Published online Jan 26, 2023. doi: 10.12998/wjcc.v11.i3.487
Peer-review started: May 6, 2022
First decision: June 9, 2022
Revised: June 13, 2022
Accepted: January 5, 2022
Article in press: January 5, 2023
Published online: January 26, 2023
Processing time: 265 Days and 8.5 Hours
Despite improvement in cardiopulmonary resuscitation (CPR) performance, cardiac arrest (CA) is still associated with poor prognosis. The high mortality rate is due to multi-organ dysfunction caused by cerebral ischemia and reperfusion injury (I/R). The guidelines for CPR suggest the use of therapeutic hypothermia (TH) as an effective treatment to decrease mortality and the only approach confirmed to reduce I/R injury. During TH, sedative agents (propofol) and analgesia agents (fentanyl) are commonly used to prevent shiver and pain. However, propofol has been associated with a number of serious adverse effects such as metabolic acidosis, cardiac asystole, myocardial failure, and death. In addition, mild TH alters the pharmacokinetics of agents (propofol and fentanyl) and reduces their systemic clearance. For CA patients undergoing TH, propofol can be overdosed, leading to delayed awakening, prolonged mechanical venti
Core Tip: Ciprofol (HSK3486) is a novel anesthetic agent that is convenient and easy to administer intravenously outside the operating room. Ciprofol is rapidly metabolized and accumulates at low concentrations after continuous infusion in a stable circulatory system compared to propofol. We hypothesize that HSK3486 can improve survival rates and achieve good neurological outcomes in cardiac arrest patients who receive therapeutic hypothermia.
- Citation: Wang YC, Wu MJ, Zhou SL, Li ZH. Protective effects of combined treatment with ciprofol and mild therapeutic hypothermia during cerebral ischemia-reperfusion injury. World J Clin Cases 2023; 11(3): 487-492
- URL: https://www.wjgnet.com/2307-8960/full/v11/i3/487.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i3.487
Cardiac arrest (CA) is associated with poor prognosis. CA accounts for approximately 15% of all deaths and 50% of all cardiac deaths worldwide[1]. Survival rates with good neurological outcomes are low, in the range between 2% and 23%, depending on a variety of regional, social, and medical factors[2,3]. From a pathophysiology perspective, post-CA myocardial injury and brain injury are induced by ischemia/reperfusion injury (I/R injury)[4,5]. The guidelines for cardiopulmonary resuscitation (CPR) suggest that therapeutic hypothermia (TH) is an effective treatment to decrease mortality, and CPR is the only approach confirmed to reduce I/R injury[6,7].
During TH, sedative agents (propofol) and analgesia agents (fentanyl) are commonly used to prevent shiver and pain[6]. However, studies have shown that propofol has been associated with a number of serious adverse effects such as metabolic acidosis, cardiac asystole, myocardial failure, rhabdomyolysis, and death[7-9]. Low-grade myotoxicity can be associated with prolonged (weeks) exposure to propofol in the intensive care unit (ICU), especial in children. Several studies reported that prolonged propofol sedation of coronavirus disease 2019 (COVID-19) patients with long-term mechanical ventilation contributed to critical illness myopathy[8-10]. In addition, it has been shown that mild TH alters the pharmacokinetics of propofol and fentanyl and reduces their systemic clearance in rats after the return of spontaneous circulation (ROSC)[11,12]. For CA patients who have undergone TH, propofol can be overdosed, leading to delayed wakening, prolonged mechanical ventilation, and other subsequent complications, often referred to as propofol syndrome[12].
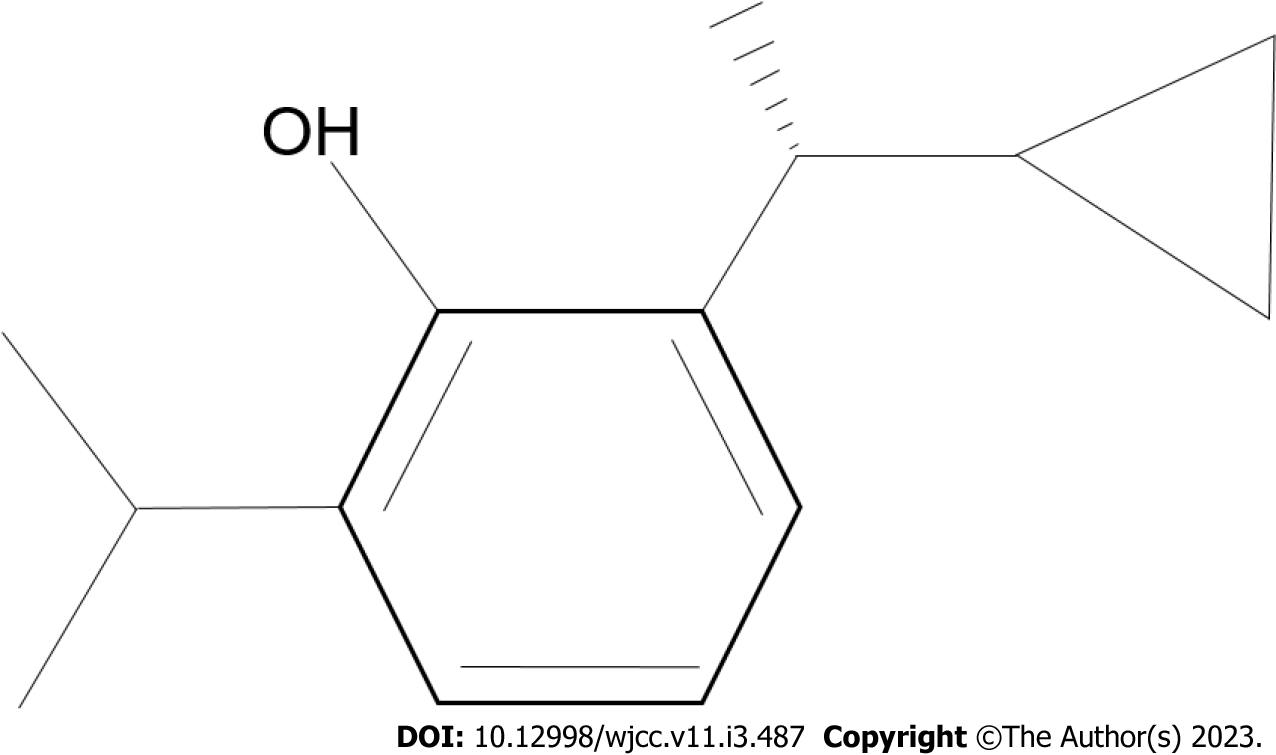
Ciprofol (HSK3486) (Figure 1), which is a 2,6-disubstituted alkylphenol phenol derivative, is a novel propofol analogue formulated in an injectable emulsion of medium- and long-chain triglycerides[13]. Its pharmacokinetics and distribution indicate that HSK3486 is rapidly metabolized and has low accumulation after continuous infusion[14]. One study reported that HSK3486, like propofol, exerts its sedative effects through binding to gamma-aminobutyric acid type A (GABAA) receptors, but showed higher liposolubility and potency than propofol[15]. Furthermore, to achieve the same level of anesthesia, fewer lipids from the HSK3486 emulsion enter into the circulatory system compared to propofol emulsion[16]. HSK3486 at a maintenance dose of 3-4 mg/kg/h showed good efficacy and safety in the treatment of long-term mechanically ventilated patients in the ICU[16,17]. However, whether HSK3486 can improve survival rates in CA patients who receive TH remains unclear.
We hypothesize that HSK3486 can improve survival rates and achieve good neurological outcomes in CA patients who receive TH.
The high mortality rate of CA is attributed to whole-body I/R induced multi-organ dysfunction that is referred to as post-cardiac arrest syndrome (PCAS)[18-20]. Post-CA myocardial dysfunction, macrocirculatory dysfunction, and brain injury are the main clinical features of this complex pathophysiological process. Myocardial dysfunction after CA commonly results in death and hemodynamic instability[19]. Increased heart rate and blood pressure after ROSC are attributed to medications and catecholamine release. These effects cause a global stunning of the myocardium that usually resolves and returns to normal after 72 h[20].
Studies have shown that propofol is associated with a number of serious adverse effects such as metabolic acidosis, cardiac asystole, myocardial failure, rhabdomyolysis, and death[7-10]. Low-grade myotoxicity can be associated with prolonged (weeks) exposure to propofol in the ICU[11]. In both clinical and experimental studies where patients and animals have been exposed to long-term (10 d) controlled mechanical ventilation, patients and animals developed critical illness myopathy[8-12]. Thus, for CA patients, propofol infusion syndrome should be reduced to maintain a stable circulatory system, especial when TH treatment has been used.
Ciprofol (HSK3486), which is a 2,6-disubstituted alkylphenol phenol derivative, is a novel propofol analogue formulated in an injectable emulsion of medium- and long-chain triglycerides[13-15]. HSK3486 acts against the α1β2γ2 subtype of GABAA receptors and inhibits a wide range of CYP450 isozymes in mammalian species. Its pharmacokinetics and distribution indicate that HSK3486 is rapidly metabolized and has low accumulation after continuous infusion. One study reported that HSK3486 showed better anesthesia potency over propofol, with an 83% lower ED50. Furthermore, fewer lipids from the HSK3486 emulsion enter into the stable circulatory system compared to a propofol emulsion. Thus, HSK3486 can achieve the same sedative depth during TH after CA and may improve survival rates compared to propofol.
This hypothesis can be tested in two ways using rat models of CA. First, we could compare the protective effects of HSK3486 and propofol directly, and second we could test whether the combination of HSK3486 and TH confers greater protection that either HSK3486 or propofol alone.
The CA animal model was established in our previous study[11,21]. Here we would adapt it as follows. After anesthesia, we will induce CA by applying six minutes of asphyxia. We will then use CPR with mechanical ventilation of 100% oxygen, at a frequency of 80 breaths/min, tidal volume of 10 ml/kg, and sternal compressions (with two fingers) at a rate of 200 times/min (attempting to generate systolic arterial pressure peaks > 50 mmHg). We will continue CPR until return of spontaneous circulation (ROSC), defined as achieving a spontaneous mean arterial pressure (MAP) of 60 mmHg, that is maintained for more than 10 min[13,21]. We will simultaneously administer epinephrine (0.02 mg/kg i.v.) with the sternal compressions every 3 min if necessary, and 5% NaHCO3 (1 mmol/kg i.v.) can also be provided if needed. If overall CPR attempts exceed 5 min, the experiment will be stopped. After ROSC, rats will be divided randomly into four groups: S group (sham-operate group), CPR group (infusion with saline 4 ml/kg for 30 min), HSK3486 group (infusion with HSK3486 at 0.4 mg/kg for 30 min), and propofol group (infusion with 2 mg/kg emulsion for 30 min). Survival conditions, behavioral evaluations, echocardiogram, and histopathologic analysis (including TUNEL staining of neurons and cardiomyocytes and Nissl staining of neurons) in each group will be evaluated on days 1 and 7 after ROSC. We will then compare the protective effects of HSK3486 and propofol post-conditioning. In the second set of experiments we will also evaluate the effects of TH. The CA model will be established as described above, but rats will be randomly divided into five groups after ROSC: CPR group, HSK3486 group (infusion with HSK3486 at 2 mg/kg for 30 min), TH group (maintaining rectal temperature at 33 ± 0.5℃ for 2 h), HSK3486-TH group (TH will be initiated with HSK3486 infusion at 33 ± 0.5℃ for 2 h), and propofol-TH group (TH will be initiated with propofol infusion at 33 ± 0.5℃ for 2 h). We will conduct the same evaluations at days 1 and 7 as described for first set of experiments. However, during these experiments, the left femoral artery and vein will be separately cannulated with catheters to measure blood pressure and for drug administration. The rectal temperature will be controlled throughout the experiment with the aid of a lamp or ice bag. Rectal temperature, MAP, electrocardiogram, and blood gas levels will be continuously monitored during the experiment.
Despite the advances in treatment of PCAS, a significant proportion of patients still have a poor prognosis. The pathophysiological processes that occur after whole-body I/R injury following CA lead to multi-organ dysfunction. The subsequent reperfusion injury after ROSC causes cardiac and brain dysfunction. HSK3486, as an intravenous anesthetic, has advantages such as rapid metabolism and low accumulation in the circulation compared to propofol.
HSK3486 is a GABAA receptor agonist that has been shown to have an improved anesthetic profile and less injection pain compared to propofol in pre-clinical studies. HSK3486 is formulated in a 10% oil-in-water emulsion with a drug concentration of 10 mg/mL. Compared with lipophilic drugs, such as midazolam, HSK3486 is metabolized at faster rate and reduces the deepening of sedation. HSK3486 has been used for clinical endoscopy, but it has also been recommended for adult patients who may need general anesthesia for fiberoptic bronchoscopy. In a previous trial, Teng et al[22] demonstrated the safety of HSK3486 during colonoscopy with good tolerance. In addition, HSK3486 has shown good tolerance in some unpublished studies (NCT03698617, NCT03808844, NCT04048811, and NCT04511728). Furthermore, based on available data, HSK3486 causes less potential damage to cardiovascular and cerebrovascular function, as evidenced by stable hemodynamics and its reported safety profile[23]. Glucuronidation, oxidation, and sulfation are the major metabolic pathways targeted by HSK3486, and its glucuronidation metabolites are generally considered to be nonhypnotic, nontoxic, and rapidly cleared in from the plasma[14]. In summary, HSK3486 may become a promising anesthetic agent.
The available studies provide some useful guidance regarding the appropriate dose for post-conditioning and HSK3486 adaptation. Teng et al[22] reported that HSK3486 could be administered at a range of 0.1-0.5 mg/kg during colonoscopy. The study by Li et al[23] showed that a sedative effect could be achieved at 0.3 mg/kg in the elderly compared to 0.4 mg/kg in the non-elderly. Thus, 0.4 mg/kg of HSK3486 would be the chosen dose to test in combination with TH. However, due to the lack of research on the appropriate dose of HSK3486 for intravenous administration in CA, more studies are needed to test our hypothesis.
We hypothesize that HSK3486 in combination with TH would confer cardio-protective effects and less risks compared to propofol. The combination of HSK3486 and TH may synergistically prevent neuronal injuries caused by I/R following CA, due to the fact that HSK3486 can be rapidly metabolized. Additionally, this combination could potentially ameliorate the side effects of hypothermia. Therefore, HSK3486 treatment in combination with TH could be a novel treatment for CA patients.
Testing our hypothesis would provide new insight about the treatment of CA, providing a promising prevention strategy for post-arrest myocardial and neuronal reperfusion injury.
Here we propose one hypothesis for using a combined TH treatment after CA, but more experimental data is needed to test our hypothesis, and further studies are needed to elucidate the cardio-protective mechanism of ciprofol in the future.
Based on the experimental laboratory data, we hypothesize that HSK3486 administered with TH after CA would improve patient survival rates and lead to good neurological outcomes.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Anesthesiology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rezus E, Romania; Seetharaman RV, India S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Woodruff TM, Thundyil J, Tang SC, Sobey CG, Taylor SM, Arumugam TV. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol Neurodegener. 2011;6:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 407] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 2. | Dalessio L. Post-Cardiac Arrest Syndrome. AACN Adv Crit Care. 2020;31:383-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Xie W, Zhou P, Sun Y, Meng X, Dai Z, Sun G, Sun X. Protective Effects and Target Network Analysis of Ginsenoside Rg1 in Cerebral Ischemia and Reperfusion Injury: A Comprehensive Overview of Experimental Studies. Cells. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 4. | Zhang WF, Jin YC, Li XM, Yang Z, Wang D, Cui JJ. Protective effects of leptin against cerebral ischemia/reperfusion injury. Exp Ther Med. 2019;17:3282-3290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Cadenas S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic Biol Med. 2018;117:76-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 591] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 6. | Peberdy MA, Callaway CW, Neumar RW, Geocadin RG, Zimmerman JL, Donnino M, Gabrielli A, Silvers SM, Zaritsky AL, Merchant R, Vanden Hoek TL, Kronick SL; American Heart Association. Part 9: post-cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S768-S786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 987] [Cited by in RCA: 960] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 7. | Kam PC, Cardone D. Propofol infusion syndrome. Anaesthesia. 2007;62:690-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 297] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 8. | Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat Med. 2011;17:1391-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1886] [Cited by in RCA: 2513] [Article Influence: 179.5] [Reference Citation Analysis (0)] |
| 9. | Lucchetta V, Bonvicini D, Ballin A, Tiberio I. Propofol infusion syndrome in severe COVID-19. Br J Anaesth. 2020;125:e441-e442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Lönnqvist PA, Bell M, Karlsson T, Wiklund L, Höglund AS, Larsson L. Does prolonged propofol sedation of mechanically ventilated COVID-19 patients contribute to critical illness myopathy? Br J Anaesth. 2020;125:e334-e336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Yuan J, Yang MC, Wu MJ, Gou YS. Sedative depth on neurological outcomes in a juvenile rat model of cardiopulmonary resuscitation. Med Hypotheses. 2019;132:109233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Zhang YJ, Wu MJ, Li Y, Yu H. Cardiocerebral protection by emulsified isoflurane during cardiopulmonary resuscitation. Med Hypotheses. 2015;84:20-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Bian Y, Zhang H, Ma S, Jiao Y, Yan P, Liu X, Xiong Y, Gu Z, Yu Z, Huang C, Miao L. Mass balance, pharmacokinetics and pharmacodynamics of intravenous HSK3486, a novel anaesthetic, administered to healthy subjects. Br J Clin Pharmacol. 2021;87:93-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 14. | Liao J, Li M, Huang C, Yu Y, Chen Y, Gan J, Xiao J, Xiang G, Ding X, Jiang R, Li P, Yang M. Pharmacodynamics and Pharmacokinetics of HSK3486, a Novel 2,6-Disubstituted Phenol Derivative as a General Anesthetic. Front Pharmacol. 2022;13:830791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 15. | Qin L, Ren L, Wan S, Liu G, Luo X, Liu Z, Li F, Yu Y, Liu J, Wei Y. Design, Synthesis, and Evaluation of Novel 2,6-Disubstituted Phenol Derivatives as General Anesthetics. J Med Chem. 2017;60:3606-3617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 16. | Liu Y, Chen C, Liu N, Tong L, Nie Y, Wu J, Liu X, Gao W, Tang L, Guan X. Efficacy and Safety of Ciprofol Sedation in ICU Patients with Mechanical Ventilation: A Clinical Trial Study Protocol. Adv Ther. 2021;38:5412-5423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Hu C, Ou X, Teng Y, Shu S, Wang Y, Zhu X, Kang Y, Miao J. Sedation Effects Produced by a Ciprofol Initial Infusion or Bolus Dose Followed by Continuous Maintenance Infusion in Healthy Subjects: A Phase 1 Trial. Adv Ther. 2021;38:5484-5500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 18. | Nolan JP, Neumar RW, Adrie C, Aibiki M, Berg RA, Böttiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC, Kern KB, Laurent I, Longstreth WT, Merchant RM, Morley P, Morrison LJ, Nadkarni V, Peberdy MA, Rivers EP, Rodriguez-Nunez A, Sellke FW, Spaulding C, Sunde K, Hoek TV. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A Scientific Statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation. 2008;79:350-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 740] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 19. | Mai N, Prifti L, Rininger A, Bazarian H, Halterman MW. Endotoxemia induces lung-brain coupling and multi-organ injury following cerebral ischemia-reperfusion. Exp Neurol. 2017;297:82-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Nagase M, Sakurai A, Sugita A, Matsumoto N, Kubo A, Miyazaki Y, Kinoshita K, Yamamoto Y. Oxidative stress and abnormal cholesterol metabolism in patients with post-cardiac arrest syndrome. J Clin Biochem Nutr. 2017;61:108-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Idris AH, Becker LB, Ornato JP, Hedges JR, Bircher NG, Chandra NC, Cummins RO, Dick W, Ebmeyer U, Halperin HR, Hazinski MF, Kerber RE, Kern KB, Safar P, Steen PA, Swindle MM, Tsitlik JE, von Planta I, von Planta M, Wears RL, Weil MH. Utstein-style guidelines for uniform reporting of laboratory CPR research. A statement for healthcare professionals from a Task Force of the American Heart Association, the American College of Emergency Physicians, the American College of Cardiology, the European Resuscitation Council, the Heart and Stroke Foundation of Canada, the Institute of Critical Care Medicine, the Safar Center for Resuscitation Research, and the Society for Academic Emergency Medicine. Resuscitation. 1996;33:69-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 139] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Teng Y, Ou MC, Wang X, Zhang WS, Liu X, Liang Y, Zuo YX, Zhu T, Liu B, Liu J. Pharmacokinetic and pharmacodynamic properties of ciprofol emulsion in Chinese subjects: a single center, open-label, single-arm dose-escalation phase 1 study. Am J Transl Res. 2021;13:13791-13802. [PubMed] |
| 23. | Li X, Yang D, Li Q, Wang H, Wang M, Yan P, Wu N, Li F, Ma S, Ding Y, Liu J. Safety, Pharmacokinetics, and Pharmacodynamics of a Single Bolus of the γ-aminobutyric Acid (GABA) Receptor Potentiator HSK3486 in Healthy Chinese Elderly and Non-elderly. Front Pharmacol. 2021;12:735700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |