Published online Oct 6, 2023. doi: 10.12998/wjcc.v11.i28.6817
Peer-review started: July 1, 2023
First decision: August 17, 2023
Revised: August 26, 2023
Accepted: September 11, 2023
Article in press: September 11, 2023
Published online: October 6, 2023
Processing time: 85 Days and 19.2 Hours
McCune-Albright syndrome (MAS) is extremely rare clinically. We here report a case of MAS with severe symptoms that have not been reported previously.
A 10-year-old boy attended our outpatient clinic due to craniofacial malformations found two years ago. He underwent temporal bone computed tomography and digital radiography photography. Based on a literature review combined with the patient's medical history and imaging examination findings, he was diagnosed with multiple fibrous dysplasia of bone. As the clinical symptoms related to MAS in this patient were not obvious, he was only followed up and not given any special treatment.
The unique clinical manifestations in this MAS patient may be related to mutations in the GNAS gene.
Core Tip: McCune-Albright syndrome is extremely rare in clinical practice, which is mainly manifested as fibrous dysplasia, café-au-lait skin spots, and precocious puberty. The clinical manifestations of the present patient were typical, with severe symptoms that have not been reported previously. This case report may provide reference for the diagnosis and treatment of this disease.
- Citation: Lin X, Feng NY, Lei YJ. Diagnosis and treatment of McCune-Albright syndrome: A case report. World J Clin Cases 2023; 11(28): 6817-6822
- URL: https://www.wjgnet.com/2307-8960/full/v11/i28/6817.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i28.6817
McCune-Albright syndrome (MAS) was initially described in 1936 as a triad of fibrous dysplasia (FD), café-au-lait skin spots, and precocious puberty (PP)[1]. In subsequent case reports, MAS was defined as varying degrees of FD with or without café-au-lait skin spots and endocrine dysfunction (such as PP, hyperthyroidism, excess growth hormone, renal phosphate depletion with or without chondromalacia, and Cushing's syndrome[2-4]). MAS is extremely rare clinically with an incidence rate of approximately 1/1000000-1/10000[5]. We here report a case of MAS with severe symptoms that have not appeared in the previous literature, hoping to provide reference for the diagnosis and treatment of this disease.
A 10-year-old boy started to develop craniofacial deformities 2 years ago.
The patient’s mother reported that the child began to develop craniofacial deformities, accompanied by nasal deformities, uneven dentition, and abnormal occlusion. No significant hearing loss or symptoms of increased intracranial pressure were observed.
At the age of 3 years, the child had a closed craniocerebral trauma without surgical treatment. There was no history of surgery, hepatitis, tuberculosis, or other special medical history.
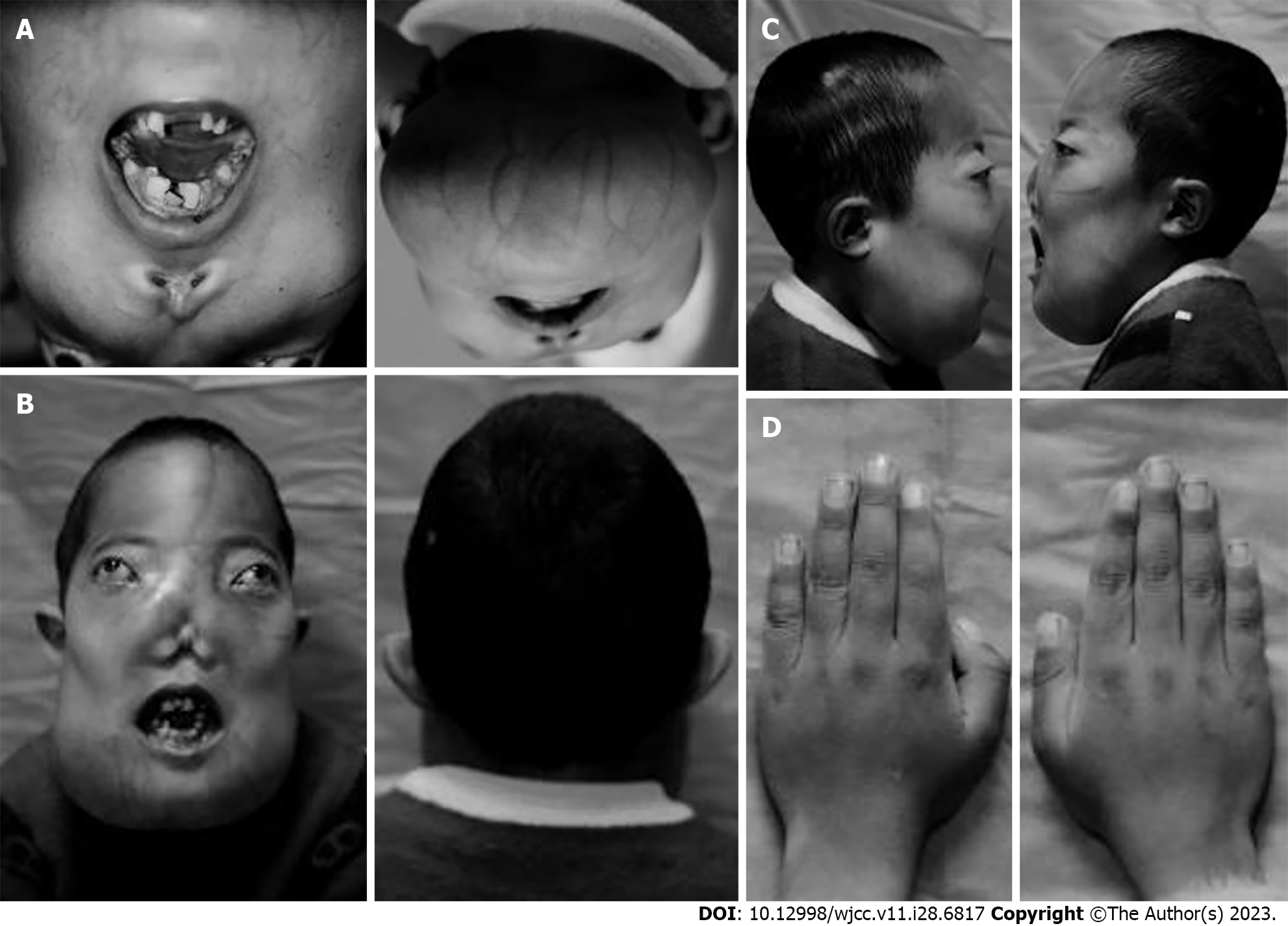
The patient had no other personal or family history. The appearance characteristics of the child at the ages of 3, 5, 8, and 10 years are shown in Figure 1.
Developmental malformation of the head and face, with a head circumference of 52.3 cm, maxillofacial malformation (Figure 2), and abnormal structure of the cheekbones, nasal bones, mandibles, and maxillary sinus bones were observed. Bilateral eyeball protrusion of 16 mm, uneven oral dentition, with most of the teeth in the entire mouth missing, and abnormal occlusion were also seen. Mouth opening was limited to only two fingers (or 3 cm), and severe wear was seen on the posterior teeth. Varicose veins were observed on the skin of the lower jaw. No coffee-like pigmentation was observed on the facial skin. There was no deformity in both auricles, the external auditory meatus on both sides was narrow, and the tympanic membrane could not be clearly seen. The nasal mucosa on both sides was congested, the total nasal meatus was narrow and clear mucus was visible, and no neoplasms were found in the middle nasal meatus. Pure tone audiometry showed binaural mixed hearing loss (left ear air conduction [AC]: 63 dB, bone conduction [BC]: 55 dB; right ear AC: 68 dB, BC: 51 dB).
Alkaline phosphatase level was 1296 U/L (normal range: 40-150 U/L), and other biochemical indicators were basically normal. Pituitary level of luteinizing hormone was 2.58 mIU/mL (normal range: 16-53 mIU/mL), follicle stimulating hormone was 1.10 mIU/mL (normal range: 3.3-8.0 mIU/mL), estradiol was 79 ng/L (normal range: 6-53 ng/L), testosterone was 15.2 μg/L (normal range: 3-5.7 μg/L), and prolactin was 52.25 μg/L (normal range: 16-53 μg/L). 25-Hydroxyvitamin D was 70.2 nmol/L (normal reference: > 75 nmol/L), osteocalcin was 385 μg/L (normal reference: 4-10 μg/L), and growth hormone was 2.105 ng/mL. The thyroid function and routine blood and urine tests were basically normal. The child's guardians refused genetic testing.
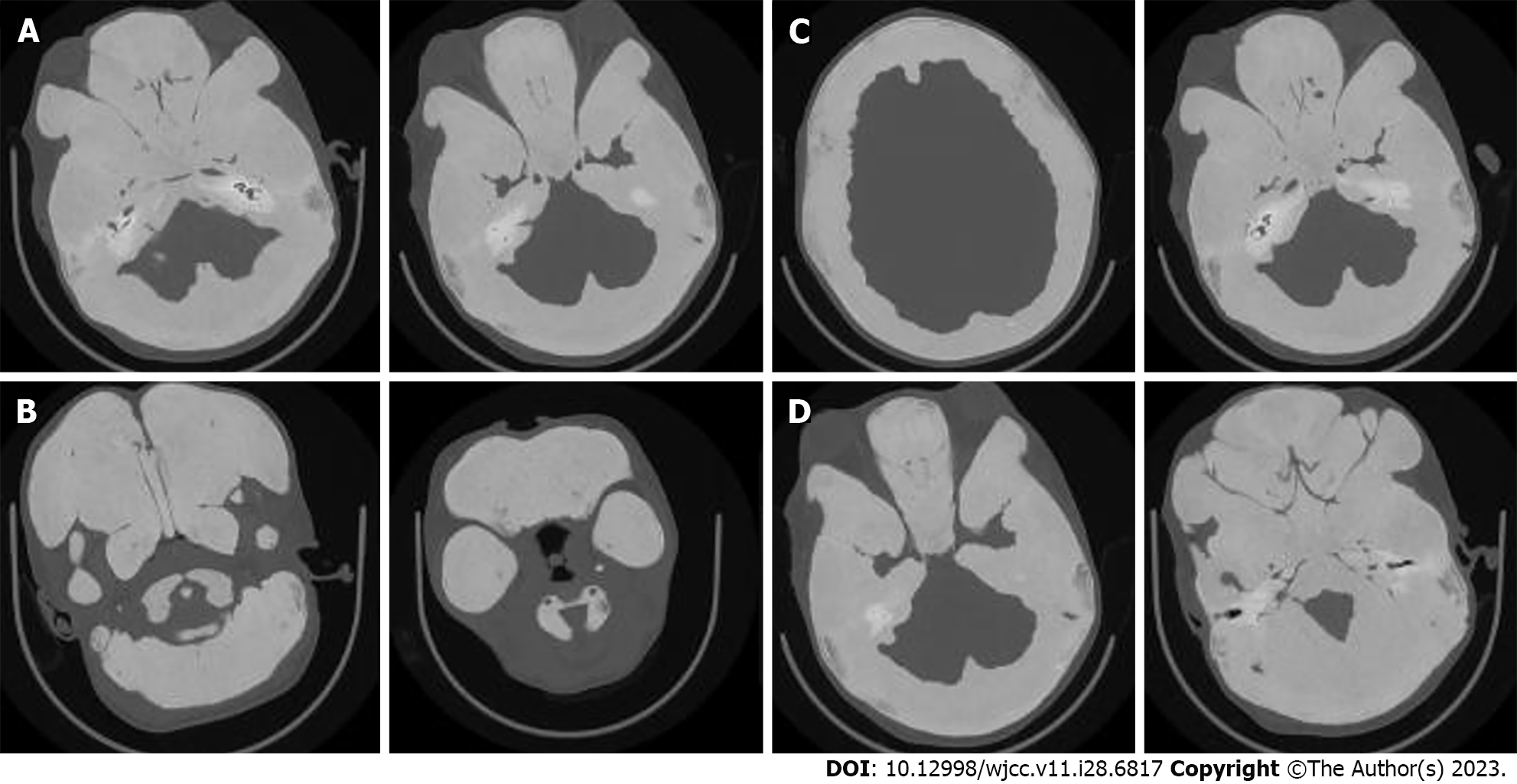
The whole body X-ray showed abnormal proliferation of multiple bone fibers in the bilateral tibia, femur, humerus, ulna, radius, and skull (Figure 3). High resolution computed tomography of the temporal bone indicated diffuse thickening and increased density of the maxillofacial bone, skull, and atlas axis. The thickest part of the frontal bone was approximately 3.7 cm, and no gasification was observed in the maxillary sinus, ethmoid sinus, frontal sinus, sphenoid sinus, and mastoid air chambers on both sides. The bony parts of the external auditory meatus on both sides were narrow, with a width of about 3.8 mm and 2.3 mm on the right and left sides, respectively. Bilateral middle ear tympanic cavities and sinuses were narrow, and there were no abnormalities in bilateral ossicles and inner ear structures (Figure 4).
Based on the patient’s symptoms combined with signs, physical examination, and relevant imaging examinations, the patient was finally diagnosed with MAS.
The child's guardians refused surgical treatment.
The child died of severe lung infection caused by coronavirus disease 2019 11 mo after diagnosis.
MAS is a rare disease which is related to a missense mutation of GNAS gene on chromosome 20. It has been reported that most patients do not have a typical triple syndrome of MAS, and more often exhibit a double syndrome. Two components in the triad (FD with café–au-lait skin spots or endocrine hyperfunction) are usually sufficient to diagnose MAS. In this case report, when comparing the appearance characteristics of the child at the ages of 3, 5, 8, and 10 years, it was found that the child’s craniofacial malformations gradually worsened (with the earliest occurrence of nasal malformation), and the degree of FD bone lesions developed rapidly, which had not been reported previously. The child's skull, maxillofacial bone, humerus, tibia, and other bones had abnormal hyperplasia of bone fibers. Testosterone and growth hormone levels were significantly higher than normal. Pituitary gland hormone levels showed the signs of PP, and the child was diagnosed with MAS.
The clinical manifestations of MAS patients vary greatly, with the most typical clinical manifestation being FD. FD is a slow progressive benign fibrous tissue disease characterized by normal bone tissue being replaced by immature bone tissue. The bone disease caused by FD can involve any part of the skeleton, and can affect single or multiple bones, mainly manifested as local bone pain, swelling, pathological fractures, and deformities. According to the literature, up to 81% of adults and 49% of children with FD suffer from bone pain, which is one of the most common complications of FD[6]. In this case report, the patient did not mention obvious symptoms of bone pain when visiting the hospital. If bone pain symptoms occur during long-term follow-up, oral bisphosphonate (the mechanism of action is inhibiting the activity of osteoclasts, but not delaying the disease process[7,8]), calcitonin, denosumab, and tocilizumab can be considered for symptomatic treatment. At the same time, the main manifestations in patients are the most common type of craniofacial FD in FD, with diffuse thickening of the craniofacial bones and poor gasification of the sinuses leading to symptoms and signs such as facial deformities, hearing impairment, and nasal congestion. Our patient's symptoms were already severe at the time of treatment, and symptoms such as increased intracranial pressure, diplopia, and hearing loss occurred successively.
Café-au-lait spots, also known as skin coffee milk spots, are commonly seen in the folds of the neck and buttocks, with serrated shapes and irregular edges that generally do not cross the midline of the body[9]. They are the earliest signs to appear. It is worth noting that café-au-lait skin spots are a common pigmented skin disease and are not specific manifestations of MAS. In the present case, no coffee milk spots on the skin were found during a systemic examination. In addition, endocrine dysfunction in MAS patients can manifest as PP, hyperthyroidism, excess growth hormone, Cushing's syndrome, etc. Of these, PP is the most characteristic manifestation, and is more common in female patients, mainly manifested as breast development, vaginal bleeding, etc., while PP is relatively rare in male patients. During childhood, MAS patients typically experience maturation of sexual organs, which is the main reason for seeking medical attention. In the present case, the family refused physical examination of the child's sexual organs, but on the hip X-ray examination (Figure 2), it could be seen that the contrast agent filled the cavernous body of the penis, the image density was uniform, the diameter was larger than normal, and the edge was smooth. It was indicated that the child had penis enlargement. In addition, the child's growth hormone level was higher than normal, and there was evidence of excess growth hormone.
MAS patients begin to develop symptoms during infancy, so early diagnosis of the disease is particularly important. Gene testing is a reliable early diagnostic method. By using PCR technology, the positive rate of peripheral blood lymphocyte detection is 20%-30%, and the positive rate of pathological tissue detection can reach over 80%[10]. Due to the mosaic characteristics of mutated cells and the sensitivity of testing techniques, it is often necessary to conduct multiple genetic tests on diseased tissues, and negative results cannot exclude MAS. The accurate diagnosis of MAS should be based on typical clinical manifestations and imaging examinations, but genetic testing can still serve as an early auxiliary diagnostic method. MAS is a slowly developing disease that often affects multiple systems and there is no effective cure. In current clinical practice, two methods are usually used in the treatment of this disease: Symptomatic drug treatment and surgical treatment. Symptomatic treatment mainly focuses on the symptoms of bone pain caused by FD, as well as the use of estrogen receptor blockers (such as tamoxifen), aromatase inhibitors (such as letrozole), and other drugs to treat PP. For the surgical treatment of MAS, the mainstream view includes selecting appropriate surgical methods during the accelerated progression of the lesion and early preventive resection of local lesions after diagnosis of MAS[11,12]. However, there is still a lack of corresponding diagnostic and treatment guidelines, and there is still controversy over the timing and method of surgery.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shafqat S, Pakistan S-Editor: Lin C L-Editor: Wang TQ P-Editor: Yu HG
| 1. | McCune, D. Osteitis fibrosa cystica: The case of a nine-year-old girl who also exhibits precocious puberty, multiple pigmentation of the skin and hyperthyroidism. Am J Dis Child. 1936;52:743-744. |
| 2. | Danon M, Crawford JD. The McCune-Albright syndrome. Ergeb Inn Med Kinderheilkd. 1987;55:81-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Diaz A, Danon M, Crawford J. McCune-Albright syndrome and disorders due to activating mutations of GNAS1. J Pediatr Endocrinol Metab. 2007;20:853-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Kirk JM, Brain CE, Carson DJ, Hyde JC, Grant DB. Cushing's syndrome caused by nodular adrenal hyperplasia in children with McCune-Albright syndrome. J Pediatr. 1999;134:789-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 88] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Honda T, Itoh F, Nakamura K, Ohba T, Katabuchi H. A case of gradually manifesting McCune-Albright syndrome with a 10-year follow-up. Reprod Med Biol. 2016;15:261-265. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Chapurlat RD, Gensburger D, Jimenez-Andrade JM, Ghilardi JR, Kelly M, Mantyh P. Pathophysiology and medical treatment of pain in fibrous dysplasia of bone. Orphanet J Rare Dis. 2012;7 Suppl 1:S3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Plotkin H, Rauch F, Zeitlin L, Munns C, Travers R, Glorieux FH. Effect of pamidronate treatment in children with polyostotic fibrous dysplasia of bone. J Clin Endocrinol Metab. 2003;88:4569-4575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Chan B, Zacharin M. Pamidronate treatment of polyostotic fibrous dysplasia: failure to prevent expansion of dysplastic lesions during childhood. J Pediatr Endocrinol Metab. 2006;19:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Song LP, Zhang Y, Guo C, Wang Y. Two cases of McCune-Albright syndrome in men with skeletal dysplasia as the main presentation. Zhongguo Youshengyuyichuan Zazhi. 2022;30:1818-1821. |
| 10. | Spencer T, Pan KS, Collins MT, Boyce AM. The Clinical Spectrum of McCune-Albright Syndrome and Its Management. Horm Res Paediatr. 2019;92:347-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Amit M, Collins MT, FitzGibbon EJ, Butman JA, Fliss DM, Gil Z. Surgery versus watchful waiting in patients with craniofacial fibrous dysplasia--a meta-analysis. PLoS One. 2011;6:e25179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Kabali TM, Moshy JR, Owibingire SS, Sohal KS, Simon ENM. Craniofacial fibrous dysplasia associated with McCune-Albright syndrome: challenges in diagnosis and treatment: case reports. BMC Oral Health. 2019;19:180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |