Published online Jan 26, 2023. doi: 10.12998/wjcc.v11.i3.635
Peer-review started: August 25, 2022
First decision: November 22, 2022
Revised: December 10, 2022
Accepted: January 9, 2023
Article in press: January 9, 2023
Published online: January 26, 2023
Processing time: 144 Days and 1.2 Hours
Tooth avulsion is one of the most severe types of dental trauma. Most avulsed teeth undergo long-term ankylosis and replacement resorption after delayed rei
Case 1 was a 14-year-old boy who fell and knocked out his left upper central inc
These cases provide examples of the successful use of PRF to reduce pathological root resorption of the avulsed teeth, and the application of PRF may provide new healing opportunities for traditionally “hopeless” avulsed teeth.
Core Tip: Tooth avulsion is one of the most severe types of dental trauma. Most avulsed teeth will undergo ankylosis and replacement resorption after delayed reimplantation and generally experience a poor prognosis. We previously demonstrated that autologous platelet-rich fibrin (PRF) could effectively help to control the occurrence and development of initial root resorption. In this report, we presented two clinical cases of avulsed teeth with delayed reimplantation that were treated with autologous PRF. Ideal periodontal healing over 12 mo of follow-up suggested that PRF, as an adjuvant therapy, may provide new insights and perspectives on the management of traditionally hopeless avulsed teeth.
- Citation: Yang Y, Liu YL, Jia LN, Wang JJ, Zhang M. Rescuing “hopeless” avulsed teeth using autologous platelet-rich fibrin following delayed reimplantation: Two case reports. World J Clin Cases 2023; 11(3): 635-644
- URL: https://www.wjgnet.com/2307-8960/full/v11/i3/635.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i3.635
Tooth avulsion is defined as a complete displacement of the tooth from its original alveolar socket and is one of the most severe types of dental trauma. Permanent tooth avulsion accounts for 0.5%-3.0% of dental trauma, whereas some studies report an incidence as high as 16.0%[1,2]. A recent study from China showed that 8.0% of all dental injuries were tooth avulsion; these injuries are more likely to occur in individuals 7-20-years-old and generally occur more often in males than females[3]. The prognosis depends on the measures taken at the site of the accident, such as immediate replantation of avulsed teeth, the use of an effective preservation medium for the avulsed tooth, and timely and professional dental treatment performed after avulsion[4]. It was noted that a delay of more than 5 min could be defined as delayed replantation, affecting tooth survival[5]. Unfortunately, in most cases, an avulsed tooth is kept out of the alveolar socket for a significantly long time or is stored under improper conditions, which eventually contribute to periodontal ligament cell necrosis and result in ankylosis and replacement resorption of the tooth root after reimplantation[6]. The commercial enamel matrix protein Emdogain has been used clinically. However, its effectiveness in preventing root resorption has not been demonstrated[7].
Platelet-rich fibrin (PRF) is a second-generation platelet concentrate that is prepared from the patient’s own blood without the use of an anticoagulant through a single-step centrifugation process[8,9]. This concentrate is classified as L-PRF or PRF based on its leukocyte content as well as standard PRF or advanced PRF depending on the centrifugation process. In addition, PRF is available in a membrane or injectable form depending on the centrifugation process and consistency of the final product[10]. The main scaffold component of PRF is fibrin, which develops a three-dimensional mesh crossover structure that is visible under a scanning electron microscope with a large interfiber space that contains numerous red blood cells, white blood cells and clusters of platelets[11,12]. The fibrin network of PRF protects platelets from immediate activation but progressively activates them during the process of fibrin degradation, slowly releasing growth factors, eventually prolonging the duration of growth factors in PRF and promoting wound healing effects[13,14]. Thus, PRF has the potential to enhance tissue regeneration, accelerate wound healing and induce stem cell differentiation through the consistent release of multiple growth factors[15,16]. Our previous study demonstrated that autologous PRF could effectively promote the periodontal healing of avulsed teeth after delayed replantation in dogs and thus control the occurrence and development of initial root resorption[15].
In this report, we presented two clinical cases of delayed reimplantation of avulsed teeth using autologous PRF granules with a 12-mo follow-up. In both cases, the avulsed teeth were separated from the alveolar socket for far longer than the optimal reimplantation time of 5 min, and the residual periodontal ligament tissue on the root surface was either damaged or seriously polluted, which would traditionally deem them as “hopeless” teeth. Upon simultaneous reimplantation of avulsed teeth with autologous PRF, the injured teeth showed no symptoms of inflammatory root resorption or ankylosis in both cases, suggesting that the application of PRF may offer new therapeutic opportunities for traditionally “hopeless” avulsed teeth.
Case 1: A 14-year-old boy was referred to the Department of General Dentistry and Emergency of the Fourth Military Medical University with complaints of pain and that his left upper central incisor fell out 18 h prior.
Case 2: A 17-year-old boy visited the Department of General Dentistry and Emergency of the Fourth Military Medical University with complaints that his left upper lateral incisor had completely fallen out 2 h prior.
Case 1: The patient accidentally fell 18 h prior to presentation, and his left upper central incisor was knocked out. The patient was conscious with stable vital signs.
Case 2: The patient suffered avulsion of the left upper lateral incisor from an accidental fall and came to the hospital 2 h later. The patient was conscious with stable vital signs.
The patients did not have a relevant medical history. They did not report any history of drug allergies or systemic diseases and exhibited no apparent dental treatment contraindications.
There were no specific family health histories.
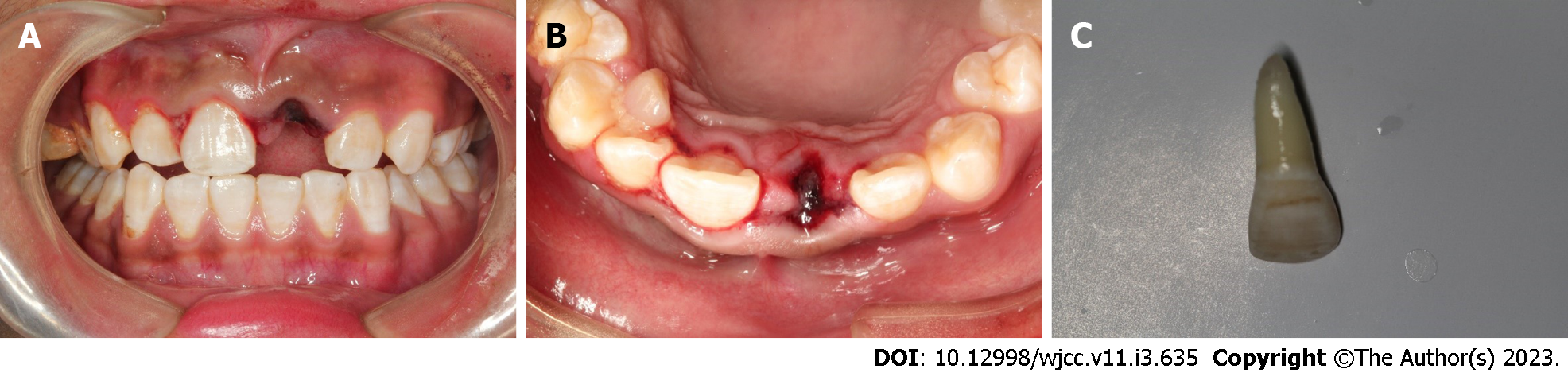
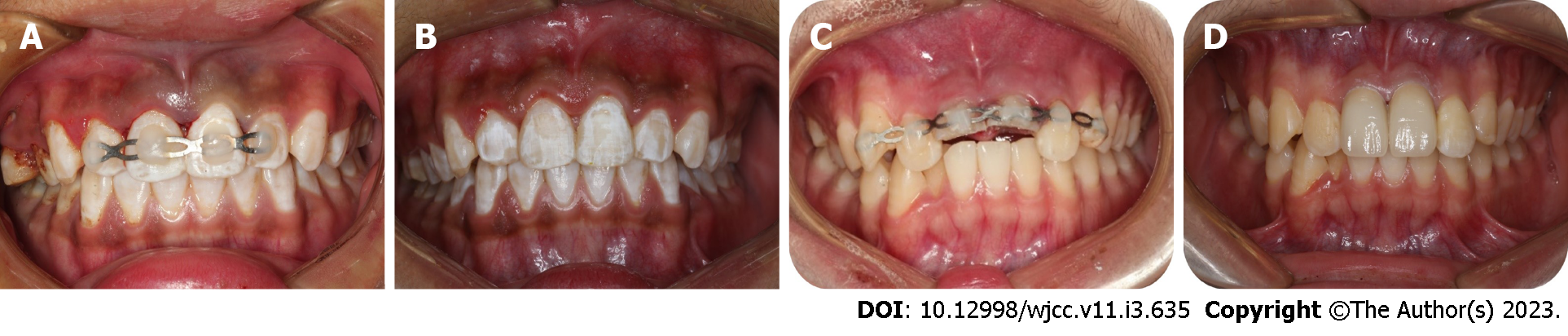
Case 1: After excluding damage to other important organs, an oral examination was performed. His general medical history did not obviously contribute to the injury, and an examination revealed no evidence of nerve injury. We performed clinical examinations, and the extraoral findings did not reveal serious wounds. Intraoral examination found that tooth 21 was missing, and the alveolar nest was empty. Blood clots had formed in the alveolar socket, and there was no obvious lacerated wound in the gums. The crown of tooth 11 was shifted to the palatal side and exhibited occlusal interference. Tooth H was retained on the lingual side of tooth 13 and was loose (Figure 1A and B). The avulsed tooth was wrapped in dirty dry paper towels (Figure 1C).
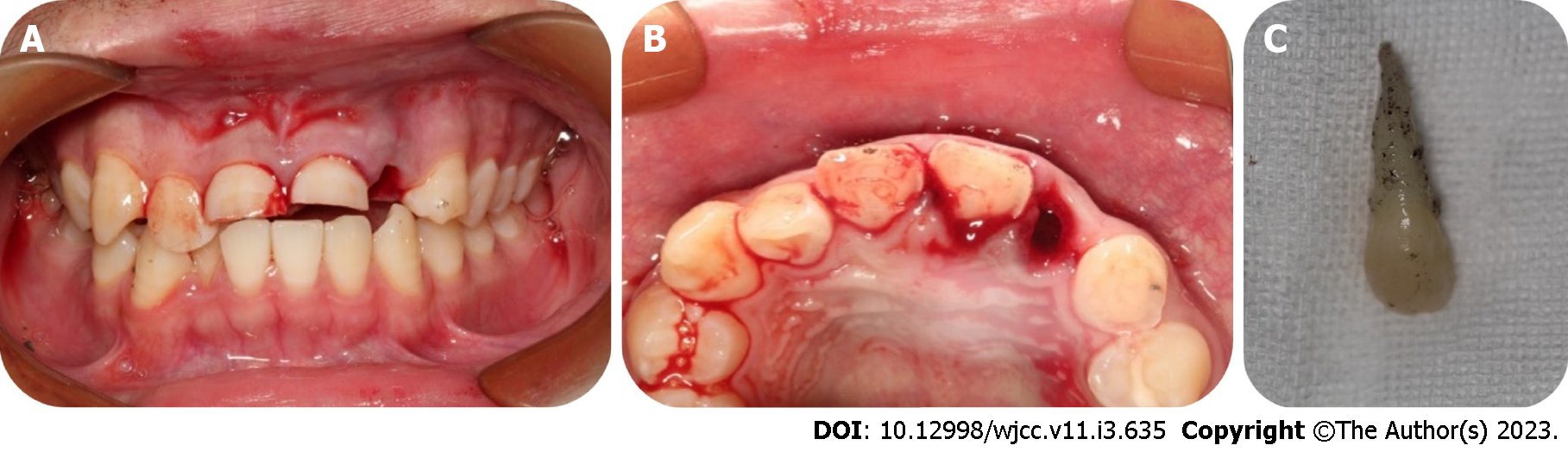
Case 2: Intraoral examination found that tooth 22 was missing, and the corresponding alveolar socket was empty with blood clots filling it. The patient had a complicated crown fracture of tooth 11 and a complicated crown-root fracture of tooth 21 with the fracture surfaces being approximately 4 mm below the enamel-dentinal junction (Figure 2A and B). The avulsed tooth was wrapped in dry paper towels, and numerous pollutants were present on the periodontal ligament tissues of the root surface (Figure 2C).
These cases did not undergo any laboratory examinations.
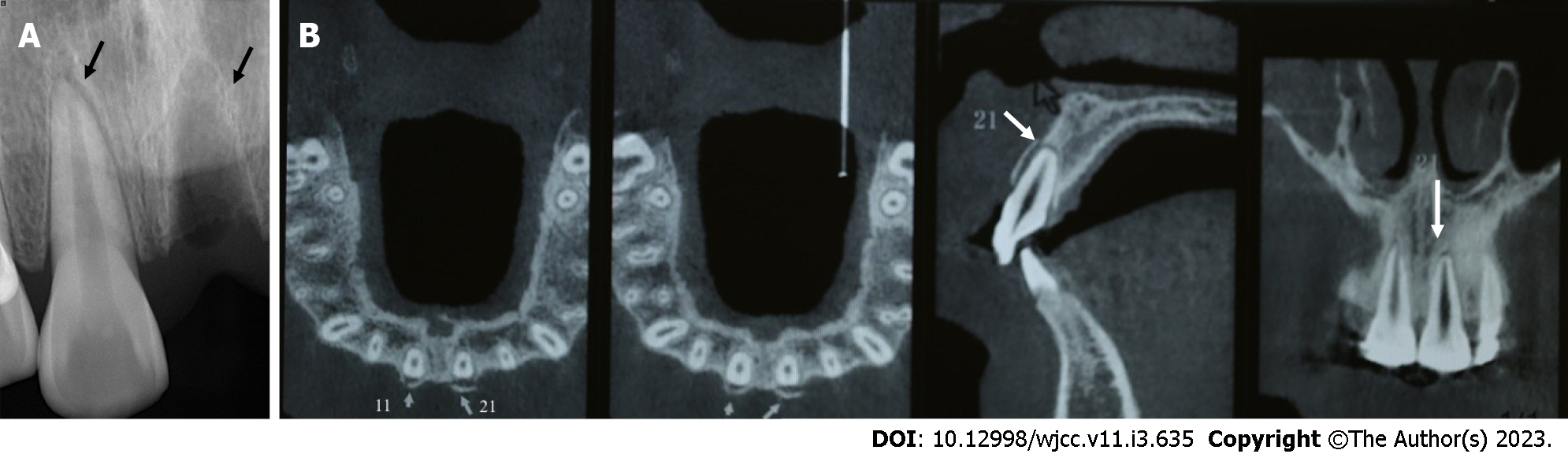
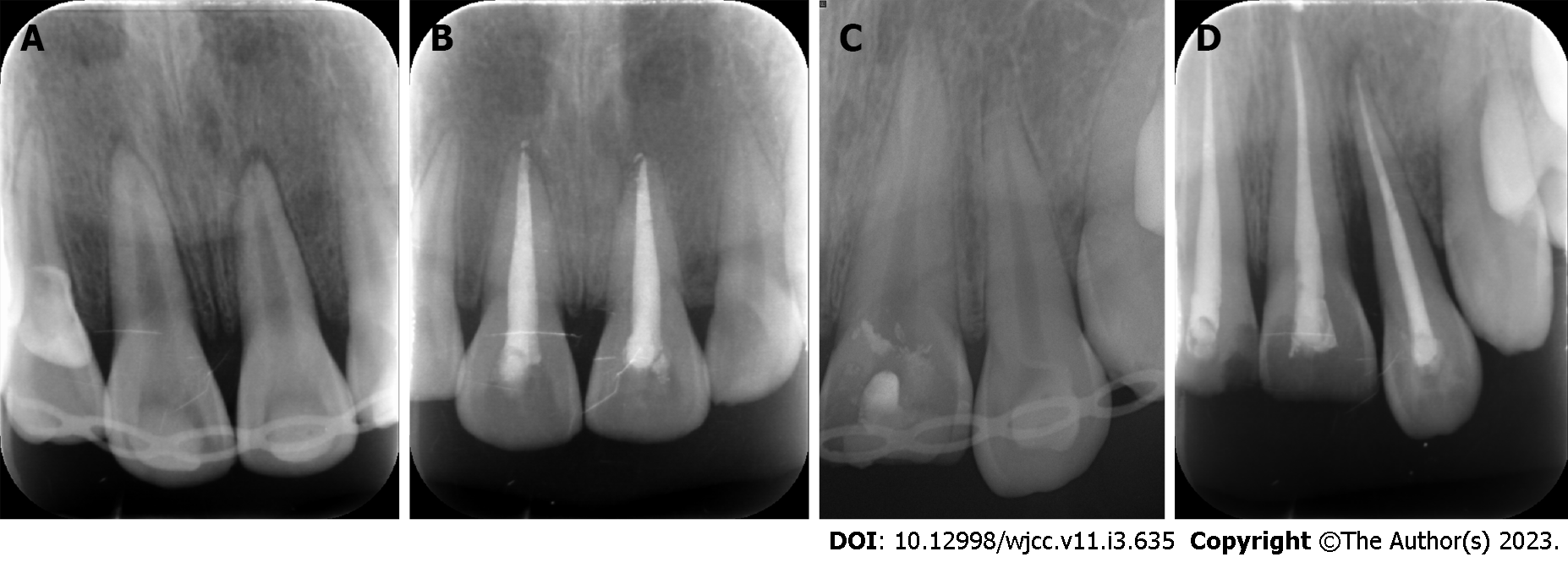
Case 1: Digital-X radiograph (FOCUS, Instrumentarium Dental, Finland) revealed that the alveolar socket of tooth 21 was empty, and no high-density foreign body images were noted. The periodontal membrane space was widened in tooth 11 without significant root fracture (Figure 3A). Cone-beam computed tomography (Hires3D, Beijing, China) revealed that the lip side of the alveolar bone wall of tooth 21 was fractured (Figure 3B).
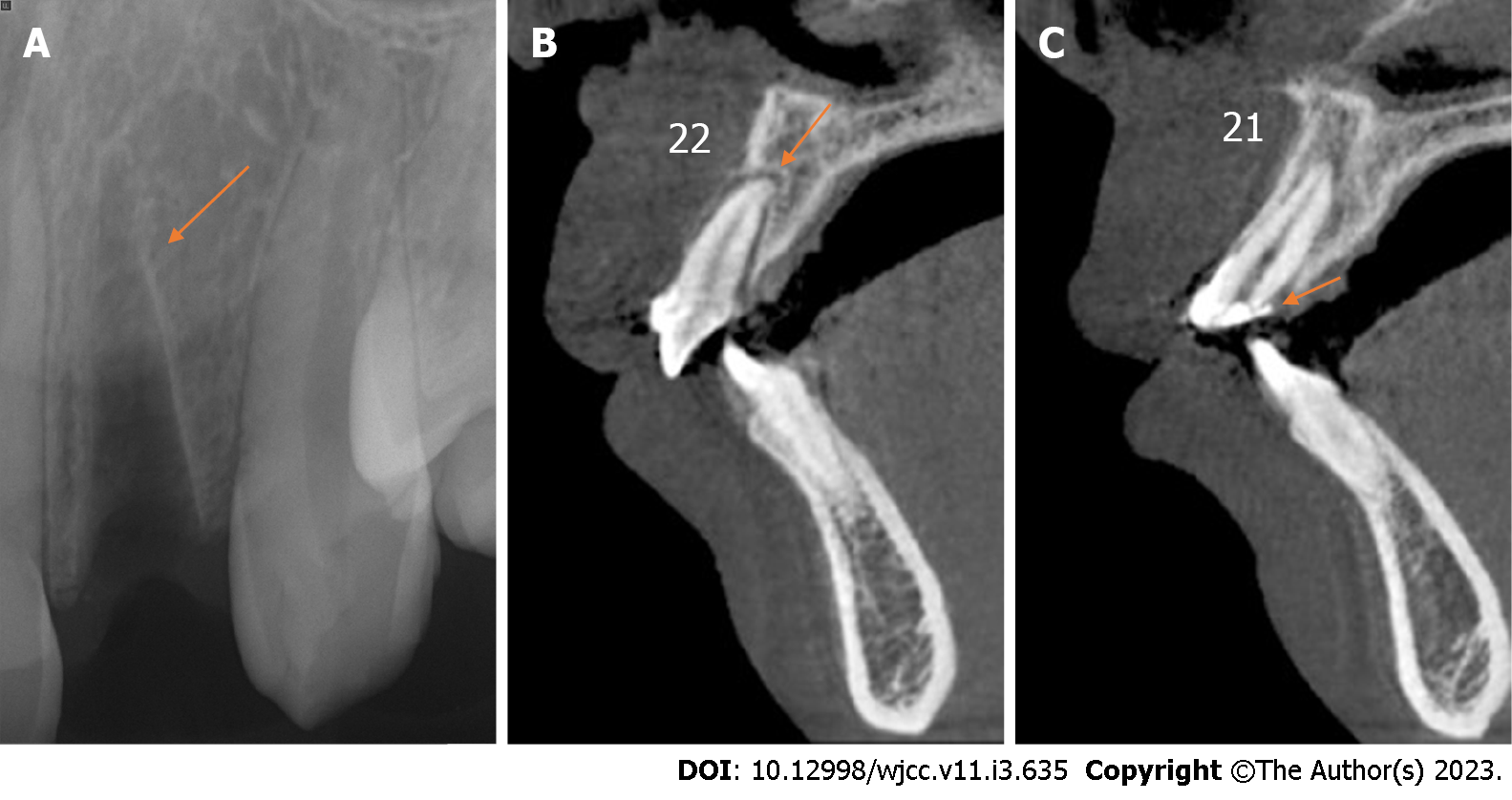
Case 2: Periapical radiography revealed that the alveolar socket of tooth 22 was empty, and no high-density foreign body images were observed (Figure 4A). Cone-beam computed tomography revealed no alveolar fracture around the empty tooth socket (Figure 4B), and the fracture position of tooth 21 was approximately 3 mm above the top of the alveolar crest (Figure 4C).
Case 1: Based on the patient’s medical history and findings of the imaging examinations, the diagnoses for this patient included lateral luxation of tooth 11, avulsion of tooth 21 and alveolar fracture of teeth 11 and 21.
Case 2: The diagnoses of this patient included avulsion of tooth 22, complicated crown fracture of tooth 11, and complicated crown-root fracture of tooth 21.
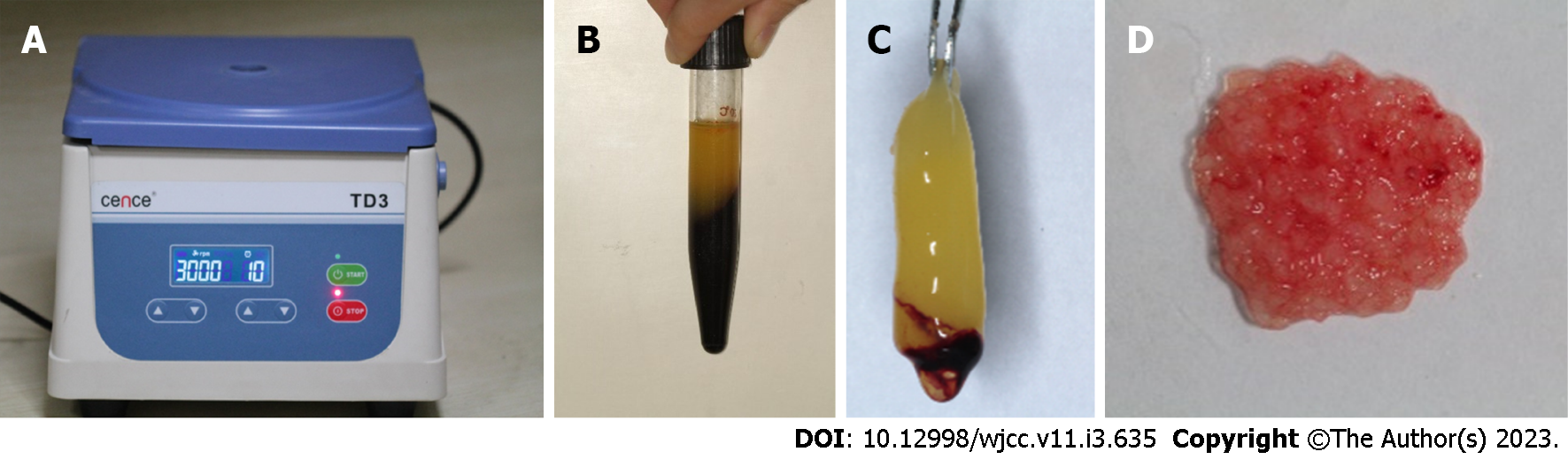
Case 1: We obtained a 10-mL blood sample from the median cubital vein and transferred the blood into a 10-mL glass tube without anticoagulation as soon as possible. The tube was immediately centrifuged at 400 × g for 10 min (TD3, CENCE, China) (Figure 5A). The fibrin clot that contains PRF formed in the middle of the tube; thus, the clot was easily separated from the red corpuscles at the bottom (Figure 5B and C). The clot was compressed with sterile dry gauze to remove the fluids trapped in the fibrin matrix. The PRF formed a very resistant autologous fibrin membrane, which was subsequently cut into approximately 1 mm3 granules (Figure 5D). Tooth 21 was reimplanted along with the PRF granules, and tooth 11 received manipulative reduction. Then, the teeth were splinted using a preshaped semiflexible titanium labial arch (Titanium Trauma Splint, Zhongbang Titanium Biological Materials Co., Ltd., Xi’an, China) for 4 wk (Figure 6A). The digital X-ray radiograph obtained immediately after the surgery showed complete reduction of teeth 21 and 11 (Figure 7A). According to the International Association of Dental Traumatology guidelines[17], root canal therapy of avulsed teeth 21 should be started within 7-14 d. For tooth 11, negative dental pulp activity was found. In addition, the tooth was sensitive to percussion, and a small transmission shadow was observed in the apical region at the return visit after 2 wk. Root canal therapy of the laterally dislocated tooth 11 and avulsed tooth 21 was performed 2 wk after the first visit. Calcium hydroxide paste was used as an intracanal medication sealant for 4 wk. Then, a biotype root canal filling sealer and hot-melt gutta-percha (SuperEndo B&L, Korea) were adopted for root canal filling. After root canal treatment, teeth 11 and 21 were restored with nanoment resin (3M Dental Products, MN, United States). The fixtures were removed 4 wk after the first treatment (Figures 6B and 7B).
Case 2: After obtaining informed consent, blood was collected from the patient, and PRF was prepared. Then, tooth 22 was reimplanted with PRF and splinted for 2 wk using a preshaped semiflexible titanium labial arch (Figure 6C). The digital X-ray radiograph obtained immediately after surgery showed complete reduction of tooth 22 (Figure 7C). After pulp vitality assessment, the dental pulp of teeth 11, 21 and 22 was removed after 2 wk, and calcium hydroxide paste was used as an intracanal medication sealant for 4 wk. Then, a biotype root canal filling sealer and hot-melt gutta-percha (SuperEndo B&L, Korea) were adopted for root canal filling. The fixtures were removed after 2 wk, and the root canal was completed after 6 wk (Figure 7D). After root canal treatment, teeth 11 and 21 were filled with fiber piles (3M Deutschland GmbH, Germany) and resin (3M Dental Products, MN, United States) and finally repaired with full crown restoration. Tooth 22 was restored with nanoment resin (3M Dental Products, MN, United States) (Figure 6D).
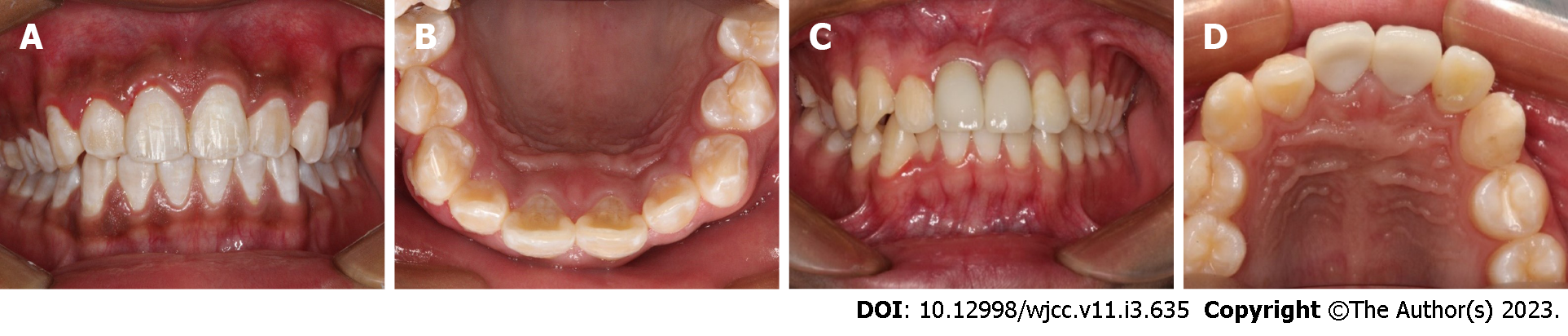
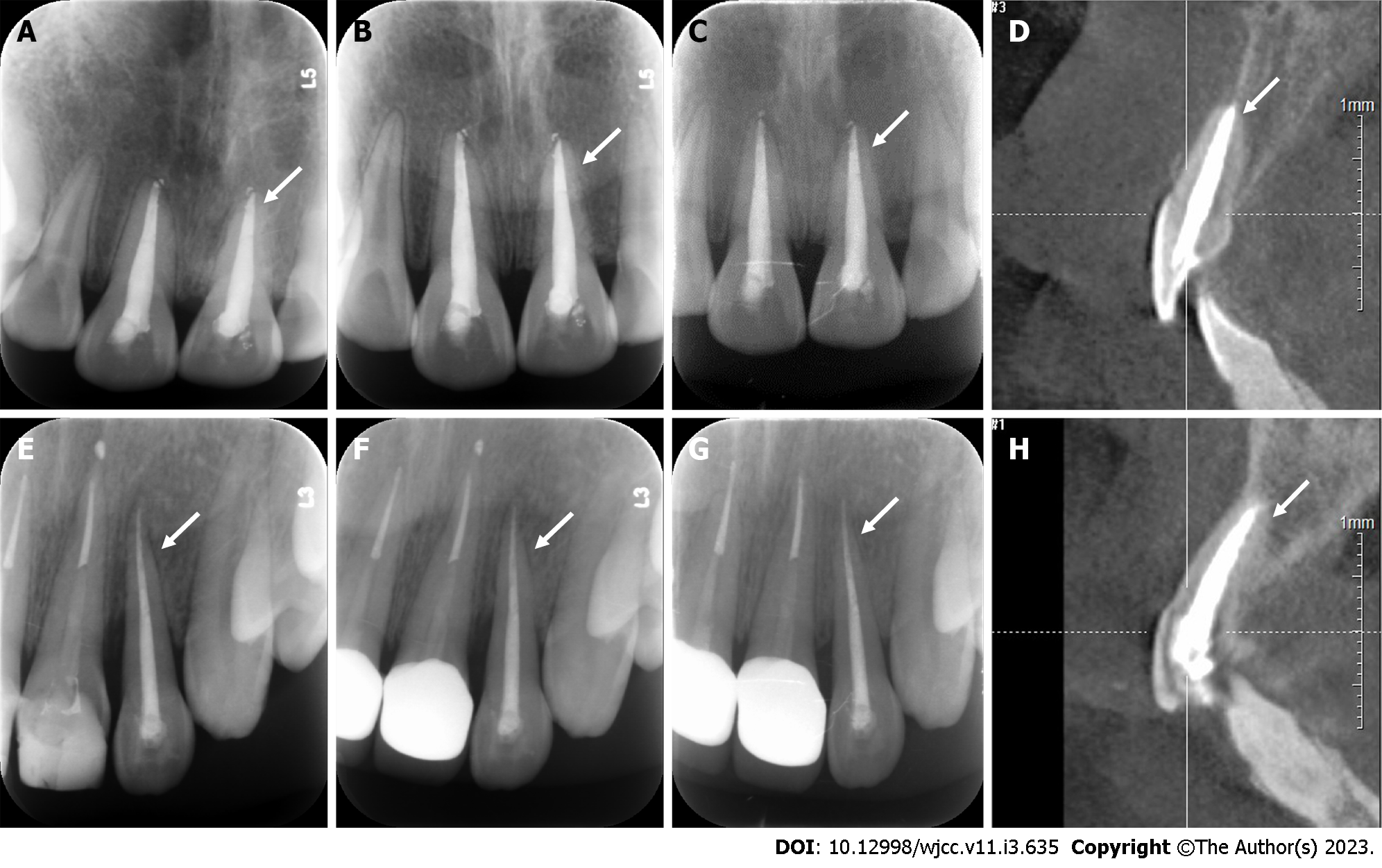
Case 1: A follow-up examination was performed 3 mo, 6 mo and 12 mo after the treatment. Clinical examination found no obvious periodontal pockets, tooth discoloration or swelling of the gums around tooth 21 (Figure 8A and B). The radiographic images obtained during the follow-up examination showed that the periodontal membrane space was continuous, and no sign of pathological root absorption was observed (Figure 9A-D).
Case 2: A follow-up examination was performed 3 mo, 6 mo and 12 mo after the treatment. Clinical examination revealed no obvious periodontal pockets, tooth discoloration or swelling of the gums around tooth 22 (Figure 8C and D). Radiographic images obtained during the follow-up examination showed that the periodontal membrane space was continuous, and no sign of pathological absorption was observed (Figure 9E-H).
Tooth reimplantation is the most important and fundamental treatment for tooth avulsion. The time interval between avulsion and reimplantation is the most important factor for successful reimplantation and is directly related to the number of live periodontal ligament cells on the root surface of the avulsed teeth[11]. If the tooth is not reimplanted as soon as possible, the residual periodontal ligament tissues on the root surface could be damaged or even exhibit necrosis. These conditions can lead to serious pathologic resorption and loss of the reimplanted tooth. Inflammation and replacement root resorption are the most common causes of reimplantation failure. The development of the lesion greatly depends on pulp vitality. When the root canal becomes infected, microbial toxins can move to the root surface through dentinal tubules, leading to the occurrence of root resorption[6,18]. Inflammatory root absorption on the outer surface can be prevented or controlled by the timely removal of the etiological origin, i.e. root canal intervention. The most effective method to prevent the replacement absorption of roots is to immediately replant or place the tooth in an appropriate storage medium[19,20].
It has been reported in the literature that ideal periodontal healing can be achieved when the avulsed tooth is reimplanted within 5 min. If reimplantation is delayed for more than 1 h after avulsion, complete necrosis of the injured periodontal ligament tissue is expected[17,21]. The storage of the avulsed tooth is also crucial to the periodontal healing process by affecting the viability of the periodontal ligament cells[22]. Unfortunately, due to the lack of common knowledge of early treatment and preservation of the avulsed teeth, few patients save the avulsed teeth in an ideal media in a timely manner. Studies have shown that 28.6% of patients place dislocated teeth in dry tissues for preservation, and only 11% of dislocated teeth were held in the mouth or placed in milk during transport to the clinic[23]. In the present cases, the patients washed the avulsed tooth with running water, which removed the periodontal tissues from the root surface. The teeth were then wrapped in paper towels, and the patients visited the doctor more than 1 h later (even up to 18 h later). Therefore, periodontal cells, which are essential for periodontal membrane healing, were almost completely destroyed.
In our previous study, we demonstrated that autologous PRF effectively promotes the periodontal healing of avulsed teeth during delayed tooth reimplantation[15]. Similarly, Hiremath et al[24] demonstrated that PRF increased the cellular activity of periodontal ligament cells in vitro[24,25]. A previous study reported that when a PRF membrane was used to wrap the root surface and condense into the canal, it promoted pulp vitality and periodontal healing of the avulsed teeth, whereas a thick radiolucent area surrounding the root was always obvious even after 24 mo of follow-up[26]. Here, PRF granules instead of PRF membranes were adopted; thus, the problem that the PRF graft might prevent the root from fitting into the alveolar bone was avoided. In case 1, the avulsed tooth was reimplanted after 18 h when its periodontal membrane was necrotic, and the probability of root absorption was greatly increased. Therefore, we used PRF together with tooth replantation to reduce the probability of root resorption. These results were also consistent with our expectations, and no signs of root resorption were noted during the 3-mo, 6-mo and 12-mo postoperative follow-up. To our knowledge, this is the longest successful delayed replantation of an avulsed tooth reported in the literature to date. These results suggest that PRF may provide a new treatment opportunity for traditionally hopeless avulsed teeth.
We assume that PRF promotes the healing of avulsed teeth via two mechanisms based on our previous in vitro and in vivo studies. First, the periodontal ligament tissues remaining within the original alveolar socket contain periodontal ligament cells and stem cells. During the tissue repair process, multiple growth factors are released by PRF. Additionally, cell homing will occur, and host stem cells from the circulation will be recruited to the injury region by factors released by the PRF to promote their proliferation and induce their differentiation toward the periodontal membrane, facilitating the formation of periodontal membrane-like structures[15,27]. Second, we demonstrated that PRF consists of concentrated blood platelets, and the α-granules are activated and degranulated. Thus, many growth factors, such as platelet-derived growth factor, transforming growth factor-β, insulin-like growth factor, epidermal growth factor and vascular endothelial growth factor, can be released for at least a week and up to 4 wk. Thus, PRF supports the regenerative and remodeling environment for a certain period of time[28-31]. These growth factors increase the mitotic activity of periodontal fibroblasts by 20%-37%[19], thereby improving the proliferation and periodontal differentiation of target cells and further promoting periodontal healing of avulsed teeth[15]. We also note that when a variety of growth factors act together, synergistic or even antagonistic effects among them cannot be ruled out. Therefore, the natural proportion of various growth factors is particularly important. This is just one of the important reasons why we chose PRF, which contains numerous active growth factors. The growth factors in PRF are not only rich in content and variety but also maintain natural proportions under normal physiological conditions. Only by their synergistic effects can they jointly maintain the balance of the tissue environment and play an important role in regulating wound healing and tissue regeneration.
Although we did not observe obvious ankylosis in these cases, it is a common finding in patients with avulsed teeth[6,15,32,33]. Previous studies have shown that PRF can inhibit the osteogenic differentiation of periodontal ligament stem cells in vitro, which might reduce the possibility of ankylosis based on three reasons. First, PRF promotes cell proliferation to promote the generation of fibroblasts and repair tissue with more seed cells instead of mobilizing bone stromal cells and bone-derived cells. Second, the main component of PRF is collagen fiber, which can act as a physical barrier when placed in the periodontal space. PRF prevents direct contact between the tooth root and the inner wall of the alveolar socket, thus reducing the bone repair between them[15]. Third, PRF inhibits the generation of osteoclasts by promoting osteoprotectin secretion. By inhibiting osteoclast activity, the opportunity for external resorption can be suppressed to some extent[26]. Of course, the present study also has some limitations, such as a short follow-up time and a small sample size. Thus, the long-term effect of PRF in promoting the periodontal healing of avulsed teeth is unclear. Future evaluations including long-term follow-up with a large sample size are planned.
Enriched with growth factors and leukocytes, PRF potentially reduces pathological resorption and promotes periodontal wound healing and periodontal ligament regeneration following delayed reimplantation of avulsed teeth. Although the viability of PRF must be demonstrated in more cases, the application of PRF may offer new therapeutic opportunities for traditionally hopeless avulsed teeth.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arumugam EAP, India; Capparè P, Italy; Ghaffar KA, Egypt; Heboyan A, Armenia; Sekhar P, India; Simbila AN, Tanzania S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Andreasen JO, Andreasen FM. Avulsions. In: Andreasen JO, Andreasen FM, Andersson L. Textbook and Color Atlas of Traumatic Injuries to the Teeth. 4th Edition. Copenhagen: Blackwell Munksgaard, 2007: 444–88. |
| 2. | Casaroto AR, Hidalgo MM, Sell AM, Franco SL, Cuman RK, Moreschi E, Victorino FR, Steffens VA, Bersani-Amado CA. Study of the effectiveness of propolis extract as a storage medium for avulsed teeth. Dent Traumatol. 2010;26:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Gong Y, Xue L, Wang N, Wu C. Emergency dental injuries presented at the Beijing Stomatological Hospital in China. Dent Traumatol. 2011;27:203-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Zhang X, Gong Y. Characteristics of avulsed permanent teeth treated at Beijing Stomatological Hospital. Dent Traumatol. 2011;27:379-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Petrovic B, Marković D, Peric T, Blagojevic D. Factors related to treatment and outcomes of avulsed teeth. Dent Traumatol. 2010;26:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Heboyan A, Avetisyan A, Karobari MI, Marya A, Khurshid Z, Rokaya D, Zafar MS, Fernandes GVO. Tooth root resorption: A review. Sci Prog. 2022;105:368504221109217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 7. | Panzarini SR, Gulinelli JL, Poi WR, Sonoda CK, Pedrini D, Brandini DA. Treatment of root surface in delayed tooth replantation: a review of literature. Dent Traumatol. 2008;24:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Verma UP, Yadav RK, Dixit M, Gupta A. Platelet-rich Fibrin: A Paradigm in Periodontal Therapy - A Systematic Review. J Int Soc Prev Community Dent. 2017;7:227-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 9. | Salgado-Peralvo AO, Mateos-Moreno MV, Uribarri A, Kewalramani N, Peña-Cardelles JF, Velasco-Ortega E. Treatment of oroantral communication with Platelet-Rich Fibrin: A systematic review. J Stomatol Oral Maxillofac Surg. 2022;123:e367-e375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Castro AB, Meschi N, Temmerman A, Pinto N, Lambrechts P, Teughels W, Quirynen M. Regenerative potential of leucocyte- and platelet-rich fibrin. Part A: intra-bony defects, furcation defects and periodontal plastic surgery. A systematic review and meta-analysis. J Clin Periodontol. 2017;44:67-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 164] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 11. | Miron RJ, Zucchelli G, Pikos MA, Salama M, Lee S, Guillemette V, Fujioka-Kobayashi M, Bishara M, Zhang Y, Wang HL, Chandad F, Nacopoulos C, Simonpieri A, Aalam AA, Felice P, Sammartino G, Ghanaati S, Hernandez MA, Choukroun J. Use of platelet-rich fibrin in regenerative dentistry: a systematic review. Clin Oral Investig. 2017;21:1913-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 293] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 12. | Mandviwala DK, Arora AV, Kapoor SV, Shah PB. Internal root resorption: A rare complication of vital pulp therapy using platelet-rich fibrin. J Oral Maxillofac Pathol. 2022;26:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Wang X, Yang Y, Zhang Y, Miron RJ. Fluid platelet-rich fibrin stimulates greater dermal skin fibroblast cell migration, proliferation, and collagen synthesis when compared to platelet-rich plasma. J Cosmet Dermatol. 2019;18:2004-2010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 14. | Chai J, Jin R, Yuan G, Kanter V, Miron RJ, Zhang Y. Effect of Liquid Platelet-rich Fibrin and Platelet-rich Plasma on the Regenerative Potential of Dental Pulp Cells Cultured under Inflammatory Conditions: A Comparative Analysis. J Endod. 2019;45:1000-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Zhao YH, Zhang M, Liu NX, Lv X, Zhang J, Chen FM, Chen YJ. The combined use of cell sheet fragments of periodontal ligament stem cells and platelet-rich fibrin granules for avulsed tooth reimplantation. Biomaterials. 2013;34:5506-5520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Chew JRJ, Tan BL, Lu JX, Tong HJ, Duggal MS. Cell-Based Therapy for Tooth Replantation Following Avulsion: A Systematic Review. Tissue Eng Part B Rev. 2022;28:351-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Fouad AF, Abbott PV, Tsilingaridis G, Cohenca N, Lauridsen E, Bourguignon C, O'Connell A, Flores MT, Day PF, Hicks L, Andreasen JO, Cehreli ZC, Harlamb S, Kahler B, Oginni A, Semper M, Levin L. International Association of Dental Traumatology guidelines for the management of traumatic dental injuries: 2. Avulsion of permanent teeth. Dent Traumatol. 2020;36:331-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 287] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 18. | Heboyan A, Avetisyan AA, Margaryan MM, Azatyan VY. Rare clinical case of tooth root external resorption as a delayed post-traumatic complication. The New Armenian Med J. 2018;12:93-98. |
| 19. | Behnaz M, Izadi SS, Mashhadi Abbas F, Dianat O, Sadeghabadi S, Akbarzadeh T, Haeri A, Kazem M, Younessian F. The impact of platelet-rich fibrin (PRF) on delayed tooth replantation: A preliminary animal study. Aust Endod J. 2021;47:457-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Day PF, Duggal M, Nazzal H. Interventions for treating traumatised permanent front teeth: avulsed (knocked out) and replanted. Cochrane Database Syst Rev. 2019;2:CD006542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Wynkoop JR 2nd, West LA, King JE, Hawley CE. An analysis of dental emergencies during combat and peacetime exercises. Mil Med. 1986;151:364-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Andreasen JO, Borum MK, Jacobsen HL, Andreasen FM. Replantation of 400 avulsed permanent incisors. 4. Factors related to periodontal ligament healing. Endod Dent Traumatol. 1995;11:76-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 437] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 23. | Hohl TH, Shapiro PA, Moffett BC, Ross A. Experimentally induced ankylosis and facial asymmetry in the macaque monkey. J Maxillofac Surg. 1981;9:199-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Hiremath H, Kulkarni S, Sharma R, Hiremath V, Motiwala T. Use of platelet-rich fibrin as an autologous biologic rejuvenating media for avulsed teeth - an in vitro study. Dent Traumatol. 2014;30:442-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Andia I, Abate M. Platelet-rich plasma: underlying biology and clinical correlates. Regen Med. 2013;8:645-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 26. | Johns DA, Shivashankar VY, Maroli RK, Vidyanath S. Novel management of avulsed tooth by pulpal and periodontal regeneration. J Endod. 2013;39:1658-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Chen FM, Wu LA, Zhang M, Zhang R, Sun HH. Homing of endogenous stem/progenitor cells for in situ tissue regeneration: Promises, strategies, and translational perspectives. Biomaterials. 2011;32:3189-3209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 266] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 28. | Khurshid Z, Asiri FYI, Najeeb S, Ratnayake J. The Impact of Autologous Platelet Concentrates on the Periapical Tissues and Root Development of Replanted Teeth: A Systematic Review. Materials (Basel). 2022;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Alves R, Grimalt R. A Review of Platelet-Rich Plasma: History, Biology, Mechanism of Action, and Classification. Skin Appendage Disord. 2018;4:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 383] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 30. | Suttapreyasri S, Leepong N. Influence of platelet-rich fibrin on alveolar ridge preservation. J Craniofac Surg. 2013;24:1088-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 31. | Ngah NA, Dias GJ, Tong DC, Mohd Noor SNF, Ratnayake J, Cooper PR, Hussaini HM. Lyophilised Platelet-Rich Fibrin: Physical and Biological Characterisation. Molecules. 2021;26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 32. | Andreasen JO. Analysis of pathogenesis and topography of replacement root resorption (ankylosis) after replantation of mature permanent incisors in monkeys. Swed Dent J. 1980;4:231-240. [PubMed] |
| 33. | Andreasen JO, Hjorting-Hansen E. Replantation of teeth. II. Histological study of 22 replanted anterior teeth in humans. Acta Odontol Scand. 1966;24:287-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 198] [Article Influence: 3.4] [Reference Citation Analysis (0)] |