Published online Sep 26, 2023. doi: 10.12998/wjcc.v11.i27.6664
Peer-review started: July 26, 2023
First decision: August 16, 2023
Revised: August 24, 2023
Accepted: August 31, 2023
Article in press: August 31, 2023
Published online: September 26, 2023
Processing time: 56 Days and 10.1 Hours
Anaplastic thyroid cancer (ATC) is a rare but aggressive type of thyroid carci
We report the case of a 63-year-old female patient diagnosed with BRAF V600E-mutant ATC. Following three surgeries—total thyroidectomy, total laryngectomy, and neck dissection—she was diagnosed with lung metastasis during follow-up. The metastatic ATC was successfully treated with dabrafenib and trametinib. The patient achieved a complete response at the 32-mo follow-up.
Adjuvant chemotherapy with dabrafenib plus trametinib is efficacious for treatment and prevention of recurrent ATC with BRAF mutation following surgery.
Core Tip: Anaplastic thyroid cancer (ATC) is a rare but aggressive type of thyroid carcinoma. The BRAF V600E-mutation, found in 10%-50% of ATCs, is associated with a poor prognosis. A recent clinical trial reported a substantial clinical benefit of dabrafenib (BRAF inhibitor) plus trametinib (MEK inhibitor) in treating BRAF V600E-mutant ATC. This report highlights the therapeutic potential of dabrafenib plus trametinib as an adjuvant chemotherapy to treat and prevent the recurrence of ATC confirmed with BRAF mutation following surgical resection.
- Citation: Lee SJ, Song SY, Kim MK, Na HG, Bae CH, Kim YD, Choi YS. Complete response of metastatic BRAF V600-mutant anaplastic thyroid cancer following adjuvant dabrafenib and trametinib treatment: A case report. World J Clin Cases 2023; 11(27): 6664-6669
- URL: https://www.wjgnet.com/2307-8960/full/v11/i27/6664.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i27.6664
Anaplastic thyroid cancer (ATC) is the fastest-growing and most aggressive type of undifferentiated thyroid carcinoma. All ATCs are considered stage IV, and the overall prognosis is poor, with a median survival time of less than a year, despite various available treatments. Many new targeted therapies for ATC have been reported in recent years; however, no definitive treatment has yet been established[1].
The BRAF V600E-mutation is found in 10%-50% of ATCs, and is associated with a poor prognosis[2,3]. Clinical phase II and open-label basket trials conducted using the combination of dabrafenib (BRAF inhibitor) and trametinib [mitogen-activated protein kinase kinase (MEK) inhibitor] for treatment of ATC with a BRAF mutation have shown substantial efficacy[2,3]. The Food and Drug Administration (FDA) of the United States has approved dabrafenib plus trametinib for the treatment of ATC with BRAF mutation. The 2021 American Thyroid Association’s (ATA) guidelines for the management of patients with ATC have also been revised to reflect the treatment option for BRAF-mutant ATC[4]. However, there are few reports of patients with ATC who have been treated using this regimen following surgery[5,6].
Here, we report a case of recurrent and metastatic ATC that was successfully treated with dabrafenib plus trametinib as an adjuvant chemotherapy.
A 63-year-old female patient presented with dyspnea and a large neck mass at the emergency department of our hospital.
Her symptoms were insidious and began one week prior to presentation.
The patient had been diagnosed with hypertension, and was taking anti-hypertensive medication at the time of her presentation. Three days prior, the patient underwent a chest computed tomography (CT) scan and was treated with steroid medication and a salbutamol inhaler, which were unable to relieve her symptoms.
The patient denied any family history of malignant tumors.
On physical examination, the patient’s vital signs were as follows: Body temperature, 37.1 ℃; blood pressure, 140/100 mmHg; heart rate, 122 beats per min; respiratory rate, 25 breaths per min; and oxygen saturation, 95%. Accessory respiratory muscle use was observed, and stridor was heard during inspiration. A hard, non-tender fixed mass with a diameter of 4 cm was found on the left side of the anterior neck. The patient’s vocal cord mobility was normal.
Subclinical hypothyroidism was noted on thyroid function tests [thyroid stimulating hormone, 9.73 µIU/mL; free thyroxin (T4), 1.4 ng/dL; triiodothyronine (T3), 1.22 ng/mL]. No abnormalities were found on routine blood analyses.
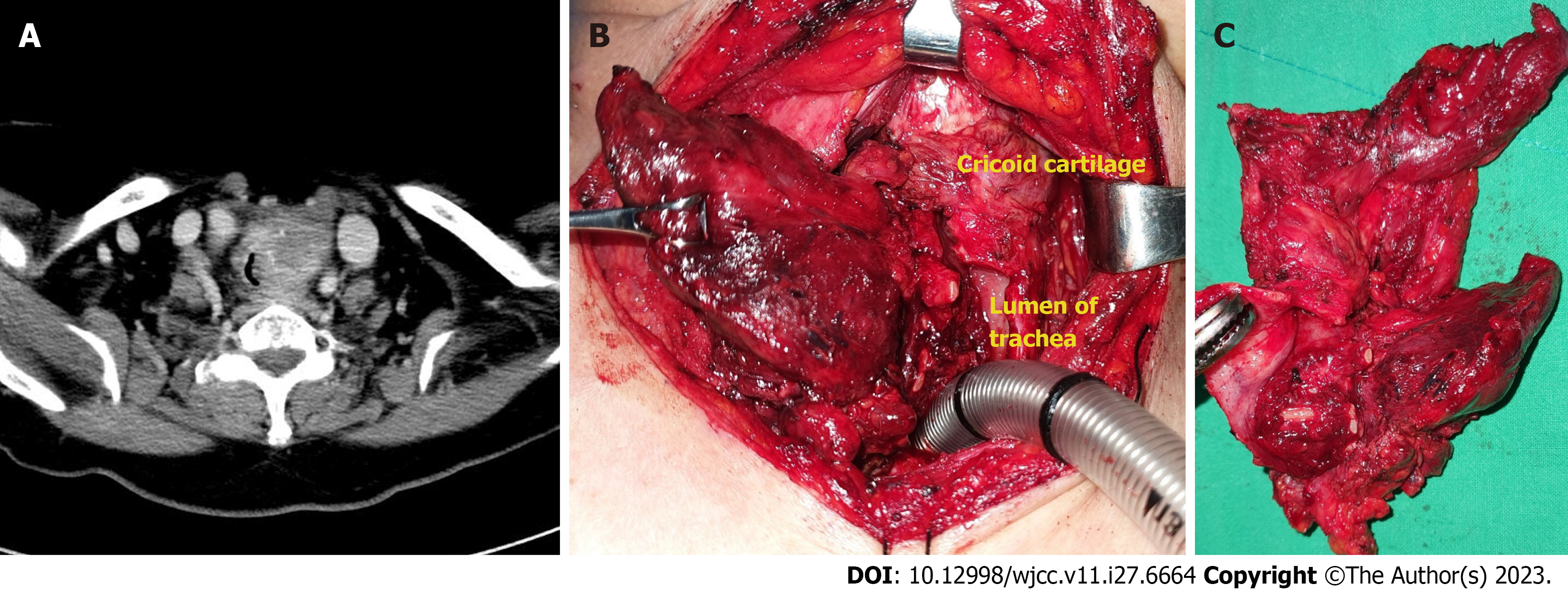
A neck CT revealed an infiltrative mass of heterogeneous density, measuring approximately 3.1 cm × 3.0 cm, in the left thyroid lobe and invading the left side of tracheal wall (Figure 1A).
Intubation was performed and the patient was admitted to the intensive care unit. A core needle biopsy was performed at the bedside, and the mass was diagnosed as papillary thyroid carcinoma (PTC). However, gross invasion of the surrounding structures from the cricoid cartilage to 6th ring of the trachea was observed during surgery (Figure 1B and C). Considering the possibility of PTC with anaplastic dedifferentiation, we performed total thyroidectomy, total laryn
The patient was diagnosed with recurrent and metastatic BRAF V600E-mutant ATC.
The patient started on debrafenib (150 mg BID) plus trametinib (2 mg QD) as an adjuvant chemotherapy.
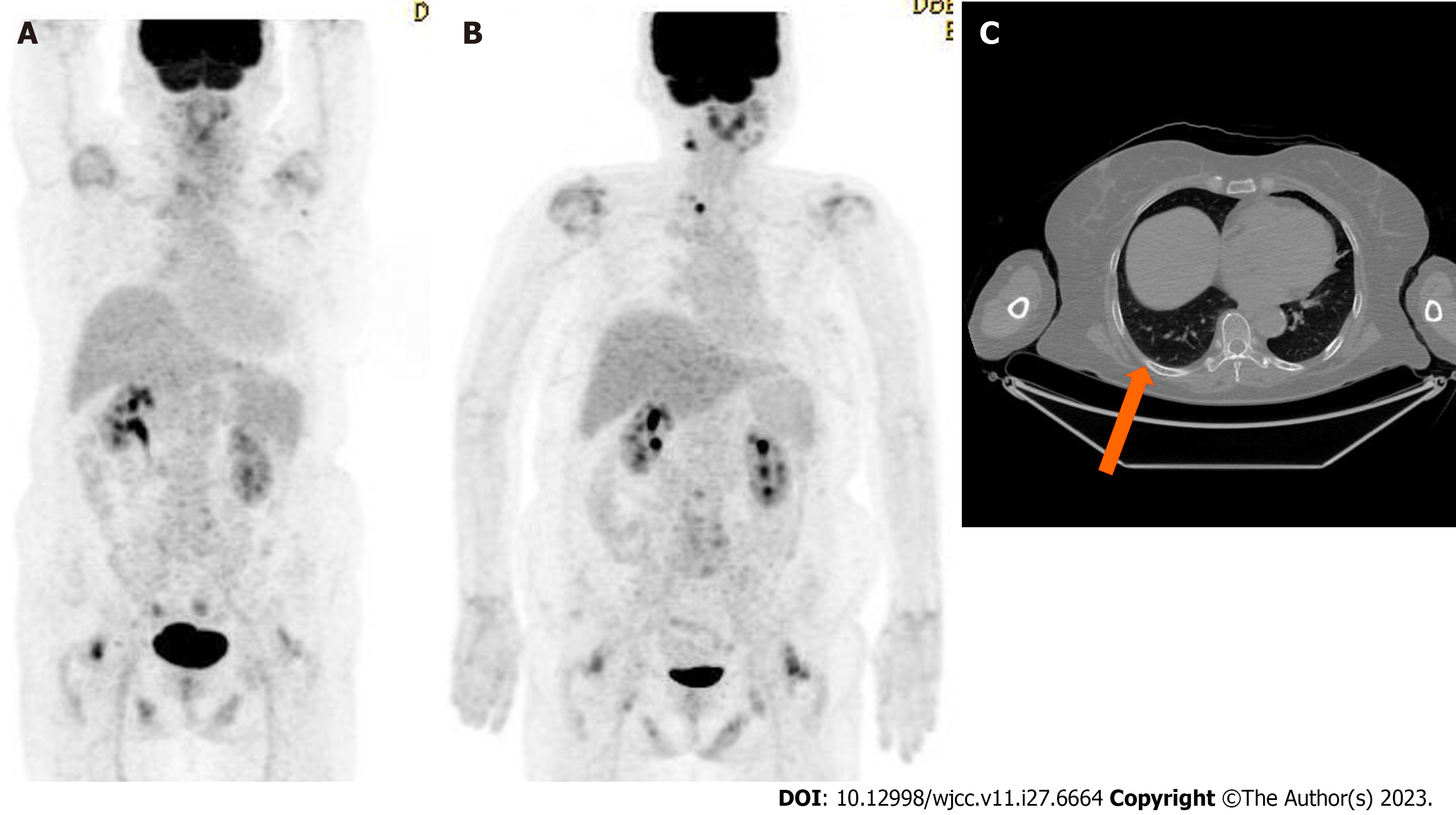
Following the administration of this treatment regimen for three months, the pulmonary nodule in the right lower lobe disappeared on a follow-up chest CT scan. The patient is currently in complete response at the 32-mo follow-up. No adverse effects except for nausea, constipation and indigestion were noted during administration of the drugs. Most of symptoms improved with symptomatic treatment. After 3 and 6 mo of surgery, the thyroglobulin (TG) levels were 0.14 and 0.21 ng/mL, respectively. (reference range for TG: 2-70 ng/mL). Medication was administered from 7 mo after the first surgery. After 5 and 16 mo of the target therapy, it measured 0.07 and 0.28 ng/mL, respectively. Throughout the follow-up period, the TG levels indicated a serologically complete response state.
A previous clinical trial reported that the overall response rate of dabrafenib plus trametinib treatment for BRAF V600E-mutant ATC was 56%, and that the 12-mo duration of the response was 50%[2,3]. Although adverse events occurred in 100% of cases, grade 3 or 4 adverse events were only noted in 58% of cases. The most common adverse event was pyrexia, and the most severe adverse events included anemia, hyponatremia, and pneumonia. Among the 34 participants in that clinical trial, medication was discontinued for six (17%) due to adverse events[2,3]. While there are some reports regarding the safety of dabrafenib and trametinib for BRAF V600E-mutant melanoma, there have been no studies on their safety and dose adjustment for treating ATC[7]. The drug manufacturer indicates that for dabrafenib, the dosage can be reduced to 150, 100, 75, or 50 mg BID; and for trametinib, dosages of 2, 1.5, or 1 mg QD can be adjusted while monitoring for side effects. A case has been reported where dosage adjustments (dabrafenib 150 mg QD + trametinib 1 mg QD) were made to prevent rhabdomyolysis and achieve therapeutic effects in BRAF V600E-mutant non-small cell lung cancer[8]. However, a study conducted on BRAF V600E-mutant melanoma reported higher side effects with full dose usage, but superior progression-free survival[9]. Due to the limited number of ATC cases that have been reported, studies involving dosage adjustments are lacking at this time. Although the rate of adverse events from this treatment has been found to be relatively high, the effectiveness of the treatment generally outweighs the severity of the adverse events. Therefore, careful observation for adverse events and appropriate symptomatic treatment are essential during treatment with dabrafenib plus trametinib.
This case exhibits several differences from the existing clinical trials[2,3]. First, the pre-operative diagnosis through core needle biopsy was PTC; however, post-operative pathological examination confirmed mixed anaplastic (70%) and papillary (30%) carcinomas. This case provided insights into appropriate treatments for patients with PTC and anaplastic dedifferentiation. Second, we were able to secure time and achieve definitive local control through multiple neck dissections in the context of recurrent neck relapses. Additionally, adjuvant chemotherapy was administered to treat the metastatic lung nodule. The ATA guidelines recommend dabrafenib plus trametinib as the optimal initial treatment for unresectable BRAF V600E-mutant ATC[4]. This case is unique because we performed complete resection through surgery and the patient received new targeted chemotherapy rather than conventional concurrent chemoradiotherapy based on a cytotoxic agent. However, the therapeutic effects of dabrafenib plus trametinib for other treatment types, such as adjuvant, neoadjuvant, and palliative treatments, have not yet been fully elucidated. Given the aggressive nature of ATC, death may occur even while treatment with dabrafenib and trametinib is being considered. It is important to diagnose and initiate treatment accurately and promptly. In the case reported here, although the patient initially refused chemotherapy, we were able to extend the survival of the patient through a salvage surgery thereby securing time. In rare but aggressive cancers such as ATC, conducting randomized controlled trials to demonstrate the efficacy of potential therapies remains a challenge. Therefore, it is important to report various clinical cases to compile global data and generate meaningful results.
PTC precedes or coexists with ATC in 50% of cases. BRAF-V600 mutations affect the transition from PTC to ATC[2,3]. ATC concurrent with PTC may be more responsive to treatment with dabrafenib and trametinib, as this combination specifically blocks the underlying mechanism behind the malignancy[7]. Owing to the limitations of the needle biopsy technique, PTC concurrent with ATC may often be misdiagnosed as PTC alone. As was observed in this case, when clinical findings show extensive invasion of PTC to the surrounding tissues, the possibility of misdiagnosis and transformation to ATC should be considered.
We present a case of recurrent and metastatic ATC following three surgical resections, which was successfully treated with dabrafenib plus trametinib, suggesting that dabrafenib plus trametinib is a promising option as an adjuvant chemotherapy. In cases where a BRAF mutation is confirmed following surgery, treatment with dabrafenib plus trametinib may prevent additional recurrences. Further studies and case reports are necessary to compare available treatment options and establish standardized guidelines for the treatment of ATC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Otorhinolaryngology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheng X, China; Liu J, China S-Editor: Yan JP L-Editor: A P-Editor: Xu ZH
| 1. | Ferrari SM, Elia G, Ragusa F, Ruffilli I, La Motta C, Paparo SR, Patrizio A, Vita R, Benvenga S, Materazzi G, Fallahi P, Antonelli A. Novel treatments for anaplastic thyroid carcinoma. Gland Surg. 2020;9:S28-S42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 2. | Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JHM, Soria JC, Wen PY, Zielinski CC, Cabanillas ME, Boran A, Ilankumaran P, Burgess P, Romero Salas T, Keam B. Dabrafenib plus trametinib in patients with BRAF V600E-mutant anaplastic thyroid cancer: updated analysis from the phase II ROAR basket study. Ann Oncol. 2022;33:406-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 181] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 3. | Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JHM, Soria JC, Wen PY, Zielinski C, Cabanillas ME, Urbanowitz G, Mookerjee B, Wang D, Rangwala F, Keam B. Dabrafenib and Trametinib Treatment in Patients With Locally Advanced or Metastatic BRAF V600-Mutant Anaplastic Thyroid Cancer. J Clin Oncol. 2018;36:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 617] [Article Influence: 77.1] [Reference Citation Analysis (1)] |
| 4. | Bible KC, Kebebew E, Brierley J, Brito JP, Cabanillas ME, Clark TJ Jr, Di Cristofano A, Foote R, Giordano T, Kasperbauer J, Newbold K, Nikiforov YE, Randolph G, Rosenthal MS, Sawka AM, Shah M, Shaha A, Smallridge R, Wong-Clark CK. 2021 American Thyroid Association Guidelines for Management of Patients with Anaplastic Thyroid Cancer. Thyroid. 2021;31:337-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 387] [Article Influence: 96.8] [Reference Citation Analysis (0)] |
| 5. | Lorimer C, Cheng L, Chandler R, Garcez K, Gill V, Graham K, Grant W, Sardo Infirri S, Wadsley J, Wall L, Webber N, Wong KH, Newbold K. Dabrafenib and Trametinib Therapy for Advanced Anaplastic Thyroid Cancer - Real-World Outcomes From UK Centres. Clin Oncol (R Coll Radiol). 2023;35:e60-e66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 6. | Arıkan R, Telli TA, Demircan NC, Başoğlu T, Ercelep Ö, Atasoy BM, Özgüven S, Dane F, Yumuk PF. Rechallenge with dabrafenib plus trametinib in anaplastic thyroid cancer: A case report and review of literature. Curr Probl Cancer. 2021;45:100668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | González-Barrallo I, Castellón Rubio VE, Medina J, España S, Mujika K, Majem M, Aguado C, Cabrera Suárez MÁ, Palacio I, Osterloh L, Martínez-Fernández A, García-Castaño A. Safety of combining dabrafenib plus trametinib in elderly BRAF V600 mutation-positive advanced melanoma patients: real-world data analysis of Spanish patients (ELDERLYMEL). Melanoma Res. 2022;32:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 8. | Adachi Y, Yanagimura N, Suzuki C, Ootani S, Tanimoto A, Nishiyama A, Yamashita K, Ohtsubo K, Takeuchi S, Yano S. Reduced doses of dabrafenib and trametinib combination therapy for BRAF V600E-mutant non-small cell lung cancer prevent rhabdomyolysis and maintain tumor shrinkage: a case report. BMC Cancer. 2020;20:156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, Hamid O, Schuchter L, Cebon J, Ibrahim N, Kudchadkar R, Burris HA 3rd, Falchook G, Algazi A, Lewis K, Long GV, Puzanov I, Lebowitz P, Singh A, Little S, Sun P, Allred A, Ouellet D, Kim KB, Patel K, Weber J. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2054] [Cited by in RCA: 2113] [Article Influence: 162.5] [Reference Citation Analysis (0)] |