count, D-dimer level, prothrombin time and fibrinogen level, which were 52 × 109/L, 20360.00 ng/mL, 15.1 s and 0.33 g/L, respectively, were abnormal.
Case 2: The pregnant woman’s laboratory examinations showed no abnormalities. The newborn’s postpartum laboratory examination results for hemoglobin concentration, platelet count and D-dimer and fibrinogen level, which were 85 g/L, 25 × 109/L, 78930.00 ng/mL and 0.84 g/L, respectively, were abnormal.
Case 3: The pregnant woman’s laboratory examinations showed no abnormalities.
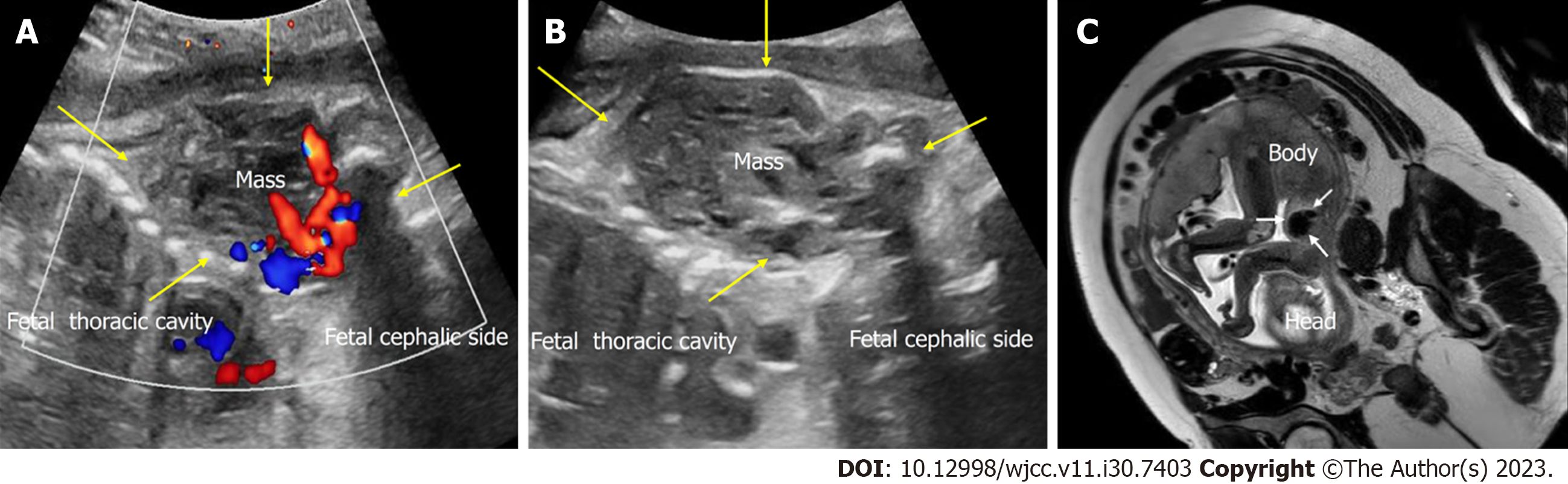
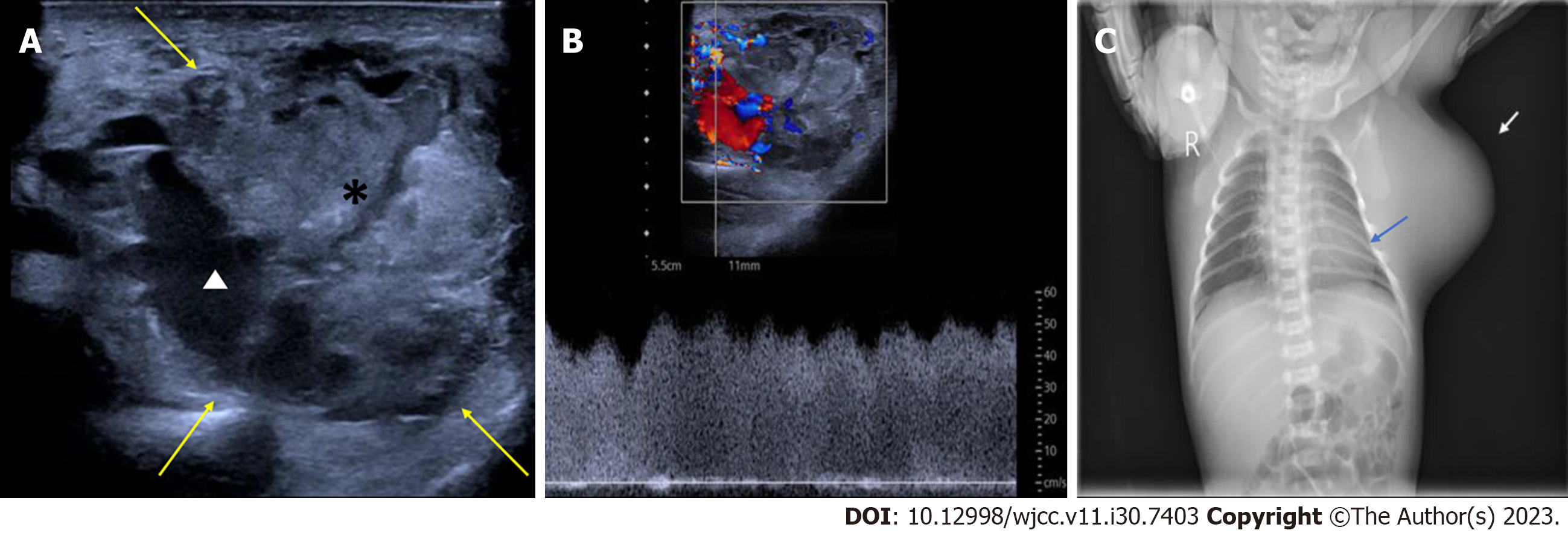
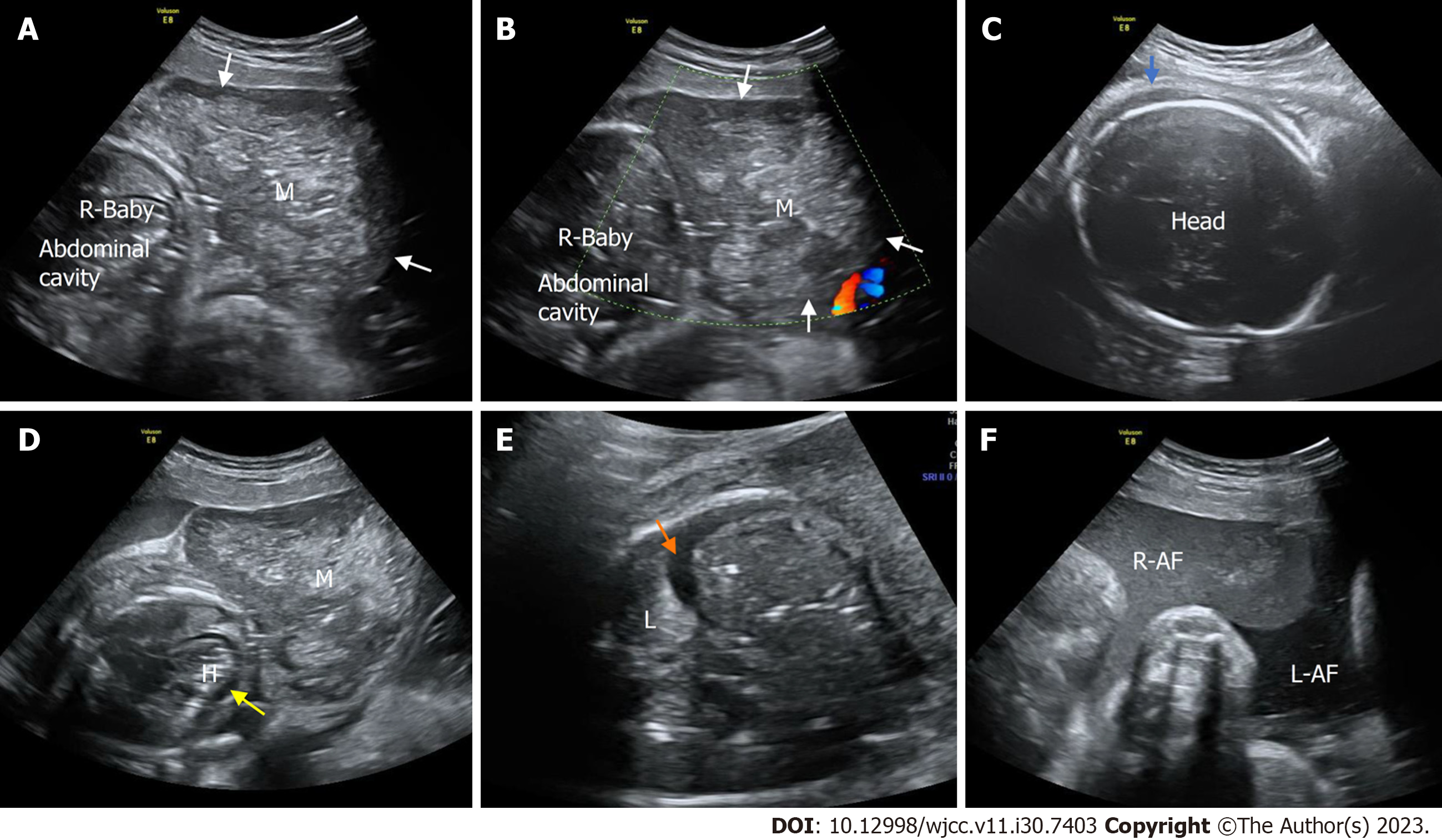
Case 1: The prenatal ultrasound images at 34+5 wk of gestation showed a well-defined, heterogeneous soft tissue mass, 5.5 cm × 4.5 cm in size, from the left axilla to the left chest wall of the fetus. The mass mainly comprised isoechoic solid components. Color Doppler flow imaging (CDFI) revealed abundant blood flow within the mass, with a dendritic distribution (Figures 1A and B). Prenatal magnetic resonance imaging revealed an irregular soft tissue mass in the fetal axilla on the T2 weighted image (Figure 1C). The size of the lesion was more extensive on postnatal ultrasound than on prenatal ultrasound, and the lesion had increased arterial blood flow. Pulse Doppler flow imaging showed low-resistance blood flow within the mass (resistance index, RI was 0.19) (Figure 2A and B). X-ray showed a vast basal arc-shaped soft tissue density shadow in the left axilla and lateral chest wall. The boundary with the chest wall tissue was unclear, and the adjacent ribs were compressed, deformed, and displaced inward (Figure 2C).
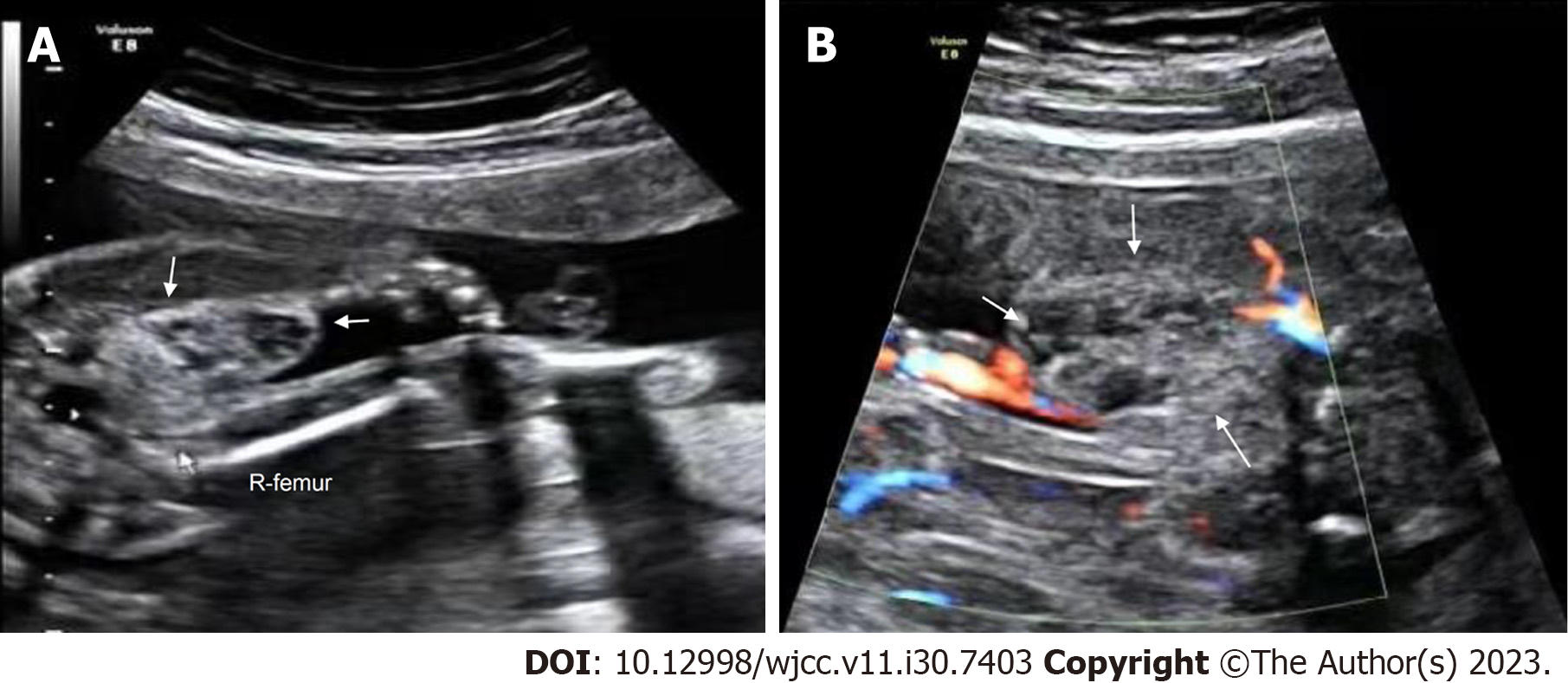
Case 2: A well-defined, heterogeneous mass (2.6 cm × 1.4 cm) was found at the root of the right thigh of the fetus on ultrasonography at 23+1 wk of gestation. CDFI showed sparse punctate blood flow around the mass (Figure 3).
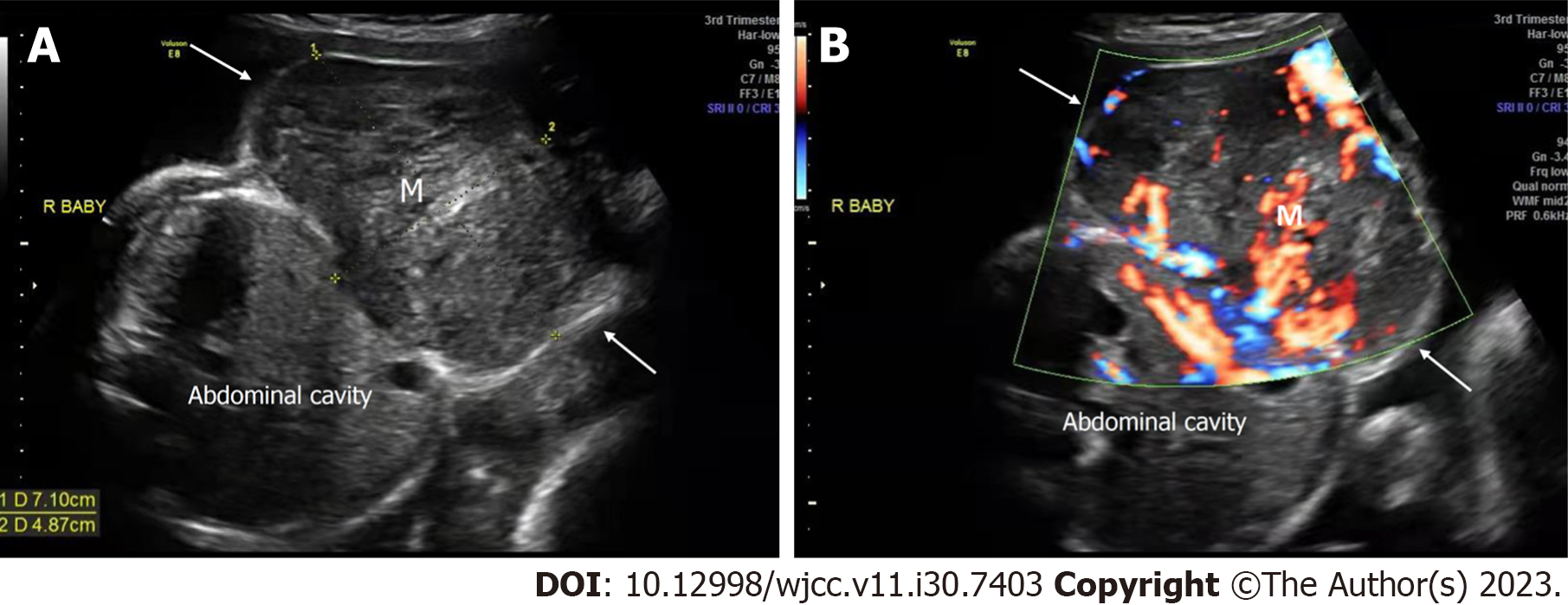
Case 3: Prenatal ultrasound at 30+0 wk of gestation showed that in one of the twins, a heterogeneous mass (7.1 cm × 4.9 cm) protruded from the subcutaneous layer of the thoracic and abdominal wall to the body surface with a clear boundary, complete capsule, smooth surface, and regular shape. The boundary between the mass and the abdominal wall was unclear. CDFI showed abundant blood flow within the mass, with a dendritic distribution (Figure 4). Prenatal ultrasound at 33+0 wk of gestation showed that the affected fetus had no heartbeat, and the volume of the mass in the thoracic and abdominal wall had increased significantly, to 9.6 cm × 5.5 cm. Compared to the previous examination, the internal echoes of the mass were more chaotic. The mass had a clear boundary and regular shape, and no apparent defect was found in the abdominal wall. CDFI showed no blood flow in the mass. At the same time, the whole body of the fetus showed edema, and the thickness of the scalp was approximately 6 mm. Effusion was found in the pleural cavity and pericardial cavity of the affected fetus, accompanied by unclear amniotic fluid (Figure 5).
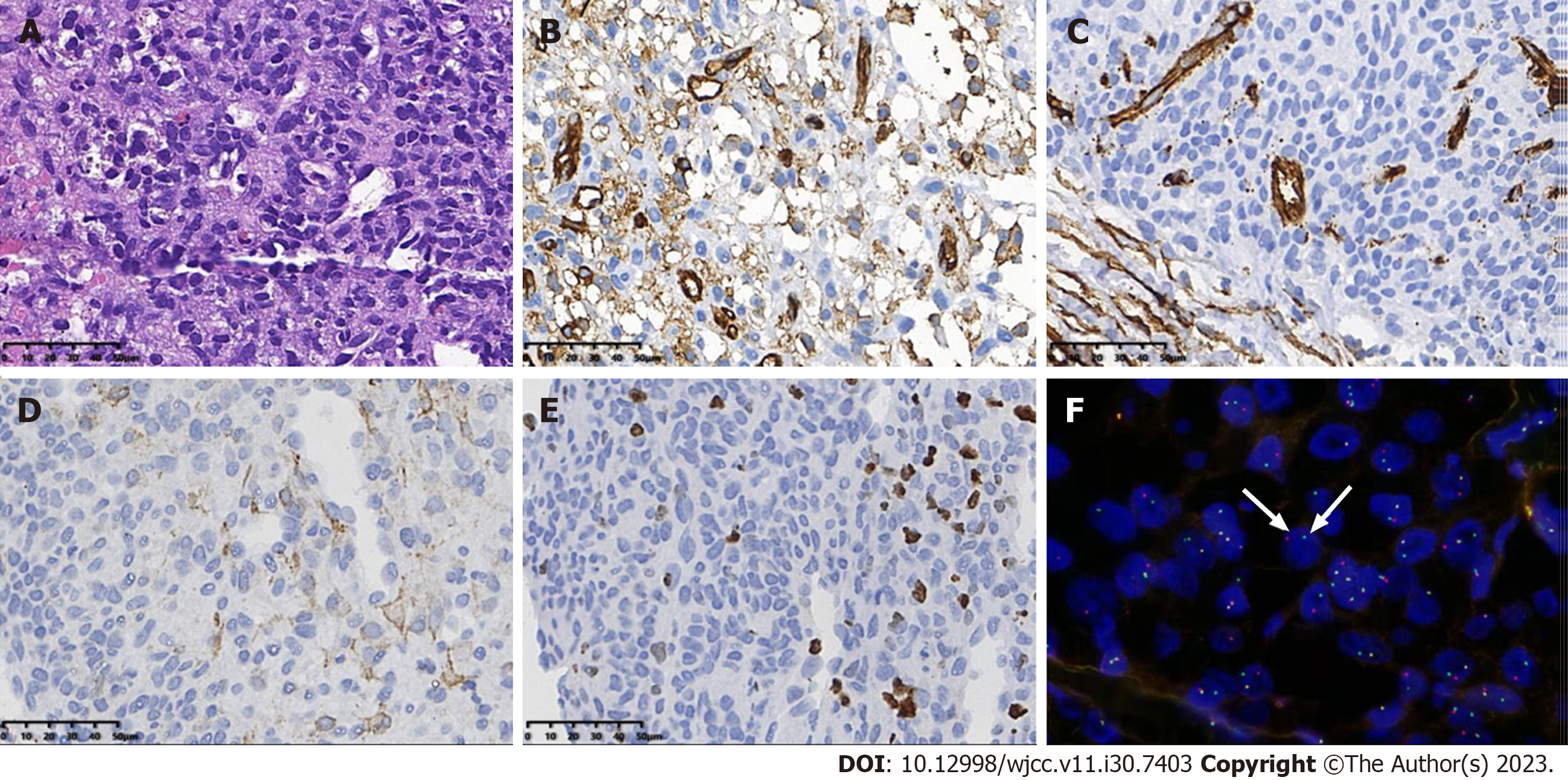
Case 1: The postoperative pathological findings in Case 1 were as follows: The tumor cells showed eosinophilic, diffuse sheets of monotonous round cells with fine chromatin and indistinct nucleoli. Immunochemistry demonstrated the expression of smooth muscle actin (SMA), CD34, and CD31 in tumor cells, and the Ki-67 proliferation index was approximately 20%. Using fluorescence in situ hybridization (FISH), the tumor cells showed NTRK3 gene rearrangement, with break-apart green (telomeric) and red (centromeric) signals, which confirmed the diagnosis of CIF (Figure 6).
Case 2: Case 2 underwent ultrasound guided biopsy of the lesion.
Case 3: Pathological examination of the lesion from the stillborn fetus was conducted.
Case 1: CIF.
Case 2 and Case 3: CH.
Case 1: The patient delivered a baby boy by cesarean section at 37+0 wk of gestation. Owing to the detection of thrombocytopenia and abnormal coagulation function, platelets, human fibrinogen and plasma were administered. After correcting coagulation dysfunction, surgical resection of the tumor was performed on Day 50.
Case 2: The patient delivered a baby boy at 40+0 wk of gestation. Owing to the detection of anemia, thrombocytopenia and abnormal coagulation function, cell suspensions and platelets were administered. Oral prednisone and propranolol were administered and were adjusted based on the changes in the CH. In addition, the tumor was closely monitored.
Case 3: After urgently promoting fetal lung maturity, the pregnant woman delivered two fetuses by cesarean section, one of which was stillborn and had a considerable mass in the thoracic and abdominal wall. The vital signs of the other fetus were stable.
Case 1: After the operation, the child recovered well, and the tumor had not recurred at 12 mo of follow-up.
Case 2: The mass on the right thigh became softer and smaller in size. At present, the child is two years old. The lesion on the right thigh has no noticeable bulge, and movement of the right leg is unrestricted.
Case 3: The newborn was discharged after a period of observation. A summary of the characteristics of the above three cases is shown in Table 1.
| Case 1 | Case 2 | Case 3 | ||
| Age (yr) | 31 | 29 | 32 | |
| History of gravida and para | G 1 P 0 | G 1 P 0 | G 2 P 1 | |
| Singleton/Twin | Singleton | Singleton | Twin | |
| GA at diagnosis (wk + d) | 28 + 0 | 23 + 1 | 28 + 3 | |
| Location | The left axilla to the left chest wall | The root of the right thigh | Thoracic and abdominal wall | |
| Prenatal fetal ultrasound findings at diagnosis | Size (cm) | 3.2 × 3.0 | 2.6 × 1.4 | 5.0 × 4.2 |
| Echoes | Heterogeneous | Heterogeneous | Heterogeneous | |
| Boundary | Clear | Clear | Clear | |
| Shape | Irregular | Irregular | Regular | |
| Blood flow (Adler) | Grade III | Grade I | Grade III | |
| Prenatal management | None | None | At 33 + 0 wk, due to intrauterine fetal death on the affected side, dexamethasone was given to promote lung maturation of the contralateral fetus | |
| GA at delivery (wk + d) | 37 + 0 | 40 + 0 | 33 + 3 | |
| Mode of delivery | Caesarean section | Normal spontaneous vaginal delivery | Caesarean section | |
| Apgar score | / | |||
| 1 min | 9 | 9 | ||
| 5 min | 10 | 10 | ||
| 10 min | 10 | 10 | ||
| Postnatal physical examination: Restricted movement of affected limbs | Yes | Yes | / | |
| Postnatal fetal ultrasound findings | / | |||
| Size (Compared to the last prenatal ultrasound) | Increase | Unchanged | ||
| Repeat ultrasound (growth trend in size in the short-term) | Yes | Yes | ||
| Blood flow (Adler) | Grade III | Grade III | ||
| Postnatal ultrasound-guided biopsy | Yes | Yes | ||
| Other postnatal imaging examinations | X-ray | MRI | / | |
| Postnatal laboratory examination: Coagulation dysfunction | Yes | Yes | / | |
| Postnatal management | Surgery + chemotherapy | Chemotherapy | / | |
| Pathological results | CIF | CH | CH | |
| Follow-up outcome (duration) | No recurrence (12 mo) | No recurrence (24 mo) | Death | |
CIF is rare, accounting for less than 1% of all childhood cancers; these tumors are mainly detected at birth (30%) or in the first year of life and are rarely detected prenatally[5]. Prenatal imaging is very useful for detecting abnormal fetal masses, but the final diagnosis relies on postpartum pathology confirmation. CIFs are usually located in subcutaneous tissues and develop in different parts of the body, most commonly in the extremities. Complete surgical resection is usually curative and sufficient in most cases[6]. Our findings were consistent with data in previous literature[7-11]. Fibrosarcoma is a highly vascular tumor, which leads to difficulties in interpretation on prenatal ultrasound and can show rapid growth. We have summarized these studies (Table 2) and compared their results to the present study. Prenatal imaging features of CIF are nonspecific, and the diagnosis of CIF is challenging. On prenatal sonography, CIF appears as a large, expansive, solid soft tissue mass. It usually presents as a heterogeneous lesion with high growth potential that tends to compress adjacent structures, and cystic echogenicity may be present within the mass. Central necrosis or hemorrhagic areas might be present depending on the rate of increase in the dimensions of the mass[12,13]. The blood supply arteries of CIFs are similar to typical tumor vessels, with irregular calibers and unorganized branching patterns[14]. The immunophenotype for CIF is nonspecific, with variable expression of desmin, muscle-specific actin, CD34, and S100 protein. ETV6/NTRK3 gene fusion is vital in making the correct diagnosis. Complete surgical resection is still the primary treatment for CIF. CIF can ulcerate within the uterus and cause many complications, such as polyhydramnios, torsion, anemia, hemorrhagic shock, hemolysis, oppression of adjacent organs, and cardiac insufficiency, all of which may threaten the life of the fetus[9]. In view of the imaging features, location and rapid growth of CIFs, we believe that prenatal diagnosis of soft tissue tumors and suspected fibrosarcoma is possible. It is essential to continuously monitor fetal vital signs and tumor development by ultrasound. For children with concomitant coagulation dysfunction, surgical resection after the correction of coagulation dysfunction can achieve good results. Long-term follow-up is necessary before it can be safely assumed that the child has been cured.
| Ref. | GA at diagnosis (wk) | Sex | Site | Size (cm) | Coagulopathy | Treatment | Outcome | GA at delivery (wk) | Modality of delivery | Birth weight (kg) | Prenatal ultrasound findings | Immunochemistry | ETV6/NTRK3 gene fusion detected | Affected organs or metastasis |
| Nonaka et al[7], 2004 | 19 | Male | Back | 6.1×2.2 | - | - | - | 29 | Pregnancy terminated at 29 wk | 1.4 | Solid mass involving the soft tissue at the rightparaspinal area | Vim(+) | Yes | Liver and left adrenal gland |
| Al-Salem et al[4], 2011 | - | Male | Sacrococcygeal | 12.0×10.0 | - | S | No recurrence during follow-up | 40 | Cesarean section | 4.1 | Not described in detail | Vim(+), Ki67(+)> 5% | Yes | Bone margin and skeletal muscle |
| Dumont et al[8], 2011 | 36 | Male | Right leg | 9.0×8.0 | Hemorrhagic shock | S* | Died at day 8 of life | 38 | Cesarean section | 3.3 | Poorly vascularized | Vim(+) | Yes | - |
| Tsang et al[9], 2013 | 37 | - | Right frontal | 9.0×7.0 | - | S | No recurrence during follow-up | 37 | Cesarean section | - | Not described in detail | Vim(+) | Yes | Skull |
| Kraneburg et al[10], 2013 | 36 | Male | Left leg | 11.8×9.3 | Bleeding, anemia, hypovolemia | S* | No recurrence during follow-up | 36 | Vaginal delivery | 2.1 | Well-circumscribed, intracavitary, homogenous solid mass | - | Yes | - |
| Sıvrıkoz et al[11], 2020 | 24 | Male | Anterior fetal neck and oropharyngeal | 5.5×5.4 | - | S+C | No recurrence during follow-up | 36 | Cesarean section | 2.9 | Hypervascular with irregular borders | Ki-67(+) 1% | Yes | Masseter muscle, parotid gland, thyroid gland |
| The present study, 2022 | 28 | Male | Left axilla to the left chest wall | 3.2×3.0 | Consumptive coagulation dysfunction | S+C | No recurrence during follow-up | 37 | Cesarean section | 2.6 | Well-defined, heterogeneous with abundant blood flow | Ki-67 (+) 20%, SMA (+), CD34 (+) and CD31 (+) | Yes | Pectoralis and adjacent ribs |
CH is the most common hypervascular benign tumor in fetuses. The prenatal imaging features of our patients were consistent with previous literature[3]. Ultrasonic features of CH included a high-vessel density, a high peak systolic velocity, and visible blood vessels. Brix performed Doppler ultrasound imaging of 3 fetuses with prenatally discovered CH, all of which showed abundant and rapid blood flow[15]. In Case 2 of our study, the lesion was small and did not have a rich blood supply at the time of prenatal ultrasound, but it grew rapidly during the monitoring process. Postnatal ultrasonography revealed a highly vascular soft tissue mass with smooth contours. In large lesions, complications such as thrombocytopenia, coagulation disorders (an increased prothrombin time, a decreased fibrinogen level, increased D-dimer levels, etc.), high cardiac output, or bleeding are usually present[3]. In Case 2, coagulation dysfunction also occurred after birth, and good results were achieved after the correction of coagulation function and conservative medical treatment. Unlike previous reports, Case 3 involved a twin fetus. Monitoring of the affected fetus and protection of the healthy fetus are key issues. In Case 3, after the prenatal discovery of a hypervascular mass on the surface of one of the twins, 5 wk later, the volume of the mass had increased significantly, and intrauterine stillbirth and fetal edema occurred. We speculated that this might be due to the large size of the lesion and high flow in tumor vessels leading to heart failure, resulting in fetal death. A previous study also showed that CH is a high-flow vascular tumor that can cause high-output heart failure[16]. This might also be one of the causes of fetal death in utero. Although CHs are benign tumors, in Case 3, the CH progressed rapidly and led to an adverse outcome. Close ultrasonography follow-up was necessary, especially when the volume of the tumor increased rapidly and when CDFI demonstrated hypervascularization. In Case 3, after the affected fetus died in utero, treatment to promote fetal lung maturation and emergency cesarean section were performed immediately, which ensured stability of the vital signs of the normal fetus and achieved good results. Based on our experience and previous studies, in the case of prenatal detection of a suspected fetal tumor, biweekly ultrasonography is recommended for the first four weeks after diagnosis to dynamically assess the likelihood of delivery and fetal cardiac status[15].
In summary, both CIF and CH may present as soft tissue masses with rich blood supplies on the fetal body surface prenatally. Once discovered, close dynamic ultrasonography is very important, especially for large lesions that grow rapidly. Prenatal ultrasound can provide accurate information such as the location, size, growth direction and blood supply of the tumor, which can provide useful information for perinatal management strategies. Although it is difficult to differentiate between CH and CIF with prenatal ultrasound, it can provide useful imaging information and is a convenient means of monitoring. Especially for lesions that occur prenatally in one twin, close ultrasonographic monitoring may allow early prediction of adverse outcomes in the affected fetus. Once adverse outcomes occur, prompt obstetric intervention can ensure successful delivery of the healthy fetus.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aydin S, Turkey; Yücel O, Turkey S-Editor: Liu JH L-Editor: Webster JR P-Editor: Zhang YL
| 1. | Parkes SE, Muir KR, Southern L, Cameron AH, Darbyshire PJ, Stevens MC. Neonatal tumours: a thirty-year population-based study. Med Pediatr Oncol. 1994;22:309-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 81] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Olsen GM, Nackers A, Drolet BA. Infantile and congenital hemangiomas. Semin Pediatr Surg. 2020;29:150969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Alluhaybi AA, Abdulqader SB, Altuhayni K, AlTurkstani A, Kabbani A, Ahmad M. Preoperative trans-arterial embolization of a giant scalp congenital hemangioma associated with cardiac failure in a premature newborn. J Int Med Res. 2020;48:300060520977589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Al-Salem AH. Congenital-infantile fibrosarcoma masquerading as sacrococcygeal teratoma. J Pediatr Surg. 2011;46:2177-2180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Farmakis SG, Herman TE, Siegel MJ. Congenital infantile fibrosarcoma. J Perinatol. 2014;34:329-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Janz TA, Nagasubramanian R, Wei JL. Pediatric head and neck fibrosarcomas: A demographical, treatment, and survival analysis and review of a rare case. Int J Pediatr Otorhinolaryngol. 2019;116:92-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Nonaka D, Sun CC. Congenital fibrosarcoma with metastasis in a fetus. Pediatr Dev Pathol. 2004;7:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Dumont C, Monforte M, Flandrin A, Couture A, Tichit R, Boulot P. Prenatal management of congenital infantile fibrosarcoma: unexpected outcome. Ultrasound Obstet Gynecol. 2011;37:733-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Tsang HH, Dolman PJ, Courtemanche DJ, Rassekh SR, Senger C, Lyons CJ. Prenatal presentation of fronto-orbital congenital infantile fibrosarcoma: a clinicopathologic report. JAMA Ophthalmol. 2013;131:965-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Kraneburg UM, Rinsky LA, Chisholm KM, Khosla RK. Emergency surgical treatment of an ulcerative and hemorrhagic congenital/infantile fibrosarcoma of the lower leg: case report and literature review. J Pediatr Orthop B. 2013;22:228-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Sıvrıkoz TS, Uygu LS, Kunt İşgüder Ç, Aygun E, Kalelioglu IH, Has R. The Giant Infantile Fibrosarcoma of Fetal Oropharynx and Anterior Neck. Fetal Pediatr Pathol. 2022;41:451-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Scheier M, Ramoni A, Alge A, Brezinka C, Reiter G, Sergi C, Hager J, Marth C. Congenital fibrosarcoma as cause for fetal anemia: prenatal diagnosis and in utero treatment. Fetal Diagn Ther. 2008;24:434-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Meizner I, Potlog-Nahari C, Mashiach R, Shalev J, Vardimon D, Ben-Sira L. In utero ultrasound detection of a large fetal sarcoma of the back. Ultrasound Obstet Gynecol. 2001;18:540-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Marguet F, Bergogne L, Laurent N, Rousseau T, Laquerrière A. Fetal presentation of congenital fibrosarcoma of the meninges: case report and literature review. Clin Neuropathol. 2015;34:70-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Brix M, Soupre V, Enjolras O, Vazquez MP. [Antenatal diagnosis of rapidly involuting congenital hemangiomas (RICH)]. Rev Stomatol Chir Maxillofac. 2007;108:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Iacobas I, Phung TL, Adams DM, Trenor CC 3rd, Blei F, Fishman DS, Hammill A, Masand PM, Fishman SJ. Guidance Document for Hepatic Hemangioma (Infantile and Congenital) Evaluation and Monitoring. J Pediatr. 2018;203:294-300.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |