Published online Oct 26, 2023. doi: 10.12998/wjcc.v11.i30.7372
Peer-review started: September 4, 2023
First decision: September 13, 2023
Revised: September 24, 2023
Accepted: October 8, 2023
Article in press: October 8, 2023
Published online: October 26, 2023
Processing time: 51 Days and 7.4 Hours
Burkholderia pseudomallei (B. pseudomallei) is a short, straight, medium-sized Gram-negative bacterium that mostly exists alone, without a capsule or spores, has more than three flagella at one end, and actively moves. B. pseudomallei confers high morbidity and mortality, with frequent granulocytopenia in B. pseudomallei sepsis-related deaths. However, mortality may be related to hemophagocytic lymphohistiocytosis (HLH) secondary to B. pseudomallei infection.
A 12-year-old female was referred from a local hospital to the pediatric intensive care unit with suspected septic shock and fever, cough, dyspnea, and malaise. After admission, supportive symptomatic treatments including fluid resus
The higher mortality rate in patients with B. pseudomallei sepsis may be related to secondary HLH after infection, wherein multiorgan dysfunction syndrome may be directly related to infection or immune damage caused by secondary HLH. Patients with B. pseudomallei can be asymptomatic and can become an infective source.
Core Tip: Given the high mortality rate associated with Burkholderia pseudomallei (B. pseudomallei), it is particularly important to fully understand the pathogenesis. This report presents the clinical characteristics of a case of B. pseudomallei infection and some clinical data of the patient’s brother, who also died from B. pseudomallei infection. The chronic carrier status of B. pseudomallei and secondary hemophagocytic lymphohistiocytosis warrants attention in research on the pathogenesis and treatment of B. pseudomallei sepsis.
- Citation: Sui MZ, Wan KC, Chen YL, Li HL, Wang SS, Chen ZF. Fatal hemophagocytic lymphohistiocytosis-induced multiorgan dysfunction secondary to Burkholderia pseudomallei sepsis: A case report. World J Clin Cases 2023; 11(30): 7372-7379
- URL: https://www.wjgnet.com/2307-8960/full/v11/i30/7372.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i30.7372
Burkholderia pseudomallei (B. pseudomallei) is a non-fermentative Gram-negative bacterium that is positive for oxidases and enzymes, does not form spores, and does not contain metachromatic particles. Approximately 165000 cases of B. pseudomallei infection and 89000 deaths are reported annually worldwide. The incidence rates in South and East Asia and the Pacific are 44% and 40%, respectively, and the mortality rates are 47% and 35%, respectively[1].
Sequential dysfunction of two or more organs is usually referred to as multiorgan dysfunction syndrome (MODS). MODS caused by sepsis likely contributes to the high mortality rates associated with B. pseudomallei infections. However, granulocytopenia, which is common in areas where such cases have been reported, has been largely neglected by the research community. Combined with the clinical data on elevated ferritin levels, there is a need to examine the status of hemophagocytosis in deaths due to B. pseudomallei[2].
Herein, we have described the clinical characteristics of a patient infected with B. pseudomallei and the clinical data of her brother, who died from the same illness.
A 12-year-old female was transferred from a local hospital to our pediatric intensive care unit after 4 d of fever, cough for 2 d, and dyspnea and malaise for 1 d.
The patient had originally presented to the local clinic with a very high fever (> 40.0 °C) and had been administered oral antibiotic treatment after a routine blood examination; however, the patient’s parents were unaware of the type of antibiotics administered. After treatment, the patient’s high fever continued and she developed a cough and other new symptoms. She then presented to a local hospital, where routine blood examination indicated agranulocytosis and blood cell and platelet levels that were significantly lower than those in the last test. After receiving ceftazidime at the hospital, the body temperature remained high and dyspnea and fatigue persisted. Arterial blood gas analysis suggested lactic acidosis, indicating that the patient was experiencing consolidated septic shock.
The patient’s parents denied a history of hepatitis, tuberculosis, measles, mumps, or other common infectious diseases. The patient had no history of surgery, trauma, or blood transfusions.
The patient did not have a history of preterm delivery, birth asphyxia, intrauterine hypoxia, intrauterine conditions, or infection. The patient’s brother had died 5 years earlier due to B. pseudomallei sepsis and septic shock (results of laboratory tests are shown in Table 1).
| Variable | Reference range | Initial value |
| Routine blood examination | ||
| White cell count as × 109/L | 4.30-11.30 | 0.60 |
| Neutrophil count as × 109/L | 1.60-7.80 | 0.40 |
| Hemoglobin in g/L | 118-156 | 104 |
| Platelet count as × 109/L | 150-407 | 184 |
| Inflammatory indicators | ||
| C-reactive protein in mg/L | 0-8.00 | 200.00 |
| Procalcitonin in ng/mL | 0-0.046 | 98.670 |
| Erythrocyte sedimentation rate in mm/h | 0-15 | 33 |
| Arterial blood gas analysis | ||
| pH | 7.350-7.450 | 7.076 |
| PaO2 in mmHg | 83.0-108.0 | 43.4 |
| PaCO2 in mmHg | 35-45 | 37 |
| HCO3- in mmol/L | 22.0-27.0 | 12.5 |
| BE in mmol/ | -3.0 to 3.0 | -19.0 |
| Lactic acid in mmol/L | 0.5-1.7 | 13.1 |
| Coagulative function | ||
| Prothrombin time in s | 9.8-13.2 | 21.1 |
| International normalized ratio | 0.85-1.20 | 1.64 |
| D-dimer in ng/mL | 0-0.50 | 4209.00 |
| Biochemical examination | ||
| Glutamic pyruvic transaminase in U/L | 7-30 | 43 |
| Albumin in g/L | 39.0-54.0 | 27.4 |
| Total bilirubin in µmol/L | 0-21.0 | 27.2 |
| Direct bilirubin in µmol/L | 0-8.0 | 20.1 |
| Urea nitrogen in mmol/L | 2.5-6.5 | 8.8 |
Body temperature, respiratory rate, and heart rate were 37.5 °C, 34 times/min, and 146 beats/min, respectively. Furthermore, the body weight was 40 kg, and skin oxygen saturation was 87% in room air. The patient’s mentality was depressed. Both pupils were equal in size and responsive to light. The nasal wings were flapped, the lips were cyanotic, breathing was rapid, the three concave signs were positive, and auscultation revealed dense moist rales. Heartbeat sounds were low. The liver and spleen were palpable under the costal margin (1.5 cm below the right midclavicular costal margin and 1.5 cm below the left midclavicular coastal margin, respectively). The extremities were cold, and capillary filling time was 6 s; however, no obvious abnormality was observed in the neurological examination.
Routine blood tests revealed agranulocytosis, thrombocytopenia, and anemia. Arterial blood gas analysis revealed sustained hypoxia and acidosis. Monitoring of coagulative parameters indicated hypofibrinogenemia. The levels of C-reactive protein, procalcitonin, erythrocyte sedimentation rate, and other inflammatory indicators significantly increased. A complete biochemical examination revealed varying degrees of multiorgan dysfunction. The levels of interleukin and inflammatory factors, such as ferritin, were higher than normal, and no biomarkers related to hemophagocytic syndrome were found in the whole-exon test (described in Table 2).
| Variable | Reference range | Initial value | Value retested after 4 h | Last value before death |
| Routine blood examination | ||||
| White cell count as × 109/L | 4.30-11.30 | 0.69 | 0.83 | - |
| Neutrophil count as × 109/L | 1.60-7.80 | 0.08 | 0.08 | - |
| Hemoglobin in g/L | 118-156 | 112 | 83 | - |
| Platelet count as × 109/L | 150-407 | 50 | 23 | - |
| Inflammatory indicators | ||||
| C-reactive protein in mg/L | 0-8.00 | 239.04 | 135.83 | - |
| Procalcitonin in ng/mL | 0-0.046 | 73.540 | - | - |
| Erythrocyte sedimentation rate in mm/h | 0-20 | 8 | - | - |
| Ferritin in ng/mL | 4.63-204.00 | 14586.00 | - | - |
| Arterial blood gas analysis | ||||
| pH | 7.350-7.450 | 7.150 | 7.230 | 6.840 |
| PaO2 in mmHg | 83.0-108.0 | 69.0 | 73.0 | 22.0 |
| PaCO2 in mmHg | 35-45 | 28 | 28 | 88 |
| BE in mmol/L | -3.0 to 3.0 | -17.6 | -14.5 | -19.2 |
| Oxygenation index | - | 191 | 202 | 36 |
| Lactic acid in mmol/L | 0.5-1.7 | 15.8 | 14.5 | 16.7 |
| Coagulation function | ||||
| Prothrombin time in s | 9.8-13.2 | 22.9 | 53.6 | - |
| International normalized ratio | 0.85-1.20 | 1.97 | 5.99 | - |
| Thrombin time in s | 14.0-21.0 | 14.7 | 240.0 | - |
| Activated partial thromboplastin time in s | 28.0-43.0 | 58.1 | 180.0 | - |
| Fibrinogen in g/L | 2.00-4 | - | 1.56 | - |
| D-dimer in ng/mL | 0-0.50 | 24.93 | 11.43 | - |
| Biochemical examination | ||||
| Glutamic pyruvic transaminase in U/L | 7.0-30.0 | 101.4 | 192.6 | - |
| Albumin in g/L | 39.0-54.0 | 27.3 | 19.7 | - |
| Total bilirubin in µmol/L | 0-21.00 | 89.07 | 54.83 | - |
| Direct bilirubin in µmol/L | 0-8.00 | 51.46 | 31.99 | - |
| Indirect bilirubin in µmol/L | 0-21.00 | 37.61 | 22.84 | - |
| Urea nitrogen in µmol/L | 2.50-6.50 | 10.75 | 11.02 | - |
| Creatinine in µmol/L | 27-66 | 157 | 82 | - |
| K+ in mmol/L | 3.50-5.50 | 3.41 | 2.65 | - |
| Na+ in mmol/L | 130.0-150.0 | 135.9 | 142.6 | - |
| Ca2+ in mmol/L | 2.10-2.80 | 1.76 | 1.45 | - |
| Triglyceride in mmol/L | 0-1.70 | 1.57 | - | - |
| Cytokines in pg/mL | ||||
| Interleukin-1B | 0-12.40 | 623.47 | - | - |
| Interleukin-6 | 0-5.30 | 1452.90 | - | - |
| Interleukin-8 | 0-53.09 | 1876.33 | - | - |
| Interleukin-10 | 0-4.91 | 5754.38 | - | - |
| Interferon-γ | 0-7.42 | 3143.99 | - | - |
| Tumor necrosis factor | 0-4.60 | 42.28 | - | - |
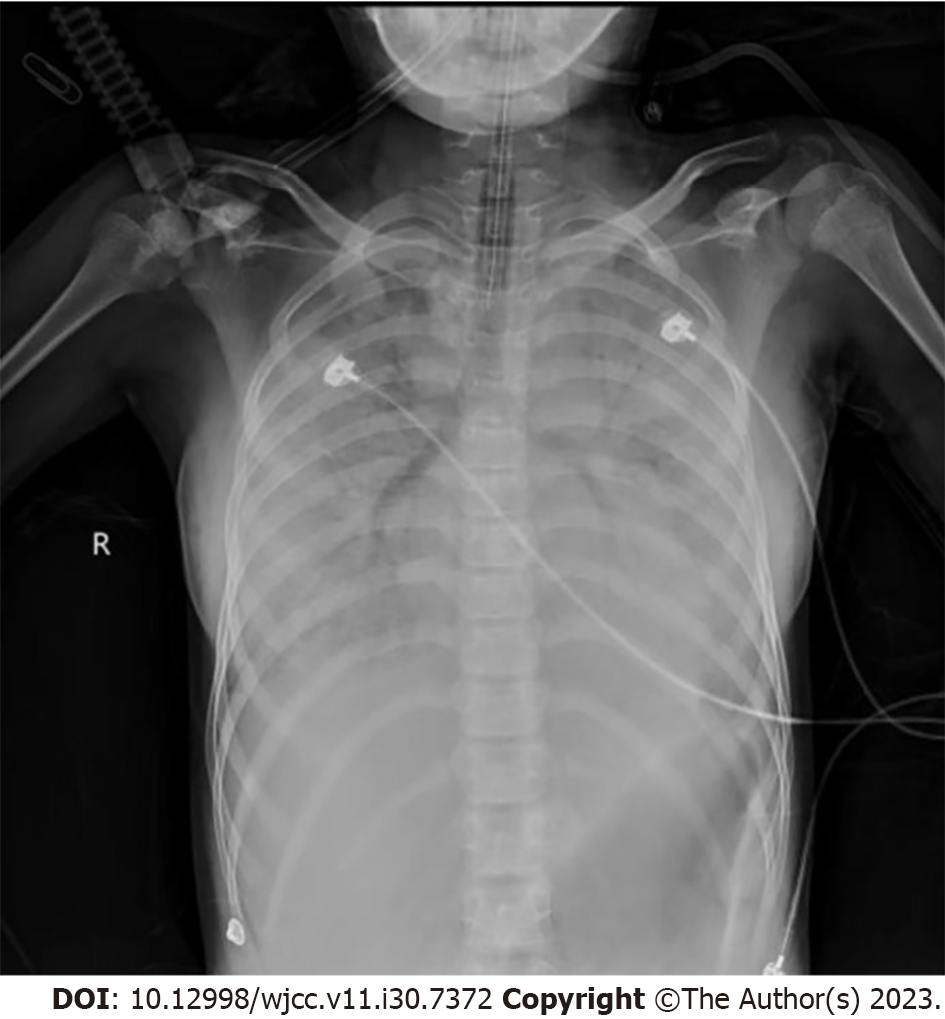
Chest radiography revealed exudative lesions in both lungs (Figure 1).
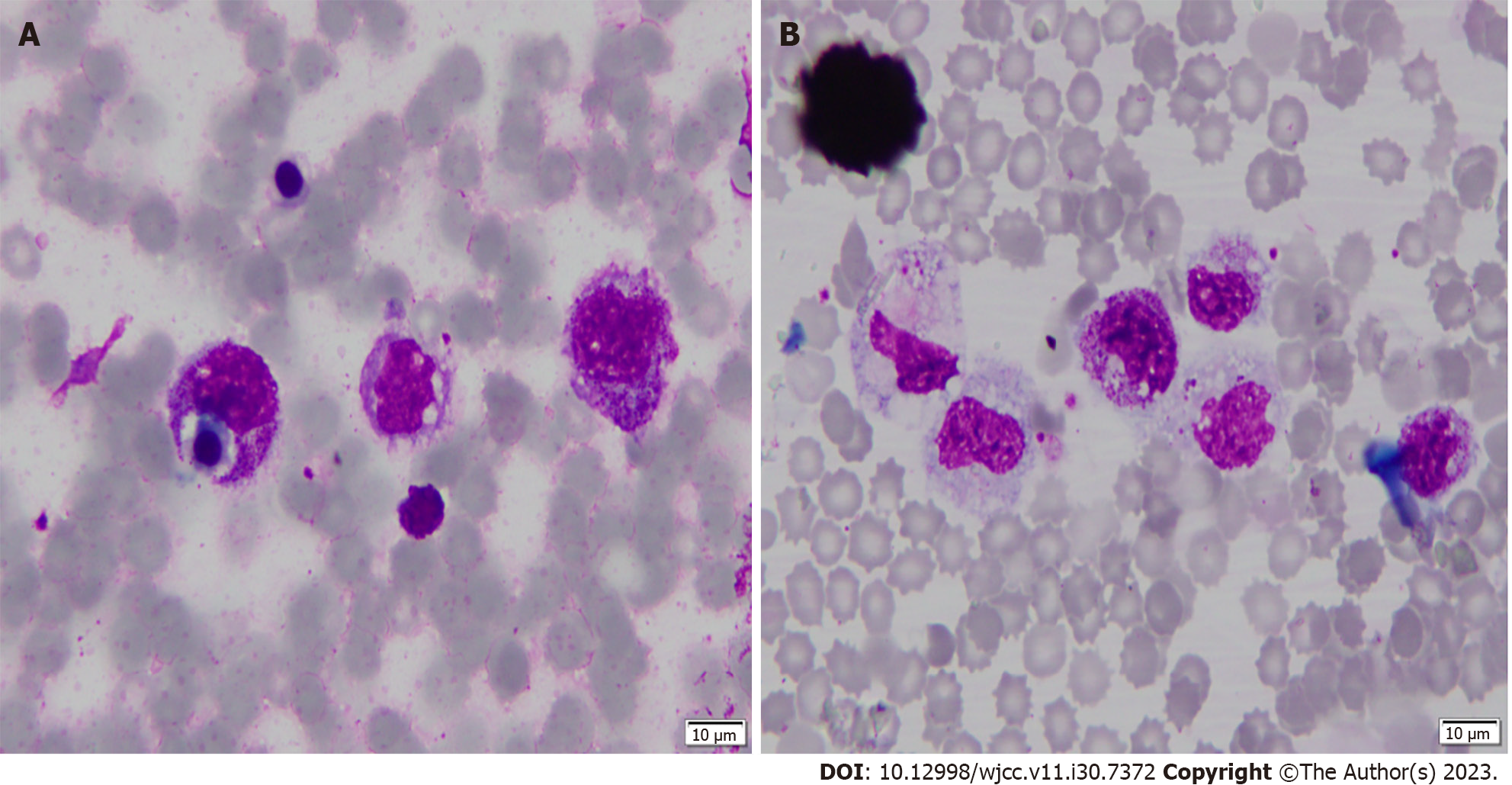
A bone marrow smear showed hemophagocytosis (Figure 2).
The patient was finally diagnosed with B. pseudomallei sepsis, septic shock, MODS, acute respiratory distress syndrome, respiratory failure, severe pneumonia, metabolic acidosis, disseminated intravascular coagulation, electrolyte metabolism disorder, agranulocytosis, and thrombocytopenia, with a high suspicion of secondary hemophagocytic lymphohistiocytosis (HLH).
Meropenem was initiated. The patient immediately received a 2:1 isotonic solution to expand the volume, twice along with nasal catheter oxygen inhalation (oxygen flow ≤ 5 L/min). We established a femoral vein infusion path to simultaneously implement subsequent fluid support. As the blood pressure of the patient could not be maintained after volume expansion and the analysis of arterial blood gas indicated continuous hypoxia due to irregular spontaneous respiration, we administered norepinephrine 0.5 µg/kg/min continuous pumping to maintain blood pressure and invasive mechanical ventilation with endotracheal intubation (PC-SIMV mode: FiO2 60%, VT 300 mL, PEEP 5 cmH2O, RR25 times/min). Supportive treatments, such as granulocyte-stimulating factor, plasma, coagulation factor cryoprecipitate, and suspended red blood cells, were also administered.
Nevertheless, the patient experienced respiratory and cardiac arrest 4 h after admission, and a pink foam-like liquid gushed from the endotracheal tube. After cardiopulmonary resuscitation and intravenous morphine administration, the patient’s heart rate recovered, but the results of arterial blood gas analysis worsened (Table 2). In addition to hypoxia, the patient had serious carbon dioxide retention; we changed the invasive mechanical ventilation mode to high-frequency mode (FiO2 60%, average airway pressure 35 cmH2O, amplitude 75 cmH2O, sighing time 0.3s, frequency 7 Hz).
After the above rescue, the patient’s condition continued to deteriorate, and norepinephrine (1 µg/kg/min) combined with dopamine (6 µg/kg/min) still failed to maintain normal blood pressure. The patient’s consciousness gradually turned into coma, and diffuse bleeding spots appeared over the patient’s entire body. We urgently punctured the bone marrow, injected vitamin K1, ethylphenesulfonate, and snake venom hemocoagulase to stop bleeding and added m-hydroxylamine to raise the blood pressure. At the same time, we actively administered bedside blood purification treatment and adjusted norepinephrine to 1.4 µg/kg/min, dopamine to 12 µg/kg/min, and m-hydroxylamine to 2 µg/kg/min during continuous renal replacement therapy. The patient’s blood pressure remained unstable, and the maintenance of transcutaneous oxygen saturation was unsatisfactory. After 12 h of hospitalization, the patient died.
The patient eventually died, and blood culture was positive for B. pseudomallei. The patient’s living brother also had a fever at the same time. The parents requested that the patient’s brother be hospitalized. Based on the patient’s brother’s blood culture and drug sensitivity results, imipenem was administered to him. Finally, the patient’s brother was discharged with a normal body temperature, and no pathogenic bacterial growth was observed in subsequent blood cultures.
HLH is a macrophage proliferative disease mostly caused by Epstein-Barr virus infection, whereas HLH caused by bacterial infections is relatively rare. According to the HLH-2004 guidelines, the diagnosis of HLH needs to meet five of the eight diagnostic criteria: (1) Fever; (2) spleen enlargement; (3) decrease of peripheral blood cells, involving 2-3 lines, that is hemoglobin < 90 g/L, platelet count < 100 × 109/L, and neutrophils < 1.0 × 109/L; (4) hypertriglyceridemia and/or low fibrinogen, with fasting triglyceride ≥ 3.0 mmol/L (≥ 2.65 g/L) and fibrinogen ≤ 1.5 g/L; (5) blood phagocytic cells in the bone marrow, spleen, or lymph nodes, and no evidence of malignant tumor; (6) activity of natural killer cells being reduced or completely absent; (7) serum ferritin ≥ 500 µg/L; and (8) soluble CD25 (interleukin-2 receptor) ≥ 2400 U/mL[3]. According to the guidelines for fever, a temperature ≥ 38.5°C for more than 7 d is required. The patient reported in this article was declared clinically dead on the 5th d of the fever. Therefore, the patient’s clinical data only met criteria 2, 3, 5, and 7. Unfortunately, the whole-exon test failed to identify the molecular biological markers supporting HLH. Many indicators cannot be reviewed or improved over time because of the rapid worsening of a patient’s condition. Although the clinical diagnosis of HLH was not confirmed, the results of the patient’s bone marrow examination and MODS caused by HLH cannot be ignored among the many factors that lead to the patient’s death.
In addition, case reports of focal infections have shown that most patients have a good prognosis, and the examination indicators for these patients are quite different from the clinical manifestations of HLH[4-6]. Systemic infections are not complicated by MODS[2]. Therefore, the final progression of secondary HLH to B. pseudomallei sepsis contributes to patient mortality, and B. pseudomallei infection is mainly observed during the rainy season in tropical and subtropical regions. The seasonal incidence may be related to the survival of bacteria in the soil. Knowledge of the history of contact between pestilence-related soil and water sources is particularly important for early treatment by doctors.
Several preclinical studies have shown that the lungs, liver, and spleen are the most common target organs for chronic B. pseudomallei infection[7]. Studies have shown that B. pseudomallei regulates phagocytic death and aids in the progression of acute or chronic infections[8]. After B. pseudomallei invade the body, they recruit host complement regulatory proteins for immune evasion[9]. Furthermore, bacteria can survive in small abscesses formed in target organs. Over time, the chronic infection site forms granulomas with neutrophils, macrophages, and lymphocytes as the main components[10]. During this process, the patient’s symptoms gradually improve, which is often considered a clinical cure. However, this patient was a chronic disease carrier. The patient reported in this article did not have any history of living in an endemic area. Based on the patient’s brother’s history, we suspected that the source of infection was an asymptomatic carrier who came into contact with the patient.
Most B. pseudomallei strains are sensitive to imipenem. The most important step is to identify the causative pathogen as soon as possible and provide effective interventions before severe clinical events occur. Early diagnosis based on bioinformatic analysis may help solve these problems[11]. Future vaccine development and bacteriophage therapy will help to reduce the incidence and mortality of B. pseudomallei[12,13].
A person infected with B. pseudomallei may be an asymptomatic carrier owing to the unique mechanism of chronic B. pseudomallei infection. The high fatality rate of B. pseudomallei may be related to MODS caused by secondary HLH, as observed in this case.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Moldovan CA, Romania S-Editor: Lin C L-Editor: Filipodia P-Editor: Yu HG
| 1. | Limmathurotsakul D, Golding N, Dance DA, Messina JP, Pigott DM, Moyes CL, Rolim DB, Bertherat E, Day NP, Peacock SJ, Hay SI. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat Microbiol. 2016;1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 673] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 2. | Bhaskaran P, Prasad V, Gopinathan A, Shaw T, Sivadas S, Jayakumar C, Chowdhury S, Dravid A, Mukhopadhyay C, Kumar A. Burkholderia pseudomallei in Environment of Adolescent Siblings with Melioidosis, Kerala, India, 2019. Emerg Infect Dis. 2022;28:1246-1249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski J, Janka G. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3075] [Cited by in RCA: 3600] [Article Influence: 200.0] [Reference Citation Analysis (1)] |
| 4. | Dimitriou NG, Flüh G, Zange S, Aytulun A, Turowski B, Hartung HP, Meuth SG, Gliem M. Case report: First case of neuromelioidosis in Europe: CNS infection caused by Burkholderia pseudomallei. Front Neurol. 2022;13:899396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Santos Júnior ELD, Moura JCR, Protásio BKPF, Parente VAS, Veiga MHND. Clinical repercussions of Glanders (Burkholderia mallei infection) in a Brazilian child: a case report. Rev Soc Bras Med Trop. 2020;53:e20200054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Verdecia JL, Jankowski CA, Isache CL, Neilsen CD, McCarter YS, Sands ML, Ravi M. A Case Report of Melioidotic Prostatic Abscess in a Traveler. Open Forum Infect Dis. 2022;9:ofac284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 7. | Amemiya K, Dankmeyer JL, Fetterer DP, Worsham PL, Welkos SL, Cote CK. Comparison of the early host immune response to two widely diverse virulent strains of Burkholderia pseudomallei that cause acute or chronic infections in BALB/c mice. Microb Pathog. 2015;86:53-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Krakauer T. Living dangerously: Burkholderia pseudomallei modulates phagocyte cell death to survive. Med Hypotheses. 2018;121:64-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Syed I, Wooten RM. Interactions Between Pathogenic Burkholderia and the Complement System: A Review of Potential Immune Evasion Mechanisms. Front Cell Infect Microbiol. 2021;11:701362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Lewis ER, Torres AG. The art of persistence-the secrets to Burkholderia chronic infections. Pathog Dis. 2016;74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Yin L, Chen Y, Fu T, Liu L, Xia Q. Identification of candidate blood biomarkers for the diagnosis of septicaemic melioidosis based on WGCNA. Artif Cells Nanomed Biotechnol. 2022;50:252-259. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Lauman P, Dennis JJ. Advances in Phage Therapy: Targeting the Burkholderia cepacia Complex. Viruses. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Wang G, Zarodkiewicz P, Valvano MA. Current Advances in Burkholderia Vaccines Development. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |