Published online Oct 26, 2023. doi: 10.12998/wjcc.v11.i30.7284
Peer-review started: August 15, 2023
First decision: August 31, 2023
Revised: September 11, 2023
Accepted: September 18, 2023
Article in press: September 18, 2023
Published online: October 26, 2023
Processing time: 70 Days and 22.5 Hours
Breast infiltrating ductal carcinoma (BIDC) represents the largest heterotypic tumor group, and an in-depth understanding of the pathogenesis of BIDC is key to improving its prognosis.
To analyze the expression profiles and clinical implications of forkhead box M1 (FOXM1), cyclooxygenase-2 (COX-2), and glucose-regulated protein 78 (GRP78) in BIDC.
A total of 65 BIDC patients and 70 healthy controls who presented to our hospital between August 2019 and May 2021 were selected for analysis. The peripheral blood FOXM1, COX-2, and GRP78 levels in both groups were measured and the association between their expression profiles in BIDC was examined. Additionally, we investigated the diagnostic value of FOXM1, COX-2, and GRP78 in patients with BIDC and their correlations with clinicopathological features. Furthermore, BIDC patients were followed for 1 year to identify factors in
The levels of FOXM1, COX-2, and GRP78 were significantly higher in BIDC patients compared to healthy controls (P < 0.05), and a positive correlation was observed among them (P < 0.05). Receiver operating characteristic analysis demonstrated that FOXM1, COX-2, and GRP78 had excellent diagnostic value in predicting the occurrence of BIDC (P < 0.05). Subsequently, we found significant differences in FOXM1, COX-2, and GRP78 levels among patients with different histological grades and metastasis statuses (with vs without) (P < 0.05). Cox analysis revealed that FOXM1, COX-2, GRP78, increased histological grade, and the presence of tumor metastasis were independent risk factors for prognostic death in BIDC (P < 0.001).
FOXM1, COX-2, and GRP78 exhibit abnormally high expression in BIDC, promoting malignant tumor development and closely correlating with prognosis. These findings hold significant research implications for the future diagnosis and treatment of BIDC.
Core Tip: Forkhead box M1, cyclooxygenase-2, and glucose-regulated protein 78 exhibit elevated expression in breast invasive ductal carcinoma (BIDC) and are associated with a poor prognosis. Their diagnostic value suggests their potential as biomarkers for BIDC detection. Understanding their clinical implications can aid in the diagnosis and treatment of BIDC, contributing to improved patient outcomes.
- Citation: Bai J, Li Y, Cai L. Clinical implications of forkhead box M1, cyclooxygenase-2, and glucose-regulated protein 78 in breast invasive ductal carcinoma. World J Clin Cases 2023; 11(30): 7284-7293
- URL: https://www.wjgnet.com/2307-8960/full/v11/i30/7284.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i30.7284
Breast cancer (BC) is the most prevalent malignancy in women, and its incidence has been increasing in recent years[1]. Among the various types of invasive BC, breast infiltrating ductal carcinoma (BIDC) represents the largest heterotypic tumor group, lacking distinct tissue characteristics and therefore being classified as a non-special type cancer[2]. BIDC is also the most common type of invasive BC, accounting for over 80% of cases[3]. While advancements in medical standards have contributed to a decrease in the overall mortality rate of BIDC, the prognosis for advanced BIDC patients remains unsatisfactory[4,5]. Researchers believe that a comprehensive understanding of the pathogenesis of BIDC and the discovery of new diagnostic and treatment methods are key to improving patient prognosis[6].
Forkhead box M1 (FOXM1), a member of the Fox protein family, plays a vital role in modulating the cell cycle[7]. Numerous studies have demonstrated that FOXM1 is overexpressed in various human tumors, promoting oncogenic transformation and participating in tumor occurrence and development[8,9]. Cyclooxygenase-2 (COX-2), a subtype of cyclooxygenase, has been identified as an essential component in the pathogenesis of malignant tumors and is closely associated with the occurrence and progression of BC based on previous data[10,11]. Additionally, glucose-regulated protein 78 (GRP78), a signature stress protein of the endoplasmic reticulum, has been found to induce tumor development, contribute to drug resistance, and facilitate tumor cell survival[12]. Although some studies have shown abnormal expression of FOXM1, COX-2, and GRP78 in BC[13-15], their specific roles in BIDC have yet to be characterized.
To develop new diagnostic and treatment protocols and identify targets for the prevention of BIDC, this study analyzed the expression profiles of FOXM1, COX-2, and GRP78 in BIDC and investigated their clinical implications in the disease. The objective was to establish the correlation between these markers and BIDC, thus laying a foundation for further research.
This study obtained approval from the Medical Ethics Committee and enrolled 65 BIDC patients [research group (RG)] and 70 healthy controls [control group (CG)], who presented to our hospital between August 2019 and May 2021. Informed consent was obtained from all participants.
Eligible patients had histopathologically confirmed BIDC[16] and exhibited normal organ function, complete clinical data, cooperation with treatment and follow-up, no contraindications to chemotherapy, and a life expectancy of ≥ 3 mo. Pregnant and lactating women were excluded from the study. Additionally, individuals with immune deficiency, inflammatory diseases, severe hematopoietic injury, a history of other malignant tumors, cardio-cerebrovascular diseases, and poor treatment compliance were excluded.
Fasting venous blood samples were collected from all participants upon admission. Total RNA was isolated from the blood using Trizol, followed by reverse transcription into cDNA for polymerase chain reaction (PCR) detection. The PCR reaction was conducted under the following conditions: 95 ℃/30 s and 40 cycles of 95 ℃/5 s, 60 ℃/30 s, and 72 ℃/30 s. The design and synthesis of primer sequences (Table 1) were performed by Tsingke Biotechnology Co., Ltd. The relative expression of the target genes to β-actin was calculated using the 2-ΔΔCT.
| Forward (5’-3’) | Reverse (5-3’) | |
| FOXM1 | CAGTCCGATTAGTCAGCTCCT | GTCATTTAGCTCCTTGTGCTG |
| COX-2 | CCGGGTACAATCGCACTTAT | GGCGCTCAGCCATACAG |
| GRP78 | GTTACAATCAAGGTAT | CATTCACATCTATCTCAA |
| β-actin | CTGATATAGCCGCGCTCG | CACTCGGTGCCGGATCATCA |
BIDC patients were followed for 1 year through regular hospital reexaminations, with reexamination intervals not exceeding 2 mo. Death was considered a termination event, and the patient’s prognosis and survival were recorded.
The expression profiles of FOXM1, COX-2, and GRP78 and their correlations in BIDC were analyzed. The diagnostic value of the three markers in BIDC patients and their correlations with clinicopathological features were also investigated. Finally, the factors influencing patient prognosis were analyzed based on the follow-up results.
Statistical analyses were performed using SPSS 22.0 software. Count data, such as previous medical history, are presented as percentages (%), and inter-group differences were assessed using the Chi-square test. Expression levels of FOXM1 and COX-2 and other measurement data are presented as the mean ± standard deviation, and inter-group and multi-group differences were identified using independent sample t-test and analysis of variance with Bonferroni post-hoc tests, respectively. Correlation analysis was conducted using the Pearson correlation coefficient, the diagnostic value was determined using receiver operating characteristic (ROC) analysis, and factors related to patient prognosis were identified using COX regression analysis. A significance level of P < 0.05 was used to indicate statistical significance.
Baseline clinical data, including age, family history, and smoking habits, were collected at admission. The analysis revealed no significant inter-group differences (P > 0.05, Table 2), confirming the comparability of the study groups.
| Control group (n = 70) | Research group (n = 65) | t/χ2 | P value | |
| Age (yr) | 60.37 ± 5.25 | 60.26 ± 7.26 | 0.101 | 0.920 |
| Family history of BC | 0.457 | 0.499 | ||
| Yes | 8 (11.43) | 10 (15.38) | ||
| No | 62 (88.57) | 55 (84.62) | ||
| Long-term smoking | 0.099 | 0.753 | ||
| Yes | 20 (28.57) | 17 (26.15) | ||
| No | 50 (71.43) | 48 (73.85) | ||
| Long-term drinking | 0.182 | 0.669 | ||
| Yes | 12 (17.14) | 13 (20.00) | ||
| No | 58 (82.86) | 52 (80.00) | ||
| History of breast disease | 0.223 | 0.637 | ||
| Yes | 20 (28.57) | 21 (32.31) | ||
| No | 50 (71.43) | 44 (67.69) |
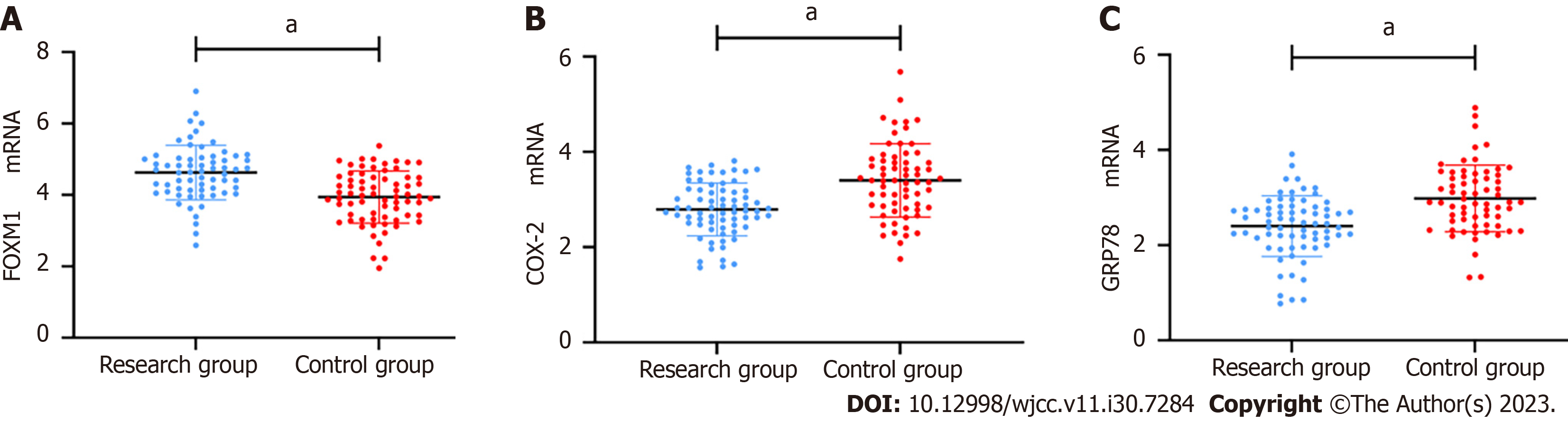
The mRNA expression level of FOXM1 was (4.63 ± 0.76) in the RG and (3.94 ± 0.73) in the CG, indicating significantly higher FOXM1 levels in BIDC patients (P < 0.05, Figure 1A). Similarly, COX-2 mRNA expression in the RG was (3.40 ± 0.77), which was also higher compared to that in the CG (P < 0.05, Figure 1B). Finally, the inter-group comparison showed a higher GRP78 mRNA level in the RG compared to the CG (P < 0.05, Figure 1C).
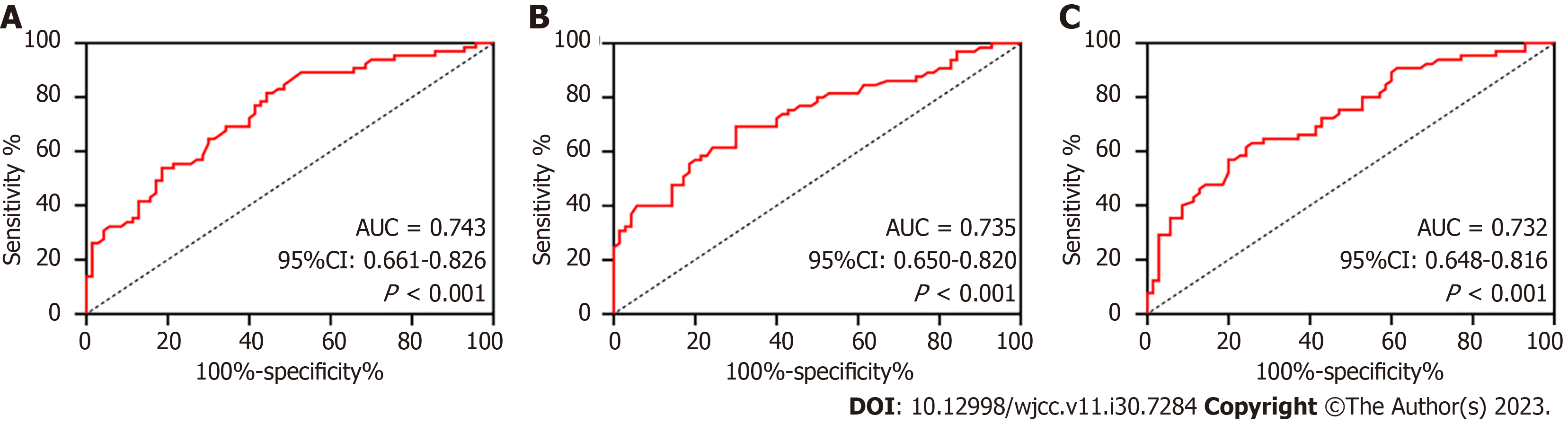
ROC analysis revealed that when the peripheral blood mRNA level of FOXM1 was > 4.05, it had a sensitivity of 81.54% and specificity of 55.71% for diagnosing BIDC (P < 0.05, Figure 2A). Similarly, COX-2 had a sensitivity of 69.23% and specificity of 70.00% for BIDC diagnosis when its mRNA level was > 3.05 (P < 0.05, Figure 2B). GRP78 exhibited a sensitivity of 63.08% and specificity of 74.29% for diagnosing BIDC when its mRNA level was > 2.76 (P < 0.05, Figure 2C).
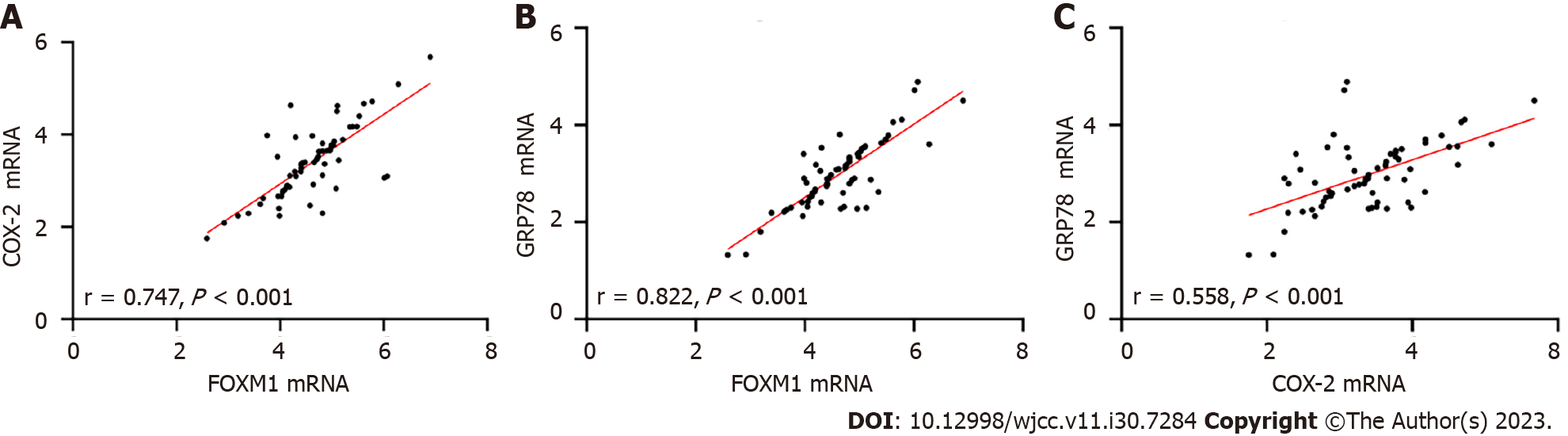
The Pearson correlation analysis demonstrated a positive correlation between FOXM1 and both COX-2 and GRP78 (P < 0.05, Figure 3A and B), as well as a positive association between COX-2 and GRP78 (P < 0.05, Figure 3C), in the peripheral blood of patients in the RG (P < 0.05, Figure 3C).
There were no significant differences in FOXM1, COX-2, and GRP78 levels among patients of different age groups (P > 0.05). However, significant differences in their levels were observed in patients with different histological grades and metastasis statuses (with vs without) (P > 0.05, Table 3), indicating a close relationship between FOXM1, COX-2, and GRP78 and the above indexes.
| n | FOXM1 | COX-2 | GRP78 | |
| Age (yr) | ||||
| ≤ 60 | 33 | 4.51 ± 0.69 | 3.22 ± 0.61 | 3.85 ± 0.71 |
| > 60 | 32 | 4.75 ± 0.83 | 3.60 ± 0.87 | 3.12 ± 0.69 |
| Family history of BC | ||||
| Yes | 10 | 4.66 ± 0.61 | 3.42 ± 0.65 | 3.05 ± 0.64 |
| No | 55 | 4.43 ± 1.37 | 3.31 ± 1.28 | 2.62 ± 0.95 |
| Long-term smoking | ||||
| Yes | 17 | 4.65 ± 0.49 | 3.59 ± 0.54 | 2.96 ± 0.49 |
| No | 48 | 4.62 ± 0.84 | 3.34 ± 0.83 | 2.99 ± 0.77 |
| Long-term drinking | ||||
| Yes | 13 | 4.36 ± 0.80 | 3.36 ± 0.91 | 2.81 ± 0.58 |
| No | 52 | 4.69 ± 0.75 | 3.41 ± 0.74 | 3.03 ± 0.73 |
| History of breast disease | ||||
| Yes | 21 | 4.49 ± 0.63 | 3.42 ± 0.61 | 2.77 ± 0.59 |
| No | 44 | 4.69 ± 0.82 | 3.40 ± 0.84 | 3.08 ± 0.74 |
| Histological grade | ||||
| Grade I | 35 | 4.39 ± 0.76 | 3.21 ± 0.72 | 2.75 ± 0.66 |
| Grade II | 19 | 4.57 ± 0.47 | 3.29 ± 0.61 | 2.91 ± 0.49 |
| Grade III | 11 | 5.48 ± 0.611,2 | 4.22 ± 0.701,2 | 3.83 ± 0.521,2 |
| Tumor metastasis | ||||
| Yes | 21 | 5.03 ± 0.75 | 3.7 ± 0.78 | 3.41 ± 0.69 |
| No | 44 | 4.43 ± 0.703 | 3.24 ± 0.713 | 2.78 ± 0.623 |
During the follow-up period, 12 patients died. Deceased patients exhibited higher age, FOXM1, COX-2, and GRP78 levels compared to the surviving patients, with a higher proportion of histological grade III and tumor metastasis (P < 0.05, Table 4). These findings suggest that age, FOXM1, COX-2, GRP78, histological grade, and tumor metastasis were individual factors affecting the prognostic death of BIDC patients.
| Surviving patients (n = 53) | Dead patients (n = 12) | t/χ2 | P value | |
| Age (yr) | 9.849 | 0.002b | ||
| ≤ 60 | 31 (58.49) | 1 (8.33) | ||
| > 60 | 22 (41.51) | 11 (91.67) | ||
| Family disease history of BC | 0.562 | 0.453 | ||
| Yes | 9 (16.98) | 1 (8.33) | ||
| No | 44 (83.02) | 11 (91.67) | ||
| Long-term smoking | 0.686 | 0.408 | ||
| Yes | 15 (28.30) | 12 (16.67) | ||
| No | 38 (71.70) | 10 (83.33) | ||
| Long-term drinking | 0.102 | 0.749 | ||
| Yes | 11 (20.75) | 12 (16.67) | ||
| No | 42 (79.25) | 10 (83.33) | ||
| History of breast disease | 1.646 | 0.200 | ||
| Yes | 19 (35.85) | 12 (16.67) | ||
| No | 34 (64.15) | 10 (83.33) | ||
| Histological grade | 37.350 | < 0.001b | ||
| Grade I | 35 (66.04) | 0 (0.0) | ||
| Grade II | 16 (30.19) | 3 (25.00) | ||
| Grade III | 2 (3.77) | 9 (75.00) | ||
| Tumor metastasis | 30.840 | < 0.001b | ||
| Yes | 9 (16.98) | 12 (100.0) | ||
| No | 44 (83.02) | 0 (0.0) | ||
| FOXM1 | 4.49 ± 0.69 | 5.22 ± 0.80 | 3.214 | 0.002b |
| COX-2 | 3.28 ± 0.70 | 3.96 ± 0.84 | 2.928 | 0.005b |
| GRP78 | 2.83 ± 0.62 | 3.66 ± 0.66 | 4.140 | < 0.001b |
Univariate indicators of BIDC (Age: ≤ 60 assigned to 0, > 60 assigned to 1; histological grade: Grade I assigned to 0, grade II assigned to 1, grade III assigned to 2; tumor metastasis: No assigned to 0, yes assigned to 1; FOXM1, COX-2, and GRP78 were analyzed using raw data) were input as covariates for COX regression analysis, with patient death used as the dependent variable. Age was not found to be an independent factor for the prognostic death of BIDC patients (P > 0.05). However, FOXM1, COX-2, and GRP78, increased histological grade, and tumor metastasis were identified as significant factors (P < 0.001, Table 5).
| β | S.E. | Wald χ2 | P value | OR | 95%CI | |
| Age (yr) | -0.426 | 2.623 | 3.621 | 0.527 | 1.642 | 0.842-3.513 |
| Histological grade | 1.124 | 0.342 | 10.624 | < 0.001 | 3.068 | 1.512-6.084 |
| Tumor metastasis | 1.824 | 0.493 | 20.184 | < 0.001 | 6.521 | 2.164-14.813 |
| FOXM1 | 1.109 | 0.384 | 8.147 | 0.004 | 3.024 | 1.541-6.038 |
| COX-2 | 0.674 | 0.281 | 5.264 | 0.021 | 1.871 | 1.064-3.257 |
| GRP78 | 1.084 | 0.573 | 11.806 | < 0.001 | 1.583 | 0.962-4.823 |
The incidence of BC has been increasing in recent years, and it is affecting women at a younger age, posing significant risks to their health and well-being[17]. Early-stage BIDC often goes undetected, leading to a diagnosis at advanced stages with tumor metastasis, contributing to the poor prognosis of BIDC[18]. Therefore, analyzing the expression profiles and clinical implications of FOXM1, COX-2, and GRP78 in BIDC is essential for advancing research in this field.
This study confirmed the high expression levels of FOXM1, COX-2, and GRP78 in BIDC, which is consistent with previous research on BC[19-21]. These findings suggest the involvement of FOXM1, COX-2, and GRP78 in the onset and progression of BIDC. Furthermore, correlation analysis revealed positive associations between the expression levels of FOXM1, COX-2, and GRP78, indicating a synergistic relationship in their abnormal expression. Previous studies have shown that FOXM1 regulates tumor cell activity, including promoting liver cancer cell growth through kinesin super-family protein 4A and influencing the quiescence and survival state of leukemia stem cells by regulating mixed lineage leukemia[22,23]. Additionally, FOXM1 plays a role in angiogenesis, endothelial cell proliferation, migration, and endothelial cell tube formation[24]. Vascular endothelial growth factor (VEGF), secreted by tumor tissues, induces tumor neovascularization, promoting tumor cell invasion and metastasis[25]. The close correlation between VEGF and tumor-distant metastasis has been well documented[26]. FOXM1 can bind to the VEGF promoter and transcriptionally regulate its expression[27]. Therefore, it is speculated that this may be the mechanism by which FOXM1 is involved in BIDC. COX-2, a subtype of COX, is minimally expressed under normal physiological conditions but is overexpressed in pathological states. By promoting prostaglandin synthesis, COX-2 increases the risk of tumor growth, proliferation, vascular permeability, and metastasis[28]. Furthermore, COX-2 enhances the anti-apoptotic abilities of tumor cells by upregulating the expression of the proto-oncogene B-cell lymphoma-2 (Bcl-2). It also inhibits lymphokine production, reduces T and B cell proliferation through the production of prostaglandin E2, weakens the immune surveillance function, and makes tumor cells prone to immune escape by inducing the expression of the immunosuppressive enzyme 3-dioxygenase[29]. Increased COX-2 represents an increased risk of cancer cell growth, proliferation, and metastasis, a stronger anti-apoptotic ability, and an increased risk of adverse prognosis in patients undergoing chemotherapy. GRP78, a molecular chaperone, assists in protein folding and transport in the endoplasmic reticulum, cytoplasm, and cell membrane. It also participates in the activation of unfolded protein response signals[30]. Studies have shown that GRP78 promotes cell proliferation and migration, and its mechanism involves inhibiting Bcl-2 and binding to caspase-7 to prevent apoptosis[31,32]. Previous studies on FOXM1, COX-2, and GRP78 have all indicated their association with tumor invasion or migration, thus suggesting that their abnormal expression in BIDC is expected. Moreover, ROC analysis revealed that the three are effective in diagnosing the occurrence of BIDC, which may significantly contribute to promoting early diagnosis in the future. Clinical data analysis demonstrated that FOXM1, COX-2, and GRP78 were closely related to the histological grade and metastasis of BIDC. This reinforced the relationship between the three markers and the pathological progression of BIDC, indicating that abnormally elevated levels of FOXM1, COX-2, and GRP78 can promote the malignant development of BIDC. Finally, the prognosis analysis revealed that FOXM1, COX-2, GRP78, histological grade, and tumor metastasis were independent factors affecting the prognostic outcomes of patients. Histological grade and tumor metastasis, being typical pathological features, naturally influence the malignant progression of BIDC[33]. The analysis results for FOXM1, COX-2, and GRP78 also support the above viewpoint, confirming their role in promoting the malignant development of BIDC. Conversely, these results suggest that targeted silencing of FOXM1, COX-2, and GRP78 may inhibit BIDC progression and facilitate the treatment of BIDC.
However, the exact mechanism by which FOXM1, COX-2, and GRP78 contribute to BIDC still needs to be further confirmed through experiments. Additionally, this study did not analyze tissue samples from BIDC patients, and the short follow-up time limits the evaluation of long-term prognosis. These areas should be addressed and supplemented in future research.
The findings of this study indicate that FOXM1, COX-2, and GRP78 exhibit abnormally high expression levels in BIDC, contributing to the malignant development of the tumor and significantly impacting the prognosis of BIDC patients. These results hold significant research implications for the future diagnosis and treatment of BIDC.
Breast infiltrating ductal carcinoma (BIDC) represents the largest heterotypic tumor group, and this study analyzed the clinical significance of forkhead box M1 (FOXM1), cyclooxygenase-2 (COX-2), and glucose-regulated protein 78 (GRP78) in BIDC, which could provide a reliable foundation for subsequent studies.
FOXM1, COX-2, and GRP78 are closely related to breast cancer development and progression, but their roles in BIDC remain unclear. They may play equally important roles and hold promise for future diagnosis and treatment of BIDC.
To analyze the clinical significance of FOXM1, COX-2, and GRP78 in BIDC, and to provide references and new research directions for future diagnosis and treatment of BIDC.
In this study, we analyzed the clinical significance of FOXM1, COX-2, and GRP78 in BIDC by detecting the expression levels of FOXM1, COX-2, and GRP78 in the peripheral blood of patients with BIDC and healthy people.
FOXM1, COX-2, and GRP78 were elevated in BIDC and demonstrated excellent diagnostic and prognostic assessment of BIDC.
FOXM1, COX-2, and GRP78 exhibit abnormally high expression in BIDC, promoting malignant tumor development and closely correlating with prognosis.
This study demonstrated the clinical significance of FOXM1, COX-2, and GRP78 in BIDC, and in the future, they can be used in the clinic as reference indexes for the diagnosis, disease evaluation, and prognosis assessment of BIDC. In addition, they can also be used as targets to study new targeted therapeutic options for BIDC in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kumar S, India; Myers JS, United States S-Editor: Qu XL L-Editor: Wang TQ P-Editor: Yu HG
| 1. | Fahad Ullah M. Breast Cancer: Current Perspectives on the Disease Status. Adv Exp Med Biol. 2019;1152:51-64. [PubMed] [DOI] [Full Text] |
| 2. | Ullah L, Hameed Y, Ejaz S, Raashid A, Iqbal J, Ullah I, Ejaz SA. Detection of novel infiltrating ductal carcinoma-associated BReast CAncer gene 2 mutations which alter the deoxyribonucleic acid-binding ability of BReast CAncer gene 2 protein. J Cancer Res Ther. 2020;16:1402-1407. [PubMed] [DOI] [Full Text] |
| 3. | Zhao H. The prognosis of invasive ductal carcinoma, lobular carcinoma and mixed ductal and lobular carcinoma according to molecular subtypes of the breast. Breast Cancer. 2021;28:187-195. [PubMed] [DOI] [Full Text] |
| 4. | Dai D, Shi R, Wang Z, Zhong Y, Shin VY, Jin H, Wang X. Competing Risk Analyses of Medullary Carcinoma of Breast in Comparison to Infiltrating Ductal Carcinoma. Sci Rep. 2020;10:560. [PubMed] [DOI] [Full Text] |
| 5. | Kader T, Hill P, Zethoven M, Goode DL, Elder K, Thio N, Doyle M, Semple T, Sufyan W, Byrne DJ, Pang JB, Murugasu A, Miligy IM, Green AR, Rakha EA, Fox SB, Mann GB, Campbell IG, Gorringe KL. Atypical ductal hyperplasia is a multipotent precursor of breast carcinoma. J Pathol. 2019;248:326-338. [PubMed] [DOI] [Full Text] |
| 6. | Chen XY, Yeong J, Thike AA, Bay BH, Tan PH. Prognostic role of immune infiltrates in breast ductal carcinoma in situ. Breast Cancer Res Treat. 2019;177:17-27. [PubMed] [DOI] [Full Text] |
| 7. | Barger CJ, Branick C, Chee L, Karpf AR. Pan-Cancer Analyses Reveal Genomic Features of FOXM1 Overexpression in Cancer. Cancers (Basel). 2019;11. [PubMed] [DOI] [Full Text] |
| 8. | Dan VM, Raveendran RS, Baby S. Resistance to Intervention: Paclitaxel in Breast Cancer. Mini Rev Med Chem. 2021;21:1237-1268. [PubMed] [DOI] [Full Text] |
| 9. | Ros S, Wright AJ, D'Santos P, Hu DE, Hesketh RL, Lubling Y, Georgopoulou D, Lerda G, Couturier DL, Razavi P, Pelossof R, Batra AS, Mannion E, Lewis DY, Martin A, Baird RD, Oliveira M, de Boo LW, Linn SC, Scaltriti M, Rueda OM, Bruna A, Caldas C, Brindle KM. Metabolic Imaging Detects Resistance to PI3Kα Inhibition Mediated by Persistent FOXM1 Expression in ER(+) Breast Cancer. Cancer Cell. 2020;38:516-533.e9. [PubMed] [DOI] [Full Text] |
| 10. | Peng Y, Wang Y, Tang N, Sun D, Lan Y, Yu Z, Zhao X, Feng L, Zhang B, Jin L, Yu F, Ma X, Lv C. Andrographolide inhibits breast cancer through suppressing COX-2 expression and angiogenesis via inactivation of p300 signaling and VEGF pathway. J Exp Clin Cancer Res. 2018;37:248. [PubMed] [DOI] [Full Text] |
| 11. | Regulski M, Regulska K, Prukała W, Piotrowska H, Stanisz B, Murias M. COX-2 inhibitors: a novel strategy in the management of breast cancer. Drug Discov Today. 2016;21:598-615. [PubMed] [DOI] [Full Text] |
| 12. | Cook KL, Clarke PA, Clarke R. Targeting GRP78 and antiestrogen resistance in breast cancer. Future Med Chem. 2013;5:1047-1057. [PubMed] [DOI] [Full Text] |
| 13. | Zhang YL, Ma Y, Zeng YQ, Liu Y, He EP, Liu YT, Qiao FL, Yu R, Wang YS, Wu XY, Leng P. A narrative review of research progress on FoxM1 in breast cancer carcinogenesis and therapeutics. Ann Transl Med. 2021;9:1704. [PubMed] [DOI] [Full Text] |
| 14. | de Souza CP, Alves B, Waisberg J, Fonseca F, Carmo AO, Gehrke F. Detection of COX-2 in liquid biopsy in patients with breast cancer. J Clin Pathol. 2020;73:826-829. [PubMed] [DOI] [Full Text] |
| 15. | Zheng Y, Liu P, Wang N, Wang S, Yang B, Li M, Chen J, Situ H, Xie M, Lin Y, Wang Z. Betulinic Acid Suppresses Breast Cancer Metastasis by Targeting GRP78-Mediated Glycolysis and ER Stress Apoptotic Pathway. Oxid Med Cell Longev. 2019;2019:8781690. [PubMed] [DOI] [Full Text] |
| 16. | Chen Y, Zhou JH, Fan HX, Luo Y, Peng YL, Ma BY. Ultrasound Diagnosis of Breast Lymphoma and the Identification of Breast Infiltrating Ductal Carcinoma. J Ultrasound Med. 2020;39:1203-1211. [PubMed] [DOI] [Full Text] |
| 17. | Odle TG. Precision Medicine in Breast Cancer. Radiol Technol. 2017;88:401M-421M. [PubMed] |
| 18. | Lan T, Lu Y, Luo H, He J, Hu Z, Xu H. Effects of Marital Status on Prognosis in Women with Infiltrating Ductal Carcinoma of the Breast: A Real-World 1: 1 Propensity-Matched Study. Med Sci Monit. 2020;26:e923630. [PubMed] [DOI] [Full Text] |
| 19. | Sun HL, Men JR, Liu HY, Liu MY, Zhang HS. FOXM1 facilitates breast cancer cell stemness and migration in YAP1-dependent manner. Arch Biochem Biophys. 2020;685:108349. [PubMed] [DOI] [Full Text] |
| 20. | Feriancová M, Walter I, Singer CF, Gazdarica J, Pohlodek K. Expression of COX-2, p16, and Ki67 in the range from normal breast tissue to breast cancer. Neoplasma. 2021;68:342-351. [PubMed] [DOI] [Full Text] |
| 21. | Lager TW, Conner C, Keating CR, Warshaw JN, Panopoulos AD. Cell surface GRP78 and Dermcidin cooperate to regulate breast cancer cell migration through Wnt signaling. Oncogene. 2021;40:4050-4059. [PubMed] [DOI] [Full Text] |
| 22. | Hu G, Yan Z, Zhang C, Cheng M, Yan Y, Wang Y, Deng L, Lu Q, Luo S. FOXM1 promotes hepatocellular carcinoma progression by regulating KIF4A expression. J Exp Clin Cancer Res. 2019;38:188. [PubMed] [DOI] [Full Text] |
| 23. | Sheng Y, Yu C, Liu Y, Hu C, Ma R, Lu X, Ji P, Chen J, Mizukawa B, Huang Y, Licht JD, Qian Z. FOXM1 regulates leukemia stem cell quiescence and survival in MLL-rearranged AML. Nat Commun. 2020;11:928. [PubMed] [DOI] [Full Text] |
| 24. | Lin JZ, Wang WW, Hu TT, Zhu GY, Li LN, Zhang CY, Xu Z, Yu HB, Wu HF, Zhu JG. FOXM1 contributes to docetaxel resistance in castration-resistant prostate cancer by inducing AMPK/mTOR-mediated autophagy. Cancer Lett. 2020;469:481-489. [PubMed] [DOI] [Full Text] |
| 25. | Liu C, Barger CJ, Karpf AR. FOXM1: A Multifunctional Oncoprotein and Emerging Therapeutic Target in Ovarian Cancer. Cancers (Basel). 2021;13. [PubMed] [DOI] [Full Text] |
| 26. | Hisada Y, Mackman N. Tissue Factor and Cancer: Regulation, Tumor Growth, and Metastasis. Semin Thromb Hemost. 2019;45:385-395. [PubMed] [DOI] [Full Text] |
| 27. | Monteiro LJ, Cubillos S, Sanchez M, Acuña-Gallardo S, Venegas P, Herrera V, Lam EW, Varas-Godoy M, Illanes SE. Reduced FOXM1 Expression Limits Trophoblast Migration and Angiogenesis and Is Associated With Preeclampsia. Reprod Sci. 2019;26:580-590. [PubMed] [DOI] [Full Text] |
| 28. | Hugo HJ, Saunders C, Ramsay RG, Thompson EW. New Insights on COX-2 in Chronic Inflammation Driving Breast Cancer Growth and Metastasis. J Mammary Gland Biol Neoplasia. 2015;20:109-119. [PubMed] [DOI] [Full Text] |
| 29. | Qin Q, Ji H, Li D, Zhang H, Zhang Z, Zhang Q. Tumor-associated macrophages increase COX-2 expression promoting endocrine resistance in breast cancer via the PI3K/Akt/mTOR pathway. Neoplasma. 2021;68:938-946. [PubMed] [DOI] [Full Text] |
| 30. | Zheng Y, Zhang J, Huang W, Zhong LLD, Wang N, Wang S, Yang B, Wang X, Pan B, Situ H, Lin Y, Liu X, Shi Y, Wang Z. Sini San Inhibits Chronic Psychological Stress-Induced Breast Cancer Stemness by Suppressing Cortisol-Mediated GRP78 Activation. Front Pharmacol. 2021;12:714163. [PubMed] [DOI] [Full Text] |
| 31. | Liao M, Wang C, Yang B, Huang D, Zheng Y, Wang S, Wang X, Zhang J, Tang C, Xu Z, He Y, Huang R, Zhang F, Wang Z, Wang N. Autophagy Blockade by Ai Du Qing Formula Promotes Chemosensitivity of Breast Cancer Stem Cells Via GRP78/β-Catenin/ABCG2 Axis. Front Pharmacol. 2021;12:659297. [PubMed] [DOI] [Full Text] |
| 32. | Zielinska HA, Daly CS, Alghamdi A, Bahl A, Sohail M, White P, Dean SR, Holly JMP, Perks CM. Interaction between GRP78 and IGFBP-3 Affects Tumourigenesis and Prognosis in Breast Cancer Patients. Cancers (Basel). 2020;12. [PubMed] [DOI] [Full Text] |
| 33. | Zhou X, Zheng Z, Li Y, Zhao W, Lin Y, Zhang J, Sun Q. The clinical features and prognosis of patients with mucinous breast carcinoma compared with those with infiltrating ductal carcinoma: a population-based study. BMC Cancer. 2021;21:536. [PubMed] [DOI] [Full Text] |