Published online Oct 6, 2023. doi: 10.12998/wjcc.v11.i28.6902
Peer-review started: July 11, 2023
First decision: August 24, 2023
Revised: August 30, 2023
Accepted: September 14, 2023
Article in press: September 14, 2023
Published online: October 6, 2023
Processing time: 76 Days and 8.7 Hours
Testicular mixed germ cell tumors (TMGCTs) are rare malignant tumors that are more common in men aged 20–40 years. TMGCTs comprise two or more types of germ cell tumors that primarily affect the testis. Their onset is undetectable; thus, early diagnosis is challenging. However, early recognition and diagnosis substantially improve patient prognosis.
We evaluated a rare case of TMGCT in a male patient presenting with recurrent fever and left supraclavicular lymphadenectasis instead of testicular enlargement and pain, which may easily lead to misdiagnosis. We report the clinical signs and symptoms, histopathological characteristics, and immunohistochemical results of this case of malignant TMGCT.
Our case, which was typical with multiple components, along with a literature review, may serve as a basis for early diagnosis.
Core Tip: We evaluated a rare case of testicular mixed germ cell tumor (TMGCT) in a male patient presenting with recurrent fever and left supraclavicular lymphadenectasis, instead of testicular enlargement and pain, which may easily lead to misdiagnosis. We report the clinical signs and symptoms, histopathological characteristics, and immunohistochemistry findings of this case of malignant TMGCT. Our case, which presented multiple components typical of TMGCT, along with a literature review, may serve as a basis for the early recognition and diagnosis of this disease.
- Citation: Xiao QF, Li J, Tang B, Zhu YQ. Testicular mixed germ cell tumor: A case report. World J Clin Cases 2023; 11(28): 6902-6907
- URL: https://www.wjgnet.com/2307-8960/full/v11/i28/6902.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i28.6902
Testicular mixed germ cell tumor (TMGCT) is a relatively rare malignant tumor of the testis with no specificity for most symptoms; thus, early diagnosis is difficult[1]. TMGCTs also demonstrate a wide variety of histopathological, genetic, pathogenetic, and immunocytochemical characteristics, as well as various clinical-biological profiles and prognoses.
We report the first case of a patient with a TMGCT with fever and lymph node enlargement in China. The patient presented with recurrent fever and enlarged left subclavian lymph nodes. An external hospital considered the possibility of lymphoma. The results of lymph node puncture biopsy suggested a malignant mixed germ cell-derived tumor (seminoma or embryonic cancer). However, the patient had no symptoms of testicular swelling or pain, and the primary lesion was hidden in the right testis after testicular sonography. This case report describes the patient’s clinical and pathological data to provide insight into the early diagnosis of TMGCT and reduce clinical misdiagnosis.
A 24-year-old man visited our hospital on January 15, 2022, with a recurrent fever and left supraclavicular lymphadenectasis that had lasted for > 1 mo.
The patient visited a local hospital on December 7, 2021, with fever after a cold. Since then, he had developed a fever four times, which was attributed to several periods of cold. The maximum body temperature was 39 °C and he reported no chills.
Ultrasonic examination revealed multiple hypoechoic nodules on the left side of the neck, with the largest nodule measuring approximately 3 cm × 2.2 cm and having a plump shape located on the supraclavicular fossa. We did not observe a hilar structure with a defined boundary in these nodules. Color Doppler flow imaging showed abundant but promiscuous blood flow signals. Based on the examination results, these nodules were considered abnormally enlarged lymph nodes. Subsequently, an ultrasound-guided puncture biopsy was performed. Postoperative pathological results indicated poorly differentiated adenocarcinoma infiltrating into the fibrous tissue.
The other hospital also considered a diagnosis of lymphoma. After admission to our hospital, adenocarcinoma was considered because the pathological results of the left supraclavicular lymph nodes were inconsistent with the diagnostic criteria for lymphoma. However, due to the lack of evidence of primary lesions, it was difficult to precisely diagnose the disease. Therefore, another lymph node puncture biopsy was required to confirm the pathology of this disease. The lesions were typical with enlarged left supraclavicular and retroperitoneal lymph nodes. Considering the high risk associated with retroperitoneal lymph node puncture, another puncture of the left supraclavicular lymph node was performed.
The patient had been healthy with no history of cryptorchidism.
The patient had no remarkable personal or family history.
The fever duration ranged from 12 h to 2 d and was accompanied by left supraclavicular lymphadenectasis.
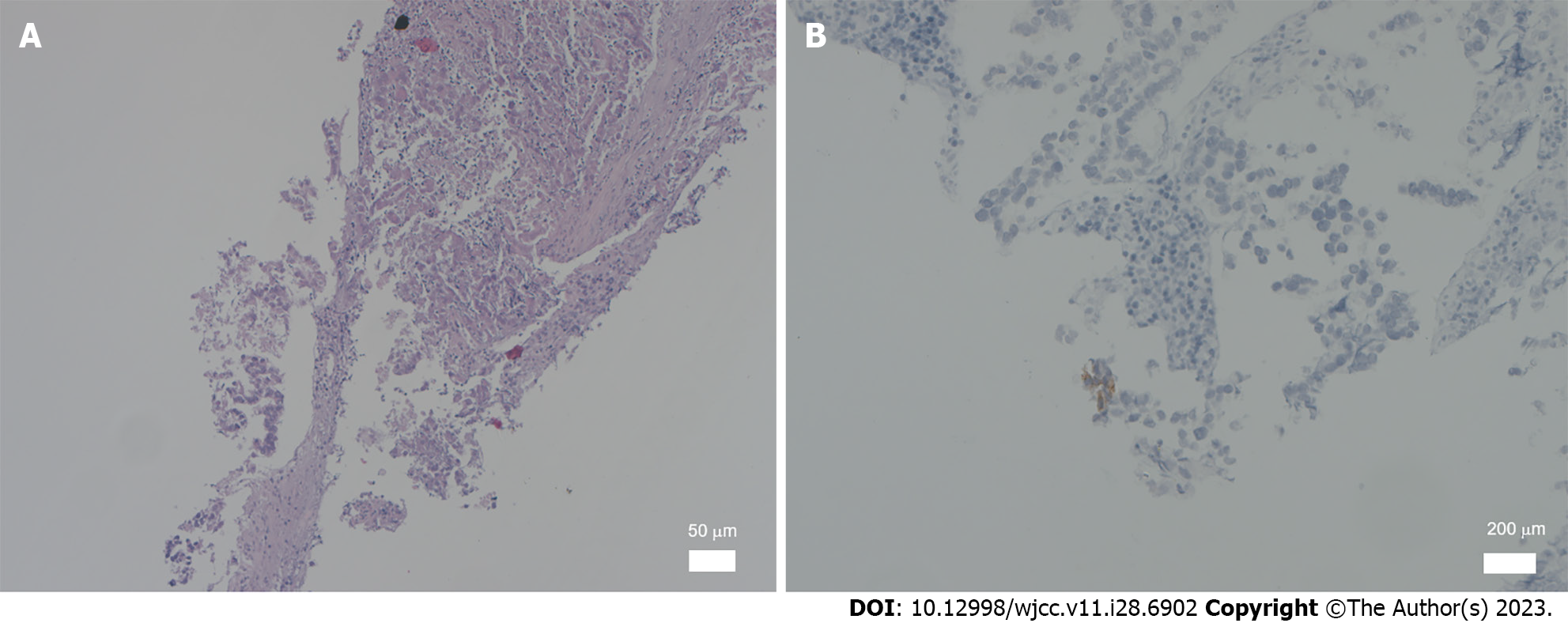
The specimens were fixed in 4% formaldehyde for 24 h, embedded in paraffin, and stained with hematoxylin and eosin. Immunohistochemical staining was performed using the EnVision two-step method with primary antibodies against β-human chorionic gonadotropin (HCG), carcinoembryonic antigen, cluster of differentiation (CD) 30, alpha-fetoprotein (AFP), creatine kinase (CK), epithelial membrane antigen, vimentin, placental alkaline phosphatase (PLAP), CK8/18, CD117, and D2-40. All antibodies and immunohistochemical test kits were obtained from the Department of Pathology, China-Japan Friendship Hospital. All primary antibodies included negative and positive controls, respectively, according to the standard. The pathological results showed metastatic malignant epithelial tumors in the lymphatic tissues, accompanied by massive tissue necrosis. Papillary lesions were present in some areas with local areas with granulomas and multinucleated giant cells. Owing to the consistency of the examination results with the diagnostic criteria for malignant germ cell-derived tumors (Figure 1A), we considered yolk sac tumor or embryonal carcinoma, which required supplementary immunohistochemistry (IHC) data owing to the small size. The IHC results [organic cation transporter (OCT)3/4 (partially +), leukocyte common antigen (-), Sal-like protein 4 (SALL4) (+), CD3 (-), CD20 (-), Wilms tumor-1 (-), anaplastic lymphoma kinase (-), AE1/AE3 (+), MC (-), CR (-), CK8 (+), CK18 (+), programmed death-ligand 1 (3%+), hepatocyte (focally weakly +), napsinA (-), P40 (-), prostate-specific antigen (-), estrogen receptor (-), CK7 (partially +), CK20 (-), renal cell carcinoma (-), and thyroid transcription factor 1 (-)] were consistent with the diagnostic criteria for malignant germ cell-derived tumors. Additional immunostaining findings for SALL4 (a small number of cells +), AFP (-), CD117 (individual cells +), PLAP (individual cells +), Ki67 (MIB-1) (20% +), CD30 (individual cells +), OCT4 (weakly +), and hepatocyte (-) were consistent with the diagnostic criteria for germ-cell tumors (seminoma + embryonal carcinoma) (Figure 1B). A targeted check of germ cell tumor-related tumor markers revealed that the patient's β-HCG level had increased to 121.97 mIU/mL.
Lymphadenectasis was attributed to metastasis of the mixed germ cell tumor[2], according to the results mentioned above. The primary lesions of germ cell tumors are usually located in the gonads, but their initial occurrence has been reported in the cranial cavity[3]. However, the examination results showed no symptoms of testicular enlargement or pain, and there was a normal size and texture of the bilateral testes without palpable masses. We did not observe thickening of the spermatic cord and the transillumination test was negative.
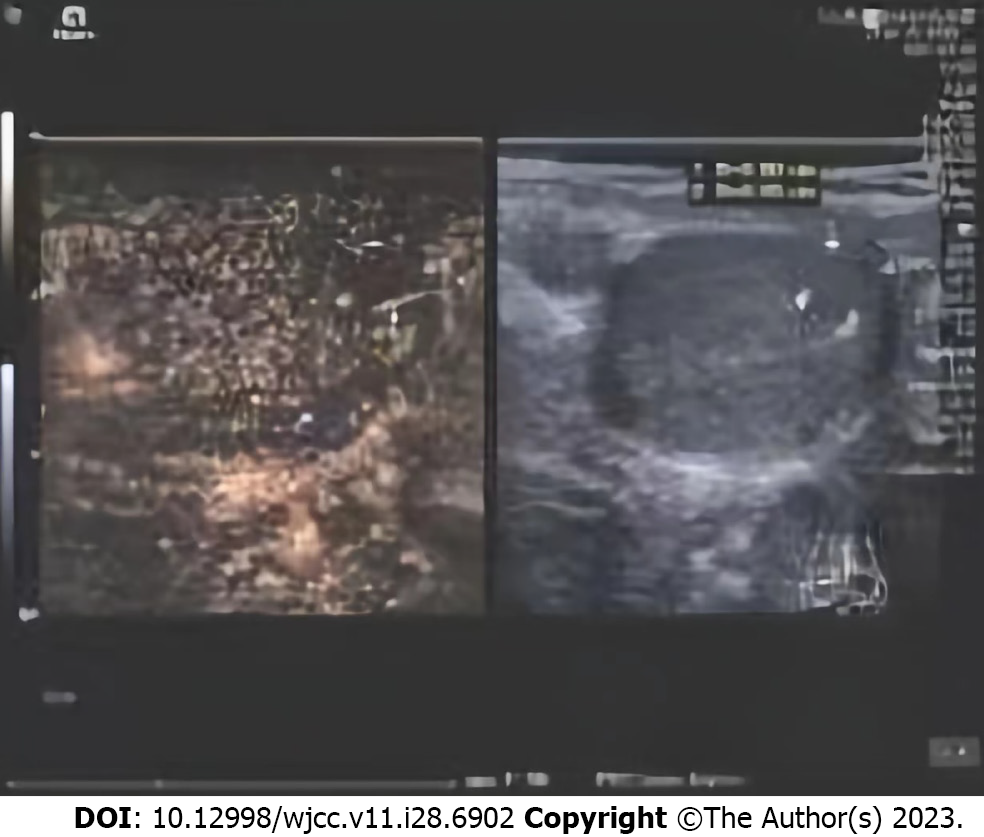
Testicular ultrasound examination did not suggest abnormalities, and no positive findings were observed in the neurological examination and brain magnetic resonance imaging examination, which was inconsistent with the presentation of germ cell tumors previously reported. Testicular contrast-enhanced ultrasound (CEUS) was performed after repeat evaluations. The contrast agent SonoVue (sulfur hexafluoride microbubbles; Bracco Spa, Milan, Italy) was injected once at a dose of 0.8 mL and twice at a dose of 1.2 mL, through the left median cubital vein bolus. A small number of microbubbles, which were obscure in boundary, irregular in shape, and approximately 0.62 cm × 0.36 cm in size, were observed in the hypoechoic areas in the right testis. Short strips of strong echoes were present inside the microbubbles, most of which had no areas of enhancement. Therefore, the lesions were considered to be tumors (Figure 2).
The patient was diagnosed with a stage III testicular germ cell tumor (seminoma + embryonal carcinoma) with multiple metastatic lymph nodes.
The first-line therapy was six cycles of chemotherapy with 100 mg etoposide, 40 mg cisplatin, and 15000 units of bleomycin, after which testicular tumor lesions and left supraclavicular and retroperitoneal lymph nodes were significantly reduced, with a decline in β-HCG to a negative level. However, the β-HCG level subsequently rebounded and increased, accompanied by multiple metastases in the lungs. Therefore, we administered second-line therapy, including four cycles of 400 mg albumin paclitaxel in combination with 200 mg tislelizumab (PD-1), followed by an injection of 200 mg tislelizumab every 3 wk. Reexamination performed one year after the disease onset showed that the primary lesion in the right testis, the left supraclavicular lymph node, and the retroperitoneal lymph node measured 0.49 cm × 0.40 cm, 0.9 cm × 0.56 cm, and 1.1 cm × 0.7 cm, respectively.
Micronodules were observed only in the inferior lobe of the right lung on plain chest computed tomography (CT). β-HCG remained negative and the evaluation suggested that the patient achieved stable disease.
TMGCTs are composed of two or more types of germ cell tumors and primarily occur in the testis. They account for only approximately 16% of all testicular tumors, and patients usually have a survival period of 20–132 mo[4], with a median survival period of 42 mo[5]. Currently, TMGCTs are mostly reported as individual cases in China and other countries. Mixed forms mainly include teratoma in combination with embryonal carcinoma, embryonal carcinoma in combination with seminoma, and teratoma in combination with embryonal carcinoma and seminoma. The combination of seminoma and yolk sac tumor is rarely observed. The onset of TMGCT is difficult to detect, particularly in the early stages. However, early recognition and diagnosis substantially improve the disease prognosis. Previous reports have shown enlarged testes among most patients with TMGCTs, and intracranial germ cell tumors are common in some cases of extragonadal germ cell tumors[6]. However, the patient in our case had a fever and lymph node enlargement but no testicular discomfort. Since we did not observe testis swelling or positive signs of the nervous system during the physical examination, this could easily result in misdiagnosis in clinical practice. Therefore, repeated evaluations of the pathology and targeted IHC are critical for the diagnosis of this disease.
TMGCTs and single germ cell tumors originate from intratubular germ cell neoplasia unclassified, which is also known as the precursor of testicular germ cell tumors. Currently, TMGCT histogenesis is believed to correlate with the translocation of the short arm of chromosome 12[7]. Hoei-Hansen et al[8] proposed the concept of testicular carcinoma in situ, i.e., the conception of the origin of intratubular germ cell tumors.
A single seminoma may differentiate into the components of a nonseminoma. TMGCTs are mainly caused by the limited differentiation of a seminoma into non-seminomatous germ cell tumors. TMGCT diagnosis relies on symptoms and signs, imaging findings, serum tumor marker detection, and pathological examinations. AFP, HCG, and lactic dehydrogenase (LDH) play significant roles in testicular tumor diagnosis, treatment, and prognosis[9]. If AFP is negative in the IHC of a single seminoma, it may contain components of a non-seminoma, such as embryonal carcinoma. Therefore, if pathological studies report seminoma with positive AFP on IHC, more careful IHC identification and interpretation are necessary. HCG is generated by the trophoblastic cells of tumor complexes. Hence, choriocarcinoma or components of choriocarcinoma are highly suspected if the HCG concentration dramatically increases in patients with testicular tumors. Furthermore, elevated levels of LDH, a key factor in poor prognosis, are correlated with tumor volume.
CEUS is of great utility when detecting a testicular mass[4]. Germ cell tumors show uneven, highly enhanced, fast-forward, and fast-backward patterns in the CEUS arterial phase, with twisted blood vessels on the edge and inside of the lesions as well as a sign of "pseudo capsule". Our patient did not exhibit testicular enlargement or pain. He also did not show testicular space-occupying lesions on routine ultrasonic, pelvic, and positron emission tomography-CT examinations. Based on a review of recent reports, CEUS, which was used to examine the bilateral testes and identify the primary lesion in the right testis, significantly contributed to the ultimate diagnosis in this case.
Radical resection combined with adjuvant chemotherapy is the classic treatment for TMGCT[10]. The components of embryonal carcinoma, yolk sac tumor, and immature teratoma revealed in pathological reports are considered high risk factors[9], for which retroperitoneal lymph node dissection should be performed after surgery. Cisplatin, bleomycin, and etoposide can be used as first-line chemotherapeutic drugs in cases with retroperitoneal lymphatic metastasis[11]. Our patient had multiple lymphatic metastases at the time of diagnosis; therefore, the tumor was classified as stage III according to the American Cancer Society staging criteria. Systemic chemotherapy was adopted as the major therapy and programmed death receptor 1 (PD-1) inhibitor immunotherapy was applied in the later period. Treatment was considered effective because the patient was in a stable condition in follow-up examinations. Moreover, his lymph nodes were significantly reduced and samples remained negative for tumor markers.
A review of the related literature in recent years reveals several reports of TMGCTs manifesting with fever and lymph node enlargement, which may be explained by the undetectable onset of the disease as well as limited knowledge of the disease to date. The diagnostic process and treatment plan for this patient may serve as a reference in clinical practice.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Exbrayat JM, France S-Editor: Lin C L-Editor: Wang TQ P-Editor: Yu HG
| 1. | Koh KN, Wong RX, Lee DE, Han JW, Byun HK, Yoon HI, Kim DS, Lyu CJ, Kang HJ, Hong KT, Lee JH, Kim IH, Phi JH, Kim SK, Wong TT, Lee HL, Lai IC, Kang YM, Ra YS, Ahn SD, Im HJ, Looi WS, Low SYY, Tan EEK, Park HJ, Shin SH, Fuji H, Suh CO, Chen YW, Kim JY. Outcomes of intracranial germinoma-A retrospective multinational Asian study on effect of clinical presentation and differential treatment strategies. Neuro Oncol. 2022;24:1389-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Ricci C, Franceschini T, Giunchi F, Borsato M, Mollica V, Massari F, Fiorentino M. A preliminary study investigating the detection of lymphovascular invasion in germ cell tumors of the testis with double staining for OCT4/CD34. Pathol Res Pract. 2021;227:153637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Wang WG, Ye H, Chinnaiyan P. Practice patterns and survival outcomes of intracranial germinoma: an analysis of the National Cancer Database. J Neurooncol. 2018;137:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Katabathina VS, Vargas-Zapata D, Monge RA, Nazarullah A, Ganeshan D, Tammisetti V, Prasad SR. Testicular Germ Cell Tumors: Classification, Pathologic Features, Imaging Findings, and Management. Radiographics. 2021;41:1698-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (2)] |
| 5. | Oruc Z, Ebinç S, Kaplan MA. Rare Tumours of the Testis: Twelve Years of Experience. Prague Med Rep. 2020;121:181-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Nassiri N, Maas M, Asanad K, Cacciamani G, Nabhani J. Testicular cancer with neurological symptoms indicates brain metastases. Lancet. 2021;397:e7. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Litchfield K, Levy M, Dudakia D, Proszek P, Shipley C, Basten S, Rapley E, Bishop DT, Reid A, Huddart R, Broderick P, Castro DG, O'Connor S, Giles RH, Houlston RS, Turnbull C. Rare disruptive mutations in ciliary function genes contribute to testicular cancer susceptibility. Nat Commun. 2016;7:13840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Hoei-Hansen CE, Rajpert-De Meyts E, Daugaard G, Skakkebaek NE. Carcinoma in situ testis, the progenitor of testicular germ cell tumours: a clinical review. Ann Oncol. 2005;16:863-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 118] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Lian X, Hou X, Yan J, Sun S, Miao Z, Liu Z, Wang W, Shen J, Hu K, Zhang F. Treatment outcomes of intracranial germinoma: a retrospective analysis of 170 patients from a single institution. J Cancer Res Clin Oncol. 2019;145:709-715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Lo AC, Hodgson D, Dang J, Tyldesley S, Bouffet E, Bartels U, Cheng S, Hukin J, Bedard PL, Goddard K, Laperriere N. Intracranial Germ Cell Tumors in Adolescents and Young Adults: A 40-Year Multi-Institutional Review of Outcomes. Int J Radiat Oncol Biol Phys. 2020;106:269-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 11. | Mazzone E, Knipper S, Mistretta FA, Tian Z, Palumbo C, Soulieres D, De Cobelli O, Montorsi F, Shariat SF, Saad F, Briganti A, Karakiewicz PI. Contemporary North-American population-based validation of the International Germ Cell Consensus Classification for metastatic germ cell tumors of the testis. World J Urol. 2020;38:1535-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |