Published online Oct 6, 2023. doi: 10.12998/wjcc.v11.i28.6715
Peer-review started: July 12, 2023
First decision: August 2, 2023
Revised: August 3, 2023
Accepted: September 4, 2023
Article in press: September 4, 2023
Published online: October 6, 2023
Processing time: 75 Days and 7.4 Hours
With the improvement of economy and living standards, the attention paid to short stature in children has been increasingly highlighted. Numerous causes can lead to short stature in children, among which growth hormone deficiency (GHD) is a significant factor.
To investigate the long-term efficacy and safety of different doses of long-acting polyethylene glycol recombinant human growth hormone (PEG-rhGH) in the treatment of GHD in children.
We selected 44 pediatric patients diagnosed with GHD who were treated at Wuhu First People's Hospital from 2014 to 2018. Total 23 patients were administered a high dose of long-acting PEG-rhGH at 0.2 mg/kg subcutaneously each week, forming the high-dose group. Meanwhile, 21 patients were given a lower dose of long-acting PEG-rhGH at 0.14 mg/kg subcutaneously each week, establishing the low-dose Group. The total treatment period was 2 years, during which we monitored the patients’ height, annual growth velocity (GV), height standard deviation score (HtSDS), chronological age (CA), bone age (BA), and serum levels of insulin-like growth factor-1 (IGF-1) and insulin-like growth factor-binding protein-3 (IGFBP-3) before treatment and at 6 mo, 1 year, and 2 years after treatment initiation. We also monitored thyroid function, fasting plasma glucose, fasting insulin, and other side effects. Furthermore, we calculated the homeostatic model assessment for insulin resistance.
After 1 year of treatment, the GV, HtSDS, IGF-1, BA, and IGFBP-3 in both groups significantly improved compared to the pre-treatment levels (P < 0.05). Moreover, when comparing GV, HtSDS, IGF-1, BA, and IGFBP-3 between the two groups, there were no statistically significant differences either before or after the treatment (P > 0.05). During the treatment intervals of 0-1.0 years and 1.0-2.0 years, both patient groups experienced a slowdown in GV and a decline in HtSDS improvement (P < 0.05).
The use of PEG-rhGH in treating GHD patients was confirmed to be effective, with similar outcomes observed in both the high-dose group and low-dose groups, and no significant differences in the main side effects.
Core Tip: The lack of growth hormone deficiency (GHD) can lead to short stature in children, and our study explored the long-term efficacy and safety of different doses of long-acting polyethylene glycol recombinant human growth hormone (PEG-rhGH) in the treatment of GHD in children. Our study demonstrates that PEG-rhGH can be initiated at a low dosage, reducing the overall medical costs for patients and providing theoretical support for the clinical use of PEG-rhGH.
- Citation: Xia W, Wang T, Pan JY. Effects of different doses of long-acting growth hormone in treating children with growth hormone deficiency. World J Clin Cases 2023; 11(28): 6715-6724
- URL: https://www.wjgnet.com/2307-8960/full/v11/i28/6715.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i28.6715
With the improvement of economy and living standards, the attention paid to short stature in children has been increasingly highlighted. Short stature in children refers to a height that is 2 standard deviations (SD) below the average for a child's same race, gender, and age in a comparable environment[1], or a height that falls below the 3rd percentile of the normal population[2]. Numerous causes can lead to short stature in children, among which growth hormone deficiency (GHD) is a significant factor. GHD is the most common pituitary hormone deficiency[3,4]. Among children, the main characteristics of morbidity are stunting[5]. Growth hormone (GH) is a peptide that is synthesized and secreted by the pituitary gland, specifically the frontal lobe[6]. The secretion of GH is regulated by various feedback signals and neurotransmitters, both directly and indirectly. The main regulator of GH release is the hypothalamus, which plays a crucial role in controlling its production and distribution.
Since 1985, when the U.S. Food and Drug Administration approved the use of recombinant human growth hormone (rhGH) for the treatment of GHD in children, nearly 40 years have passed[7]. Over this period, rhGH has been utilized in the treatment of short stature caused by various reasons, with children suffering from GHD[8].
However, with the widespread use of long-acting polyethylene glycol recombinant human growth hormone (PEG-rhGH) as a replacement therapy for GH[9], studies have revealed various drug side effects associated with PEG-rhGH in recent years. These side effects include endocrine disorders such as diabetes, hypothyroidism, and increased bone density[10,11]. Moreover, previous research has also observed cases of hypothyroidism in patients receiving PEG-rhGH replacement therapy[12]. It was discovered that kids receiving PEG-rhGH treatment had increased blood glucose levels and a small rise in type 2 diabetes. The same risk factors for poor glucose tolerance may be more prevalent in youngsters than in adults.
The primary treatment for GHD at present involves daily subcutaneous injections of rhGH. While the compliance rate with this medication is relatively low, it is closely linked to the effectiveness of the treatment[13]. With the advancement of pharmaceutical technology in China, the use of long-acting PEG-rhGH, which only requires a weekly injection, is gradually increasing[14]. However, further comparative research is needed on the application dosage, long-term efficacy, and side effects of PEG-rhGH. It is therefore important to find a safer, more stable, and relatively reasonable dose of PEG-rhGH. Our study applies high and low doses of PEG-rhGH to treat 44 cases of children with GHD, comparing the long-term efficacy and safety of medication use between the two groups.
We selected 44 children with GHD who were treated at Wuhu First People's Hospital between 2014 and 2018. Table 1 showed the baseline characteristics of the 44 GHD children.
| Characteristics | mean ± SD |
| Age (yr) | 7.11 ± 2.73 |
| Female (n = 7, yr) | 7.18 ± 2.56 |
| Male (n = 37, yr) | 7.06 ± 2.62 |
| Height (cm) | 110.38 ± 14.17 |
| Body mass index (kg/m2) | 15.91 ± 1.45 |
Inclusion criteria: (1) The height of the child was less than 2 SD of the average height for children of the same age and gender, with a annual growth velocity (GV) of ≤ 5 cm/year; (2) Clonidine and arginine stimulation test, with a GH peak < 10 ng/mL; (3) Prepubescent, age ≥ 3 years; bone age (BA) ≤ 9 years for girls, ≤ 10 years for boys; (4) No GH treatment in the last 6 mo; and (5) The subject agreed to participate in this study and signed an informed consent form.
Exclusion criteria: (1) Abnormal liver and kidney function; (2) Positive tests for HBcAg, HBsAg, and anti-HBc of hepatitis B virus; (3) Severe diseases like hematological, cardiopulmonary, malignant tumors, systemic infections, immunodeficiency; (4) Potential tumor patients; (5) Diabetes; (6) Drug allergy; and (7) Growth and developmental anomalies such as Turner syndrome.
All 44 children underwent routine urine tests and skull MRI examinations. In the high-dose group (HDG): 20 males, 3 females, with an average age of 7.13 ± 2.82years. In the low-dose group (LDG): 17 males, 4 females; with an average age of 7.08 ± 2.67 years. There was no statistically significant difference between the two groups in terms of age and gender (P > 0.05).
The study was conducted by the Helsinki Declaration and ethical guidelines for good clinical practice. Since all participants were under the age of 18, written informed consent was obtained from legally authorized patient representatives. The study was approved by the Ethics Committee of Wuhu First People's Hospital.
Participants were randomly divided into two groups: the HDG was given PEG-rhGH 0.2 mg/kg/w via subcutaneous injections, while the LDG was given PEG-rhGH 0.14 mg/kg/w via subcutaneous injections. The total course of treatment was 2.0 years for both groups.
Standardized measurement tools and methods were used to measure height at times specified by the study. BA was determined using X-ray imaging of the left wrist, and the TW3 method was used for evaluation. Venous blood was collected before treatment and 3 mo, 6 mo, 1 year, 1.5 years and 2 years after treatment. The Siemens IMMLITE2000 automatic analyzer and corresponding reagents were used to detect serum insulin-like growth factor-1 (IGF-1) and insulin-like growth factor-binding protein-3 (IGFBP-3) through chemiluminescence. The Siemens ADVIA Centaur XPT automatic analyzer and corresponding reagents were used to test thyroid function and insulin, while the Siemens ADVIA2400 automatic analyzer and corresponding reagents were used to test fasting plasma glucose (FPG), blood lipids, and liver and kidney function.
Homeostatic model assessment (HOMA) is a mathematical model established by Matthews et al[15] in 1985, which can reflect the mutual influence of insulin and glucose in different organs in the body. HOMA insulin resistance (HOMA-IR) can be calculated from FPG, fasting insulin (FINS), and a correction factor. As a result of a formula HOMA-IR = FPG (mmol/L) × FINS (uu/mL)/22.5[16]. According to a clinical study of insulin resistance in 3203 children, the HOMA-IR value corresponding to the 95th percentile in healthy children is 3.0, so this study uses a HOMA-IR value > 3.0 to indicate insulin resistance[17].
Both groups of children were monitored for height, GV, height standard deviation score (HtSDS), BA, IGF-1, IGFBP-3, and other efficacy indicators, as well as thyroid function, fasting blood glucose, HOMA-IR, and other side effect indicators before and after treatment at specified times. Here, HtSDS = (evaluation time point height - average height of children of the same age and gender)/standard deviation of height of children of the same age and gender.
SPSS 26.0 software was used for statistical analysis. Measurement data are represented as mean ± SD. The independent samples t-test was used for comparing the two groups, with P < 0.05 indicating statistical significance. The incidence of side effects between the two groups was compared using the Fisher's exact test, with P < 0.05 indicating statistical significance.
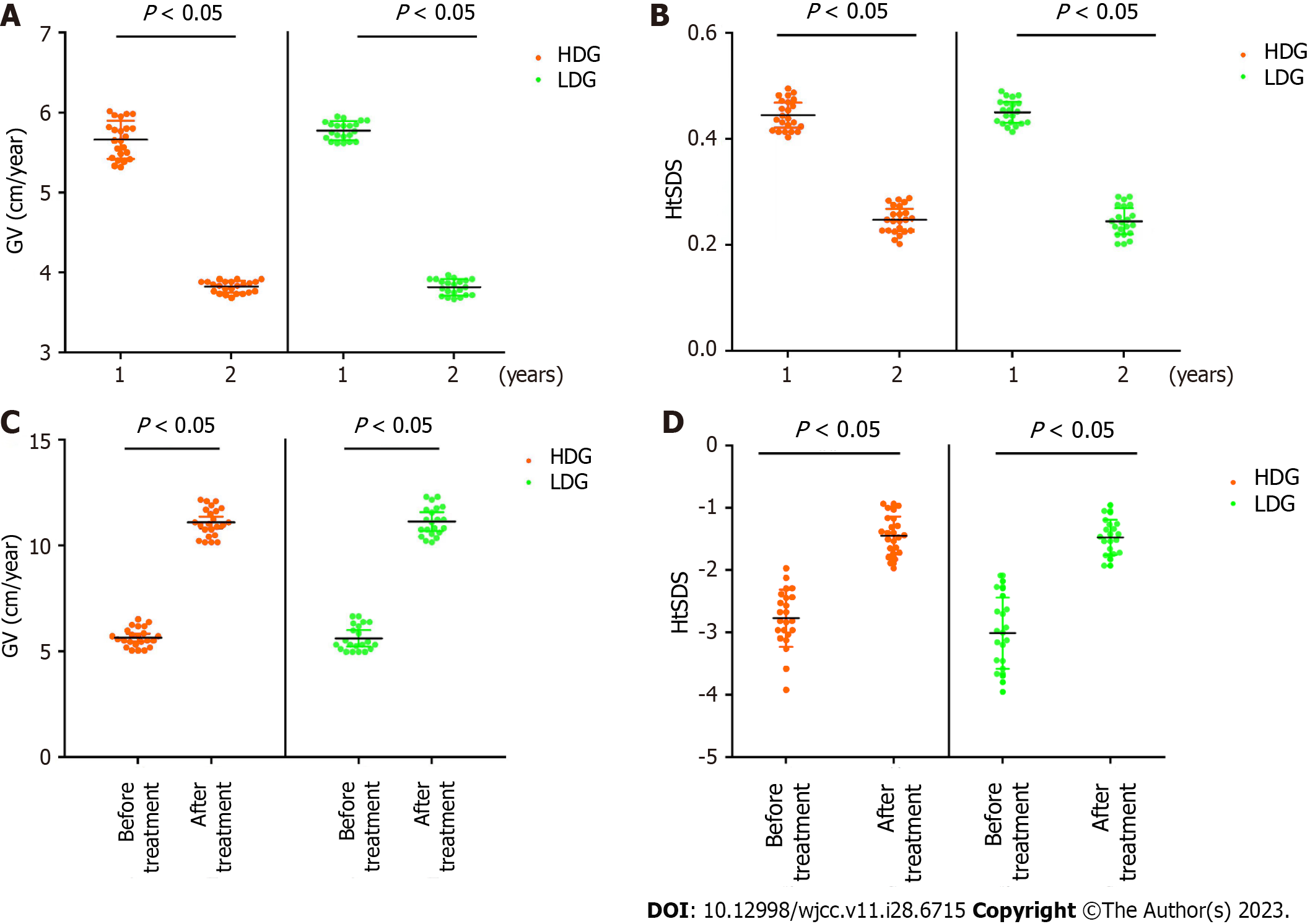
Our results shown a significant decrease in patient GV and HtSDS after 2 years of PEG-rhGH treatment compared to 1 year treatment (Figure 1A and B), suggesting a decrease in PEG-rhGH efficacy in year 2. As is shown in Table 2, after 1 year of treatment, the GV in both groups increased, with the difference being statistically significant (P < 0.05). In addition, there was no statistically significant difference in GV between the two groups before and after the treatment (P < 0.05) (Figure 1C). The HtSDS in both groups also improved (P < 0.05). And there was no statistically significant difference in HtSDS between the two groups after the treatment (P > 0.05) (Figure 1D).
| Group | GV (cm/year) | HtSDS | ||||||
| Before | After | t value | P value | Before | After | t value | P value | |
| HDG (n = 23) | 3.87 ± 0.63 | 9.77 ± 1.89 | 14.183 | 0.000 | -2.41 ± 0.74 | -1.56 ± 0.72 | -3.947 | 0.000 |
| LDG (n = 21) | 3.90 ± 0.66 | 9.06 ± 1.45 | 18.847 | 0.000 | -2.12 ± 0.46 | -1.39 ± 0.53 | -4.779 | 0.000 |
| t value | -0.181 | 1.392 | -1.568 | -0.927 | ||||
| P value | 0.857 | 0.171 | 0.124 | 0.359 | ||||
To monitor biochemical changes in GHD to determine the effects of PEG-rhGH on physiological function. After 1 year of PEG-rhGH treatment, we collected patients' peripheral blood and measured changes in levels of IGF-1 and IGFBP-3 in the blood. Figure 2 shown a significant increase in the expression of IGF-1 (Figrue 2A) and IGFBP-3 (Figure 2B) in serum after 1 year of PEG-rhGH treatment. Moreover, our results shown that after one year of treatment, the levels of IGF-1 and IGFBP-3 in children from both groups significantly increased (Table 3), with the difference being statistically significant (P < 0.05). Also, there was no statistically significant difference in IGF-1 and IGFBP-3 Levels between the two groups before and after treatment (P > 0.05).
| Group | IGF-1 | IGFBP-3 | ||||||
| Before | After | t value | P value | Before | After | t value | P value | |
| HDG (n = 23) | 127.15 ± 63.39 | 259.17 ± 124.86 | 4.522 | 0.000 | 4.18 ± 0.86 | 5.64 ± 1.41 | 4.237 | 0.000 |
| LDG (n = 21) | 115.39 ± 62.65 | 253.11 ± 149.52 | 3.893 | 0.000 | 3.57 ± 0.72 | 5.37 ± 1.11 | 6.269 | 0.000 |
| t value | 0.618 | 0.146 | 2.547 | 0.691 | ||||
| P value | 0.540 | 0.884 | 0.015 | 0.493 | ||||
Previous studies have shown that PEG-rhGH treatment increases BA in patients [18,19]. Here, our results shown that after 1 year of PEG-rhGH treatment, BA is significantly higher than chronological age (CA) (Figure 2C). As is shown in Table 4, after 2 years of treatment, the growth of BA in children from both groups accelerated more than the growth of CA, with the difference being statistically significant (P < 0.05). However, there was no statistically significant difference in the growth of BA between the two groups after treatment (P > 0.05). Comparing the GV between 0-1 years and 1-2 years of treatment, children in both groups shown a slowdown in GV and a decrease in the improvement of HtSDS (Table 5), with the difference being statistically significant (P < 0.05).
| Group | BA growth (yr) | CA growth (yr) | t value | P value |
| HDG (n = 23) | 2 | 2.45 ± 0.55 | 3.392 | 0.003 |
| LDG (n = 21) | 2 | 2.41 ± 0.43 | 3.355 | 0.003 |
| t value | 0.629 | |||
| P value | 0.629 |
| Group | GV | HtSDS | ||||||
| 0-1 year | 1-2 year | t value | P value | 0-1 year | 1-2 year | t value | P value | |
| HDG (n = 23) | 10.56 ± 1.15 | 7.78 ± 0.75 | 4.875 | 0.000 | 1.12 ± 0.28 | 0.42 ± 0.15 | 5.328 | 0.000 |
| LDG (n = 21) | 10.1 ± 0.99 | 8.41 ± 0.84 | 2.942 | 0.005 | 0.96 ± 0.18 | 0.52 ± 0.18 | 3.852 | 0.000 |
As is shown in Table 6, During the 2-year treatment period for both groups of children, efficacy indicators such as thyroid function, FPG, and HOMA-IR were closely monitored for side effects. No statistically significant difference was found between the groups (P > 0.05). There are 2 cases of FMPG damage, 2 cases of hypothyroidism and 4 cases of HOMA-IR in HDG and LDG groups, which suggest that both doses of PEG-rhGH have side effects.
| Group | HDG (n = 23) | LDG (n = 21) | χ2 | P value |
| FPG damage | 2 | 2 | 0.009 | 1.000 |
| Subclinical Hypothyroidism | 3 | 2 | 0.135 | 1.000 |
| HOMA-IR | 4 | 4 | 0.020 | 1.000 |
The rhGH is currently the most widely used drug for the treatment of GHD in clinical practice, with fewer side effects. However, the medication adherence of rhGH cannot be ignored. According to previous study, for every week of rhGH administration, height increases decrease by 0.11SD for each missed injection[20]. Therefore, various long-acting rhGHs have emerged as a result, and the common methods for extending the half-life of peptide drugs at home and abroad mainly include chemical modification techniques (PEG chemical modification), microsphere encapsulation sustained-release techniques, and fusion protein techniques. PEG-rhGH extends the serum half-life of rhGH by attaching a 40 KDa hydrophilic PEG residue to rhGH and reducing its immunogenicity. Because it is injected once a week, so that it can significantly improve the medication adherence of patients[21], but the dosage of PEG-rhGH is still being explored.
In the clinical treatment of GHD, we have noticed that the height growth in most children displays seasonal variations. Therefore, to eliminate the influence of seasonal factors, we assessed the therapeutic effects on an annual basis. This study evaluated the annual growth rate, HtSDS, IGF-1, IGFBP-3, and other indicators in children treated with high and low doses of PEG-rhGH for 1 year. The results shown a significant increase in all therapeutic indicators in both groups compared to before treatment (P < 0.05), confirming the effectiveness of PEG-rhGH treatment for children with GHD, which is consistent with reports[14,22]. However, there were no statistically significant differences between the high and low dose groups in terms of GV, HtSDS, IGF-1, and IGFBP-3 after 1 year of treatment (P > 0.05). Furthermore, our study found no significant difference in the treatment effect of GHD between the high and low doses of PEG-rhGH we set. This is in contrast to other related studies in the CNKI database of China, which suggest that the HDG had superior effects to the low dose group. This discrepancy might be due to variations in treatment duration and dosage. Consequently, we recommend starting with a low dose of PEG-rhGH (0.14 mg/kg/w) when treating GHD clinically, taking into account the heterogeneity of the GHD population. The dosage can be personalized based on the growth rate and improvement in height SDS to achieve optimal clinical treatment outcomes[23].
According to the BA data of patients treated for 2 years, both the high and low dose groups showed varying degrees of BA growth, and the difference was statistically significant when compared with CA growth (P < 0.05). Moreover, there was no statistically significant difference in BA growth between the two groups (P > 0.05). Study have reported no significant increase in BA after GH therapy[24], which might be due to the short treatment duration. Previous studies have shown that in adult GH deficiency, long-term rhGH replacement therapy induces an increase in bone mineral density, which is reflected in the lumbar spine and femoral neck, especially more pronounced in male patients[25]. And our results found the effect of PEG-rhGH treatment on BA in children with GHD, finding a significant increase in BA in children treated with PEG-rhGH, which is consistent with the results found by Zak et al[26]. According to research related to long-term rhGH treatment of GHD, in the first year of treatment, BA may increase to varying degrees, and the dose of GH has no impact on the progression of BA. After five years of treatment, the BA of most patients gradually increases to match their chronological age, which is considered normal as the BA increases with the extension of treatment time[27].
Taking seasonal factors into account, this study compared the height growth and improvement of HtSDS within groups in 0-1 years and 1-2 years of treatment, and found that both the high and low dose groups experienced a slowdown in growth rate and a decline in HtSDS improvement. Here, we observed the main reasons for this decline are thought to be related to the following three aspects: (1) In conventional rhGH treatment, the reported non-adherence rate can reach 52.8%, and this rate tends to decrease significantly with the extension of treatment time. The main reasons include the route of administration, pain at the injection site, and other factors, and adherence has been proven to be closely related to treatment effectiveness[6]. Injecting PEG-rhGH once a week can significantly improve adherence, but due to its increased molecular weight, higher dose and concentration, and increased difficulty in injection compared to conventional rhGH, problems like pain at the injection site still affect adherence. Long-term treatment may cause psychological fatigue in parents and patients, which may lead to a decline in treatment adherence in the second year and affect treatment effectiveness[20]; (2) The issue of antibodies has always troubled clinicians in the early treatment of GH. With the advancement of pharmaceutical technology, the current rhGH has greatly reduced the production of antibodies. However, according to this study, GH neutralizing antibodies may still be produced during the treatment process with rhGH, inhibiting the expected drug effect and leading to a significant decline in growth rate[28]. Since PEG-rhGH is a PEG-conjugated rhGH, it still faces the issue of neutralizing antibody production; and (3) GHD patients, due to the lack of GH, have a slower growth rate than normal children. In the first year of GH treatment, the patients' IGF-1 gradually rises to normal levels, and the growth potential is maximized. Later, as the GH level stabilizes, the growth rate gradually slows down. According to statistics from long-term large-scale GH treatment, the younger the age at the start of treatment, the more ideal the treatment effect. The impact of GH on height is most significant in the first year, while the influence of the GH dose on the growth rate is relatively small[20,29].
Common side effects of rhGH mainly include impact on glucose metabolism, decreased thyroid function, benign intracranial hypertension, transient peripheral edema, joint pain, and skeletal changes[9,28-30]. Due to PEG-rhGH being conjugated with large molecular weight PEG residues, in addition to the common side effects of rhGH, fat loss also one of the side effects[18,30]. This study focused on abnormal thyroid function, FPG, and insulin resistance, and detailed records of other rare adverse reactions reported by patients were kept. During the 2 years of treatment, there were 3 cases of subclinical hypothyroidism, 2 cases of impaired FPG, and 4 cases of insulin resistance in the HDG, and 2 cases of subclinical hypothyroidism, 2 cases of impaired FPG, and 4 cases of insulin resistance in the low dose group. No other side effects such as fat atrophy or benign intracranial hypertension occurred. There was no statistically significant difference in the incidence of side effects between the high and low dose groups (P > 0.05). For the fat atrophy reported in some literature, it is considered likely related to repeated injections in nearby locations. However, it is noteworthy that the incidence of insulin resistance is significantly higher than impaired FPG and subclinical hypothyroidism, so more attention should be paid to the condition of insulin resistance when treating GHD in children. Apart from impaired FPG, subclinical hypothyroidism, and insulin resistance, no other side effects occurred in this study. The safety of the medication is relatively good, which is consistent with other report[9].
However, there are some limitations to our study. We only included a small sample size of 44 cases, which resulted in only a high-dose group and a low-dose group being established for the PEG-rhGH dosing. This restricted the range of PEG-rhGH doses and prevented us from identifying the most cost-effective and efficient minimum dosage.
In summary, our study revealed that treating children with GHD using various doses of PEG-rhGH substantially influenced their growth patterns. Notably, there was no significant difference in HtSDS, BA, and serum concentrations of IGF-1 and IGFBP-3 across different doses of PEG-rhGH. Moreover, our safety analysis, which included an evaluation of adverse events, indicated no significant clinical difference between low-dose and high-dose PEG-rhGH treatments. Given the current high cost of long-acting growth hormones, investigating the impact of dosage on treatment effectiveness has economic implications. Our results indicate that the effects of low-dose treatment are closely comparable to those of high-dose treatment. Therefore, initiating therapy with a lower dosage can achieve similar treatment outcomes at a reduced cost, thus alleviating the financial burden on the families of patients.
The attention paid to short stature in children has been increasingly highlighted. Numerous causes can lead to short stature in children, among which growth hormone deficiency (GHD) is a significant factor.
The use of polyethylene glycol composite human growth hormone (PEG rhGH) has certain side effects, and its cost can cause certain economic pressure on patients.
This study aimed to investigate the long-term efficacy and safety of different doses of long-acting PEG-rhGH in the treatment of GHD in children.
The authors selected 44 pediatric patients diagnosed with GHD. 23 patients were administered a high dose of long-acting PEG-rhGH at 0.2 mg/kg subcutaneously each week, forming the high-dose group. Meanwhile, 21 patients were given a lower dose of long-acting PEG-rhGH at 0.14 mg/kg subcutaneously each week, establishing the low-dose group. The patients’ height, annual growth velocity (GV), height standard deviation score (HtSDS), chronological age, bone age (BA), serum levels of insulin-like growth factor-1 (IGF-1), insulin-like growth factor-binding protein-3 (IGFBP-3), thyroid function, fasting plasma glucose, fasting insulin, and other side effects were monitored.
After 1 year of treatment, the GV, HtSDS, IGF-1, BA, and IGFBP-3 in both groups significantly improved compared to the pre-treatment levels. Moreover, when comparing GV, HtSDS, IGF-1, BA, and IGFBP-3 between the two groups, there were no statistically significant differences either before or after the treatment. During the treatment intervals of 0-1.0 years and 1.0-2.0 years, both patient groups experienced a slowdown in GV and a decline in HtSDS improvement.
Initiating treatment with a low dosage of PEG-rhGH can achieve similar therapeutic outcomes at lower costs, thereby alleviating the financial burden on patients and their families.
Observe the therapeutic effect of GHD based on different doses of PEG-rhGH.
We would like to express our gratitude to all the patients and their families who participated in our study, as well as the editors and staff involved in the publication of this manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kaesmacher J, Switzerland; Mendonca G, United States S-Editor: Wang JL L-Editor: A P-Editor: Zhao S
| 1. | Jiang S, Qu X, Liu S, Wei J, Yi X, Liu Y, Gao C. Proteomic Identification of Plasma Components in Tachypleus tridentatus and Their Effects on the Longitudinal Bone Growth Rate in Rats. Mar Drugs. 2023;21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Wong HS, Lin YJ, Lu HF, Liao WL, Chen CH, Wu JY, Chang WC, Tsai FJ. Genomic interrogation of familial short stature contributes to the discovery of the pathophysiological mechanisms and pharmaceutical drug repositioning. J Biomed Sci. 2019;26:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Alatzoglou KS, Webb EA, Le Tissier P, Dattani MT. Isolated growth hormone deficiency (GHD) in childhood and adolescence: recent advances. Endocr Rev. 2014;35:376-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 4. | Berberoğlu M, Sıklar Z, Darendeliler F, Poyrazoğlu S, Darcan S, Işgüven P, Bideci A, Ocal G, Bundak R, Yüksel B, Arslanoğlu I. Evaluation of permanent growth hormone deficiency (GHD) in young adults with childhood onset GHD: a multicenter study. J Clin Res Pediatr Endocrinol. 2008;1:30-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Binder G, Reinehr T, Ibáñez L, Thiele S, Linglart A, Woelfle J, Saenger P, Bettendorf M, Zachurzok A, Gohlke B, Randell T, Hauffa BP, Claahsen van der Grinten HL, Holterhus PM, Juul A, Pfäffle R, Cianfarani S. GHD Diagnostics in Europe and the US: An Audit of National Guidelines and Practice. Horm Res Paediatr. 2019;92:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 6. | Acerini CL, Wac K, Bang P, Lehwalder D. Optimizing Patient Management and Adherence for Children Receiving Growth Hormone. Front Endocrinol (Lausanne). 2017;8:313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | U.S. Food and Drug Administration. FDA Drug Safety Communication: Ongoing safety review of Recombinant Human Growth Hormone (somatropin) and possible increased risk of death. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-ongoing-safety-review-recombinant-human-growth-hormone-somatropin-and. |
| 8. | Felício JS, Janaú LC, Moraes MA, Zahalan NA, de Souza Resende F, de Lemos MN, de Souza Neto NJK, Farias de Franco II, Leitão LTC, Silva LSD, de Oliveira MCNI, de Alcântara AL, Contente Braga de Souza AC, da Silva WM, Dos Santos MC, de Queiroz NNM, de Moraes LV, de Figueiredo AB Jr, Farinassi ALP, Farias LMDC, da Silva DD, Felício KM, Abrahão Neto JF. Diagnosis of Idiopathic GHD in Children Based on Response to rhGH Treatment: The Importance of GH Provocative Tests and IGF-1. Front Endocrinol (Lausanne). 2019;10:638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Yang Y, Bai X, Yuan X, Zhang Y, Chen S, Yang H, Du H, Zhu H, Pan H. Efficacy and safety of long-acting growth hormone in children with short stature: a systematic review and meta-analysis. Endocrine. 2019;65:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Capalbo D, Esposito A, Improda N, Wasniewska MG, Di Mase R, De Luca F, Bruzzese D, Salerno M. Glucose homeostasis in GHD children during long-term replacement therapy: a case-control study. Endocrine. 2018;59:643-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Meazza C, Elsedfy HH, Pagani S, Bozzola E, El Kholy M, Bozzola M. Metabolic parameters and adipokine profile in growth hormone deficient (GHD) children before and after 12-month GH treatment. Horm Metab Res. 2014;46:219-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Witkowska-Sędek E, Kucharska AM, Rumińska M, Paluchowska M, Pyrżak B. Decreased Thyroxine Levels during rhGH Therapy in Children with Growth Hormone Deficiency. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 13. | Johannsson G, Gordon MB, Højby Rasmussen M, Håkonsson IH, Karges W, Sværke C, Tahara S, Takano K, Biller BMK. Once-weekly Somapacitan is Effective and Well Tolerated in Adults with GH Deficiency: A Randomized Phase 3 Trial. J Clin Endocrinol Metab. 2020;105:e1358-e1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 14. | Chen WJ, Zhang J. [A comparative study om efficacy of long - and short - term recombinant growth hormones in pediatric patients with growth hormone deficiency]. Linchuang He Shiyan Yixue Zazhi. 2019;18:171-174. [DOI] [Full Text] |
| 15. | Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22373] [Cited by in RCA: 24456] [Article Influence: 611.4] [Reference Citation Analysis (0)] |
| 16. | Zhao Z, Shi A, Wang Q, Zhou J. High Oleic Acid Peanut Oil and Extra Virgin Olive Oil Supplementation Attenuate Metabolic Syndrome in Rats by Modulating the Gut Microbiota. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Yin J, Li M, Xu L, Wang Y, Cheng H, Zhao X, Mi J. Insulin resistance determined by Homeostasis Model Assessment (HOMA) and associations with metabolic syndrome among Chinese children and teenagers. Diabetol Metab Syndr. 2013;5:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 18. | Qiao Y, Wang Z, Han J, Li G. Use of PEGylated Recombinant Human Growth Hormone in Chinese Children with Growth Hormone Deficiency: A 24-Month Follow-Up Study. Int J Endocrinol. 2019;2019:1438723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Thaker V, Haagensen AL, Carter B, Fedorowicz Z, Houston BW. Recombinant growth hormone therapy for cystic fibrosis in children and young adults. Cochrane Database Syst Rev. 2013;6:CD008901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | van Dommelen P, Koledova E, Wit JM. Effect of adherence to growth hormone treatment on 0-2 year catch-up growth in children with growth hormone deficiency. PLoS One. 2018;13:e0206009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Hou L, Chen ZH, Liu D, Cheng YG, Luo XP. Comparative pharmacokinetics and pharmacodynamics of a PEGylated recombinant human growth hormone and daily recombinant human growth hormone in growth hormone-deficient children. Drug Des Devel Ther. 2016;10:13-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Sun C, Lu B, Liu Y, Zhang Y, Wei H, Hu X, Hu P, Zhao Q, Ye K, Wang K, Gu Z, Liu Z, Ye J, Zhang H, Zhu H, Jiang Z, Wan N, Yan C, Yin J, Ying L, Huang F, Yin Q, Xi L, Luo F, Cheng R. Reduced Effectiveness and Comparable Safety in Biweekly vs. Weekly PEGylated Recombinant Human Growth Hormone for Children With Growth Hormone Deficiency: A Phase IV Non-Inferiority Threshold Targeted Trial. Front Endocrinol (Lausanne). 2021;12:779365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Grimberg A, DiVall SA, Polychronakos C, Allen DB, Cohen LE, Quintos JB, Rossi WC, Feudtner C, Murad MH; Drug and Therapeutics Committee and Ethics Committee of the Pediatric Endocrine Society. Guidelines for Growth Hormone and Insulin-Like Growth Factor-I Treatment in Children and Adolescents: Growth Hormone Deficiency, Idiopathic Short Stature, and Primary Insulin-Like Growth Factor-I Deficiency. Horm Res Paediatr. 2016;86:361-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 431] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 24. | Luo X, Zhao S, Yang Y, Dong G, Chen L, Li P, Luo F, Gong C, Xu Z, Xu X, Gong H, Du H, Hou L, Zhong Y, Shi Q, Chen X, Xu L, Cheng R, Su C, Ma Y, Zhang L, Lu H. Long-acting PEGylated growth hormone in children with idiopathic short stature. Eur J Endocrinol. 2022;187:709-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 25. | Claessen KM, Appelman-Dijkstra NM, Adoptie DM, Roelfsema F, Smit JW, Biermasz NR, Pereira AM. Metabolic profile in growth hormone-deficient (GHD) adults after long-term recombinant human growth hormone (rhGH) therapy. J Clin Endocrinol Metab. 2013;98:352-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Zak T, Basiak A, Zubkiewicz-Kucharska A, Noczyńska A. [The effect of one year therapy with recombinant human growth hormone (rhGH) on growth velocity, calcium-phosphorus metabolism, bone mineral density and changes in body composition in children with growth hormone deficiency (GHD)]. Pediatr Endocrinol Diabetes Metab. 2010;16:39-43. [PubMed] |
| 27. | Ross JL, Lee PA, Gut R, Germak J. Attaining genetic height potential: Analysis of height outcomes from the ANSWER Program in children treated with growth hormone over 5 years. Growth Horm IGF Res. 2015;25:286-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Christiansen JS, Backeljauw PF, Bidlingmaier M, Biller BM, Boguszewski MC, Casanueva FF, Chanson P, Chatelain P, Choong CS, Clemmons DR, Cohen LE, Cohen P, Frystyk J, Grimberg A, Hasegawa Y, Haymond MW, Ho K, Hoffman AR, Holly JM, Horikawa R, Höybye C, Jorgensen JO, Johannsson G, Juul A, Katznelson L, Kopchick JJ, Lee KO, Lee KW, Luo X, Melmed S, Miller BS, Misra M, Popovic V, Rosenfeld RG, Ross J, Ross RJ, Saenger P, Strasburger CJ, Thorner MO, Werner H, Yuen K. Growth Hormone Research Society perspective on the development of long-acting growth hormone preparations. Eur J Endocrinol. 2016;174:C1-C8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 29. | Reiter EO, Price DA, Wilton P, Albertsson-Wikland K, Ranke MB. Effect of growth hormone (GH) treatment on the near-final height of 1258 patients with idiopathic GH deficiency: analysis of a large international database. J Clin Endocrinol Metab. 2006;91:2047-2054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 135] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 30. | Touraine P, D'Souza GA, Kourides I, Abs R, Barclay P, Xie R, Pico A, Torres-Vela E, Ekman B; GH Lipoatrophy Study Group. Lipoatrophy in GH deficient patients treated with a long-acting pegylated GH. Eur J Endocrinol. 2009;161:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |