Published online Sep 26, 2023. doi: 10.12998/wjcc.v11.i27.6603
Peer-review started: July 2, 2023
First decision: August 16, 2023
Revised: August 24, 2023
Accepted: August 31, 2023
Article in press: August 31, 2023
Published online: September 26, 2023
Processing time: 80 Days and 8.4 Hours
Angioedema is a disorder characterized by edema of the face, lips, tongue, and extremities due to increased vascular permeability. Angioedema of the tongue usually occurs bilaterally, and the incidence of unilateral angioedema of the tongue is rare. This study reports a rare case of unilateral angioedema of the tongue with no identifiable cause and repeated recurrence even after discontinuation of an angiotensin-converting enzyme inhibitor.
The patient was a 65-year-old woman with pre-existing hypertension and hyper
Careful monitoring and identification of the underlying mechanism play a crucial role in the treatment of angioedema.
Core Tip: This study describes the case of a 65-year-old woman who presented with unilateral angioedema of the tongue with no identifiable cause and who showed repeated recurrence of angioedema after discontinuation of an angiotensin-converting enzyme (ACE) inhibitor. She had a history of hypertension and hyperlipidemia for 32 years and had been receiving 20 mg/d of lisinopril for 32 years. The ACE inhibitor was suspected as the cause of angioedema; therefore, its use was discontinued. However, angioedema of the left unilateral tongue continued to recur. Recurrence of the unilateral angioedema did not occur following the administration of 500 mg of tranexamic acid.
- Citation: Matsuhisa Y, Kenzaka T, Shimizu H, Hirose H, Gotoh T. Recurrence of unilateral angioedema of the tongue: A case report. World J Clin Cases 2023; 11(27): 6603-6612
- URL: https://www.wjgnet.com/2307-8960/full/v11/i27/6603.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i27.6603
Angioedema, a disorder characterized by localized swelling of the skin and subcutaneous tissues, is caused by a transient increase in capillary permeability. It was first described by Quinche[1] in 1882 and is also known as Quinche’s edema[1,2]. Angioedema primarily occurs on the face, lips, tongue, and extremities. The causes of angioedema include trauma; infection; drugs, such as angiotensin-converting enzyme (ACE) inhibitors; allergic reactions; hereditary factors; and acquired C1 inhibitor deficiency[2]. Angioedema in the oral cavity can cause airway obstruction and may require airway clearance.
Angioedema of the tongue usually occurs bilaterally, and cases of unilateral angioedema of the tongue are rare. The most frequently reported cause of unilateral tongue angioedema is drugs, such as ACE inhibitors and recombinant tissue-type plasminogen activators (rtPA), and recurrence of angioedema has not been reported after discontinuation of these drugs[3-16]. Herein, we describe a rare case of unilateral angioedema of the tongue with no identifiable cause that recurred repeatedly even after discontinuation of an ACE inhibitor.
A 65-year-old Japanese woman was presented to the emergency department at 1:00 a.m. with unilateral swelling on the left side of the tongue.
The patient complained of sudden development of unilateral swelling on the left side of the tongue while falling asleep.
She had a history of hypertension and hyperlipidemia for 32 years and had been receiving 20 mg/d of lisinopril, 5 mg/d of amlodipine, 1 mg/d of doxazosin, 2 mg/d of pitavastatin, and 500 mg/d of polyenephosphatidylcholine. She had been receiving lisinopril, an ACE inhibitor, for 32 years. There was no history of food allergies, allergic rhinitis, atopic dermatitis, or bronchial asthma. She had eaten her dinner the previous day at around 6:00 p.m. and had not consumed anything other than her usual diet. She had no history of alcohol consumption or smoking.
There was no family history of angioedema.
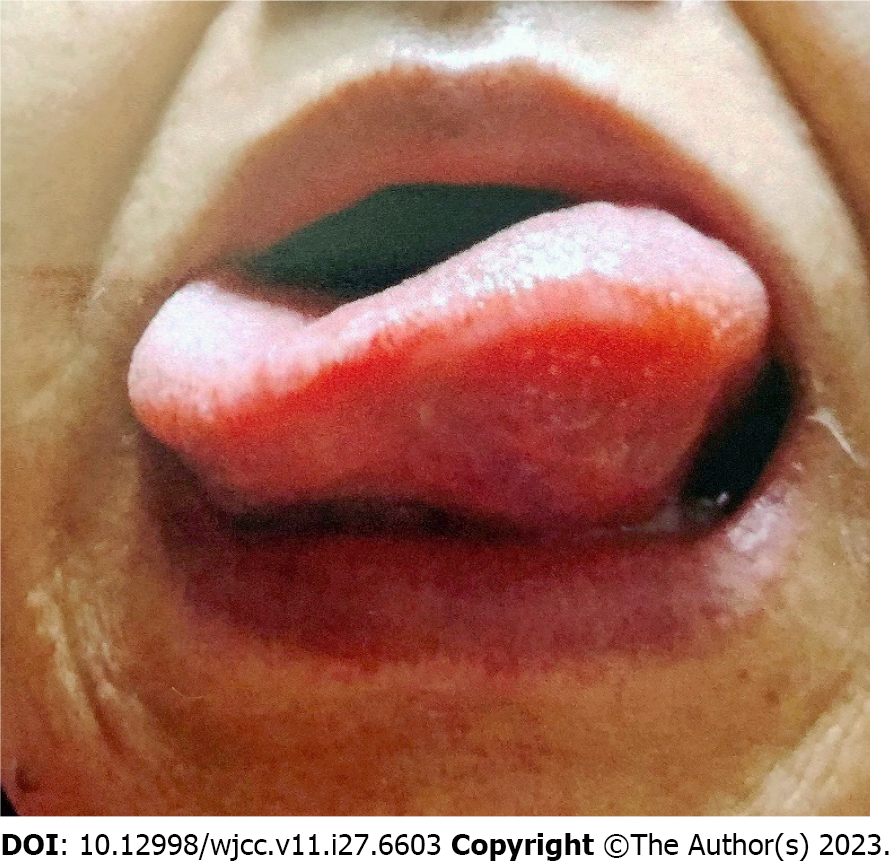
The patient’s vital signs on arrival at our hospital were as follows: Conscious with a Glasgow Coma Scale score of 15 points (E4V5M6); body temperature, 35.9 ℃; blood pressure, 137/78 mmHg; pulse, 88 beats/min; no tachypnea; and SpO2 level, 98% (room air). The patient was obese; her height was 164.6 cm, weight 101.2 kg, and body mass index 37.4 kg/m2. Although she had difficulty in speaking due to unilateral tongue edema, the airway was open, and she was able to breathe (Figure 1). No abnormalities were detected in the heart and respiratory sounds. Edema was not observed in any region other than the tongue. Skin rashes, including wheals, were also not observed.
Table 1 presents the results of laboratory examinations at the time of her visit. The white blood cell (WBC) count was 5560/μL (eosinophils 0.4%), C-reactive protein (CRP) level was 0.16 mg/dL, platelet count was 91000/μL, serum albumin level was 4.0 g/dL, total bilirubin level was 1.0 mg/dL, prothrombin time activity was 69.7%, and the prothrombin time-international normalized ratio was 1.25.
| Parameter | Recorded value | Standard value |
| White blood cell count (µL) | 5560 | 4000-9000 |
| Neutrophils (%) | 91.1 | 40.0-71.9 |
| Lymphocytes (%) | 7.4 | 26.0-46.6 |
| Monocytes (%) | 1.1 | 2.3-7.7 |
| Eosinocytes (%) | 0.4 | 0.2-6.8 |
| Hemoglobin (g/dL) | 13.7 | 11.3-15.2 |
| Platelet count (µL) | 9.1 × 104 | 15.0-35.0 × 104 |
| Prothrombin time/activity (%) | 69.7 | 80-120 |
| International normalized ratio | 1.25 | 0.90-1.20 |
| Activated partial thromboplastin time (s) | 37.0 | 26.0-35.0 |
| C-reactive protein (mg/dL) | 0.16 | < 0.30 |
| Total protein (g/dL) | 6.9 | 6.7-8.3 |
| Albumin (g/dL) | 4.0 | 3.9-4.9 |
| Total bilirubin (mg/dL) | 1.0 | 0.2-1.2 |
| Aspartate aminotransferase (IU/L) | 64 | 13-33 |
| Alanine aminotransferase (IU/L) | 49 | 8-42 |
| Lactase dehydrogenase (IU/L) | 253 | 124-222 |
| Creatine kinase (IU/L) | 213 | 45-163 |
| Blood urea nitrogen (mg/dL) | 14.4 | 8.0-20.0 |
| Creatinine (mg/dL) | 0.52 | 0.40-0.80 |
| Sodium (mEq/L) | 143.6 | 135.0-147.0 |
| Potassium (mEq/L) | 3.8 | 3.4-4.8 |
| Chloride (mEq/L) | 107 | 98-110 |
| Glucose (mg/dL) | 126 | 70-109 |
| Hemoglobin A1c (%) | 5.0 | 4.6-6.2 |
| Ferritin (ng/mL) | 116.5 | 5.0-157.0 |
| Total cholesterol (mg/dL) | 157 | 130-219 |
| Triglyceride (mg/dL) | 43 | 30-150 |
| High-density lipoprotein cholesterol (mg/dL) | 76 | 48-103 |
| Low-density lipoprotein cholesterol (mg/dL) | 73 | 70-139 |
Computed tomography (CT) of the head did not reveal the presence of any ischemic lesions, and echocardiography did not reveal stenosis or dissection of the carotid artery. Abdominal echocardiography, performed due to a history of liver injury, revealed an irregular liver surface and coarse parenchyma, with no ascites. The Child-Pugh score was 6 points, grade A, and the fibrosis-4 index (FIB-4) was 6.53[17]. The patient tested negative for hepatitis B surface antigen and hepatitis C virus antibodies. Based on the abdominal echocardiography images and the FIB-4, she was diagnosed with cirrhosis of the liver due to non-alcoholic steatohepatitis.
Additional tests were performed to identify the cause of angioedema, and the data are shown in Table 2. The complement test revealed normal C1 inhibitor activity of 73%, C3 level of 75 mg/dL, C4 level of 10 mg/dL, CH50 level of 26.7 U/mL, and C1q level of 12.6 mg/dL. Blood tests revealed a nonspecific immunoglobulin E level of 71.81 IU/mL, an antinuclear antibody level of < 40 ×, and an estradiol level of 16.8 pg/mL. A diagnosis of unilateral angioedema of the tongue was made; however, the cause of angioedema remained unclear.
| Parameter | Recorded value | Standard value |
| C1 inhibitor activity (%) | 73 | 70-130 |
| C3 (mg/dL) | 75 | 86-160 |
| C4 (mg/dL) | 10 | 17-45 |
| CH50 (U/mL) | 26.7 | 25.0-48.0 |
| C1q (mg/dL) | 12.6 | 8.8-15.3 |
| Nonspecific IgE (IU/mL) | 71.81 | < 170 |
| Antinuclear antibody | < 40 × | < 40 × |
| Estradiol | 16.8 | < 47.0 pg/mL (postmenopausal) |
The patient was considered to be at risk of developing airway obstruction based on the possibility of anaphylaxis. Therefore, 0.3 mg of epinephrine was administered intramuscularly, and 125 mg of methylprednisolone was admi
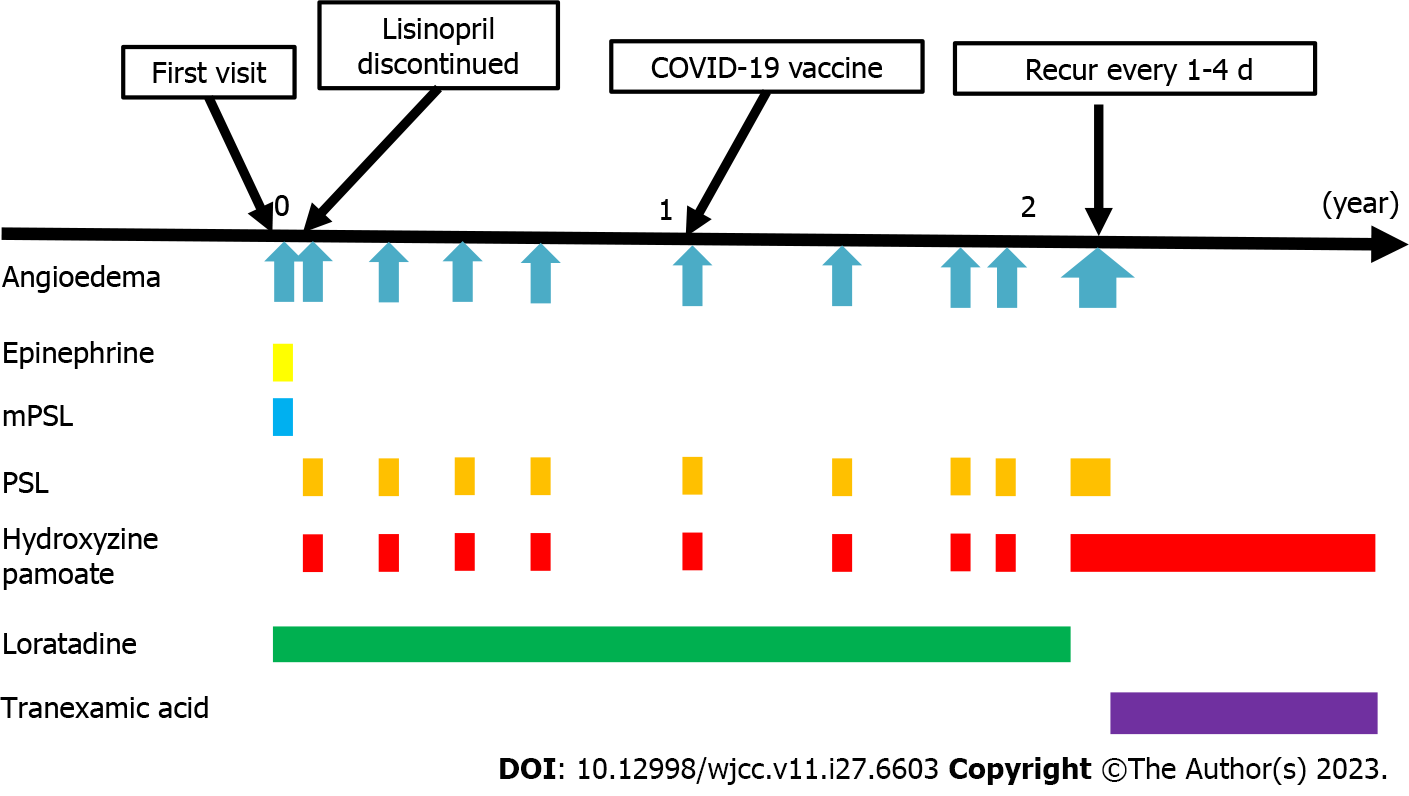
The ACE inhibitor (lisinopril) was suspected to be the cause of angioedema; therefore, its use was discontinued. However, angioedema of the left unilateral tongue recurred after 12 d. Consequently, 20 mg of prednisolone (PSL) and 25 mg of hydroxyzine pamoate, an antihistamine, were administered. The symptoms resolved within 8 h.
Although the cause of angioedema could not be identified, treatment with loratadine 10 mg/d was initiated based on the assumption of an allergic mechanism involving histamine. However, unilateral angioedema of the tongue continued to recur every 2-5 mo. The involvement of the left and right sides was not constant. The symptoms improved within 30 min to 12 h after receiving a single dose of 20 mg of PSL and 25 mg of hydroxyzine pamoate, without any symptoms of respiratory disturbance or airway obstruction. In total, 10 recurrent episodes of unilateral angioedema of the tongue were reported. The sixth recurrence occurred on the day after receiving the third dose of the coronavirus disease 2019 vaccine; however, no apparent trigger was noted for the other episodes. Notably, all episodes of recurrences occurred only between midnight and 6 a.m. and did not occur during the day. During the tenth episode, the symptoms improved within hours following the administration of PSL and an abortive dose of antihistamine; however, angioedema continued to recur every 1-4 d, resulting in three more episodes of recurrence. Therefore, the administration of loratadine was discontinued, and regular oral administration of hydroxyzine pamoate, a sedating antihistamine, was initiated. However, recurrences continued every few days. Thus, the administration of 500 mg of tranexamic acid daily after dinner was initiated, following which the recurrence of the unilateral angioedema ceased, and the patient has not experienced any recurrence for 5 mo since starting tranexamic acid (Figure 2).
This report presents the case of a patient with recurrent unilateral angioedema of the tongue. Unilateral angioedema of the tongue is a rare condition; to the best of our knowledge, this is the first case report to present multiple episodes of unilateral angioedema of the tongue after discontinuation of an ACE inhibitor.
The reasons underlying the unilateral presentation of the angioedema of the tongue are not well understood. However, it is speculated that unilateral edema precedes bilateral angioedema as its origin[4] and that the asymmetry of the lingual nerve results in a left-right difference in the chemical microenvironment, resulting in unilateral susceptibility to the action of the inflammatory mediators[8].
Since the incidence of unilateral angioedema of the tongue is rare, we performed a comprehensive literature review. A search of the database of PubMed using the keyword “unilateral tongue angioedema” retrieved 21 articles and 17 case reports (last retrieved on August 23, 2023). Among the retrieved results, details of the 15 cases that were reported in English are presented in Table 3[3-16]. The median age of the patients was 68 years (range: 30-80 years). Eight patients were male and seven were female. Regarding angioedema caused by ACE inhibitors in 11 cases, administration of ACE inhibitors alone was the cause in nine cases, whereas administration of rtPA for cerebral ischemic disease in addition to ACE inhibitors was the cause in 1 case. In the remaining one case, the patient receiving an ACE inhibitor developed a subdural hematoma, and angioedema developed as a result of the removal of the blood specimen. In the other cases, angioedema was caused by the administration of rtPA for cerebral infarction, acetaminophen, aspirin, and an angiotensin receptor blocker.
| Case | Ref. | Age (yr) /sex | Causes of Angioedema | Comorbidity | Type of ACEI and duration of use | Other medication | Treatment of angioedema | Time to symptom improvement | Recurrence |
| 1 | Mlynarek et al[3] | 73/F | ACEI | Diabetes mellitus, hypertension, hypothyroidism, Bell's palsy, rectal surgery for rectal cancer | Enalapril, 3 yr | Nifedipine, lorazepam, clonidine, levothyroxine, aspirin, and hydrochlorothiazide | MPSL, diphenhydramine, and penicillin. Enalapril was not discontinued initially | Overnight | Symptoms recurred after 3 wk. Enalapril was discontinued subsequently. No further recurrence at the 1-, |
| 2 | Kaptanoglu and Aytas[4] | 44/M | Aspirin | Headache | None | None | Epinephrine subcutaneously, mPSL, chlorphenoxamine | 2 h | No recurrence at the 10-d follow-up |
| 3 | Chan et al[5] | 68/F | ACEI | Type 2 diabetes mellitus and hypertension | Benazepril, several mon | Humulin 70/30, amlodipine, and chlorthalidone | Intravenous diphenhydramine, mPSL, and famotidine. PSL, famotidine, and diphenhydramine | Within 24 h | No recurrence at the 2-mo follow-up |
| 4 | Ee et al[6] | 71/M | ACEI, evacuation of the subdural hematoma | Subdural hemorrhage, hypertension, history of deep vein thrombosis and right temporal subdural hemorrhage secondary to warfarin therapy | Perindopril, 3 mo | Pantoprazole (40 mg once daily), one dose of intravenous ceftriaxone (2 g), and pre-operative prophylaxis (evacuation of subdural hematoma) | Intravenous dexamethasone. Perindopril was discontinued | 48 h | No recurrence at the 2-wk follow-up |
| 5 | Kuhlen and Forcucci[7] | 62/M | ACEI | Hypertension, type 2 diabetes, vascular dementia, end-stage renal disease after cadaveric renal transplant | Lisinopril, recently | Other medications were not mentioned. He had undergone a kidney transplant | Diphenhydramine, famotidine, and mPSL. Intubation | 48 h | NA |
| 6 | Leung et al[8] | 64/F | ACEI | Liver transplant | Lisinopril, 2 d | Mammalian target of rapamycin inhibitor for the liver transplant | Intravenous steroids and antihistamine. Lisinopril was discontinued | Within h | No recurrence at the 4-wk follow-up |
| 7 | Amey et al[9] | 76/M | ACEI | Allergic rhinitis, hypertension, ischemic heart disease, and two percutaneous coronary interventions | Perindopril, 10 yr | Clopidogrel, aspirin, pravastatin, and diclofenac for a rotator cuff injury. Clopidogrel and atorvastatin | Intravenous steroids and antihistamine. Intubation. Perindopril was discontinued. Intubation. Perindopril was discontinued | 48 h | No recurrence |
| 8 | Amey et al[9] | 78/M | ACEI | Asthma with moderate airflow obstruction, percutaneous coronary interventions performed 7 yr prior, epileptic | Perindopril, 7 yr | Clopidogrel, atorvastatin, and phenytoin | Epinephrine nebulizations, steroids, and antihistamines. Perindopril was discontinued | 24 h | No recurrence |
| 9 | Deepthi et al[10] | 30/F | Acetaminophen | Tension headache | None | Acetaminophen | Acetaminophen was discontinued, intravenous dexamethasone, intravenous diphenhydramine | 2 d | NA |
| 10 | Arts et al[11] | 67/M | ACEI, rtPA, cerebrovascular ischemia of the left hemisphere | Right-sided hemiparesis, and cerebrovascular ischemia of the left hemisphere | NA | NA | Intubation, clemastine, and adrenaline | NA | NA |
| 11 | Imai et al[12] | 69/F | ARB | Hypertension, diabetes mellitus, and dysarthria | None (ARB used) | Nicardipine, azilsartan, glimepiride, metformin, vildagliptin, and aspirin | Azilsartan was changed to a carvedilol. Aspirin was discontinued | NA | NA |
| 12 | Al-Hoqani et al[13] | 78/F | ACEI | Hypertension, hyperlipidemia, ischemic heart disease, bilateral knee osteoarthritis, and urinary incontinence | Lisinopril, 2 yr | Amlodipine, rosuvastatin, aspirin, calcium with vitamin D, bisoprolol, diclofenac sodium, and a multivitamin | Chlorpheniramine maleate intramuscularly, Lisinopril was stopped | 12 h | No recurrence at the 2-mo follow-up |
| 13 | Wollmach et al[14] | 49/F | Acute ischemic stroke, rtPA | Pulmonary sarcoidosis and post liposarcoma resection. Angioedema after receiving NSAIDS | None | None | mPSL, clemastine, ranitidine, intubation, and icatibant | 48 h | NA |
| 14 | Lee and Bryant[15] | 80/M | ACEI | Hypertension | Benazepril, 4 yr | Amlodipine | mPSL, diphenhydramine, and famotidine | 48 h | NA |
| 15 | Gil Braga et al[16] | 55/M | ACEI | Hypertension | Enalapril, 3 yr | None | Observation, enalapril was discontinued | Several h | No recurrence at the follow-up a few mon later |
| 16 | This present case | 65/F | Unknown | Hypertension, hyperlipidemia, obesity, NASH, and liver cirrhosis | Lisinopril, 32 yr | Amlodipine, doxazosin, pitavastatin, and polyene phosphatidylcholine | Epinephrine intramuscular injection, mPSL. Lisinopril was discontinued | 17 h | Recurrences occurred 10 times at the 2-yr follow-up |
Recurrence was observed in one case with continuation of ACE inhibitors[3]; no further recurrences were observed following discontinuation of the ACE inhibitor. In the other cases, the causative agent or invasion was discontinued, and no recurrence was reported in any of the cases.
The mechanisms underlying the development of angioedema can be divided into histamine-mediated and bradykinin-mediated mechanisms[18]. Bradykinin-mediated angioedema has a late onset, no urticaria, and no known or suspected allergic triggers[18]. Since the patient in the present case had not presented with urticaria, the mechanism underlying the development of angioedema was suspected to be bradykinin-mediated rather than histamine-mediated mechanism. However, the symptoms improved with the administration of PSL and antihistamines; therefore, a histamine-related mechanism for the onset of the disease could not be ruled out.
ACE inhibitors cause angioedema by inhibiting the breakdown of bradykinin[19]. The incidence of angioedema in patients receiving ACE inhibitors is reported to be 0.20%[19]. Although angioedema occurs within the first week of treatment in most cases, it can occur at any time. Notably, there have been reports of angioedema occurring after receiving 23 years of continuous treatment[19]. Angioedema can also occur after discontinuation of treatment. Although the duration is unclear, there have been reports of angioedema recurring more than 6 mo after discontinuation of ACE inhibitors[19]. Since the involvement of a bradykinin-related mechanism was suspected in the present case, the ACE inhibitor was initially considered the cause of angioedema. However, unlike previous reports, other causes were also suspected, as the angioedema had developed after 32 years of continuous ACE inhibitor use, and its recurrence was observed more than 2 years after discontinuation.
The differential diagnoses of the cause of angioedema other than ACE inhibitors in this case are discussed below. Histamine is involved in the mediation of allergy and anaphylaxis, and it can cause bronchospasm, wheezing, urticaria, and hypotension. However, these symptoms were not observed in our patient, and there was no history of food intake or other factors that could have triggered them. In addition, there was no history of allergic rhinitis, which seems unlikely, or trauma.
Infection was unlikely, as there was no fever or hyperinflammatory response, such as increased WBC counts or elevated CRP levels. Similarly, hereditary or acquired angioedema was considered unlikely due to the absence of C1 inhibitor activity or decreased C1q levels.
The literature review yielded no reports of angioedema associated with cirrhosis. There have been no reports of hereditary angioedema type 3 in Japan, with no increase in estrogen level associated with cirrhosis reported in the present case.
The incidence of angioedema-like unilateral enlargement of the tongue due to acute neurodegeneration associated with internal carotid artery dissection has been reported[20,21]. In the present case, considering the absence of carotid dissection or stenosis on carotid artery echocardiography, absence of ischemic changes on CT, and repeated recurrences, these causes were ruled out.
The possibility of drugs other than ACE inhibitors causing angioedema was also examined. Adverse reactions to drugs were assessed using the naranjo adverse event causality rating scale[22], with each drug receiving the following scores: Lisinopril, 2 points; amlodipine, 2 points; doxazosin, 2 points; pitavastatin, 2 points; and polyenephosphatidylcholine, 2 points. Thus, these drugs were unlikely to cause adverse reactions. There have been several reports of the development of angioedema after the administration of statins, and the development of angioedema due to the administration of pitavastatin has also been reported[23]. Dose-dependent development of angioedema with the administration of statins has been reported[24]. However, angioedema developed within a short period of initiating or increasing the dose in these cases, unlike that in the present case, wherein angioedema recurred after dose reduction and discontinuation; thus, it was considered an unlikely cause. The development of angioedema due to the administration of polyenephosphatidylcholine has not been reported; however, the incidence of angioedema has been reported with the addition of benzoic acid[25]. Since the symptoms persisted after discontinuing the drug in the present study, polyenephosphatidylcholine was considered an unlikely cause.
Reports of angioedema caused by the administration of amlodipine are rare but have been increasing in recent years[26]. There have been no reports of doxazosin-induced angioedema; however, its incidence has been reported in interventional studies[27]. The incidence of angioedema after the administration of these drugs is rare, and the Naranjo Causality scores[22] of these drugs were low in the present case; thus, these drugs were unlikely to be the cause of angioedema. Nevertheless, discontinuation of the drugs was considered in the present study.
The abovementioned factors were highly unlikely to be the cause in the present case; thus, the cause remained unclear. Based on the lack of recurrence with the regular oral administration of tranexamic acid, the risks associated with the discontinuation or modification of antihypertensive medications, and the causal relationship of each drug with angioedema, antihypertensive medications should not be discontinued, and no further verification or intervention should be undertaken. Tranexamic acid inhibits the fibrinolytic system, which is assumed to be involved in the mechanism underlying the relative increase in C1 inhibitor level[28]. Although there was no decrease in C1 inhibitor level during the symptomatic period in the present case, the decrease in bradykinin level with the increase in C1 inhibitor level is assumed to have prevented the onset of angioedema. In the future, increasing the dose of tranexamic acid, discontinuation of amlodipine or doxazosin, and regular oral administration of steroids may be considered if angioedema recurs.
This study has several limitations. First, as this was a case report, it is difficult to generalize the findings to other patients with unilateral tongue angioedema. In addition, the cause of the recurrence of the disease was not identified, and its long-term health effects remain unknown. In the future, we aim to study the long-term health effects in this patient by conducting long-term observations and examining a population of patients with recurrent angioedema. Further research is required to identify the exact changes in the local environment (i.e., the affected half of the tongue).
We report a case of a patient with recurrent unilateral angioedema of the tongue. Although ACE inhibitors are the most common causes of unilateral angioedema of the tongue, other causes may also result in the development of angioedema. Thus, other causes should be considered if the patient shows relapses after discontinuation of ACE inhibitors. In addition, if no cause can be identified, the mechanism of the relapse should be considered during treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Japanese Medical Specialty Board, 29-00000289; Japan Pediatric Association, 30744.
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Barik R, India; Mehta V, India S-Editor: Qu XL L-Editor: A P-Editor: Wu RR
| 1. | Quinche H. Uber akutes umschriebens. Hautoderm Mschr Prakt Dermatol. 1882;1:160-169. [DOI] [Full Text] |
| 2. | Reshef A, Kidon M, Leibovich I. The Story of Angioedema: from Quincke to Bradykinin. Clin Rev Allergy Immunol. 2016;51:121-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Mlynarek A, Hagr A, Kost K. Angiotensin-converting enzyme inhibitor-induced unilateral tongue angioedema. Otolaryngol Head Neck Surg. 2003;129:593-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 4. | Kaptanoglu AF, Aytas H. Aspirin-induced unilateral angioedema of the tongue. J Eur Acad Dermatol Venereol. 2006;20:617-618. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Chan YF, Kalira D, Hore P. Angiotensin-converting enzyme inhibitors as a cause of unilateral tongue angioedema in a 68-year-old woman. Am J Emerg Med. 2006;24:249-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Ee YS, Sow AJ, Goh BS. Unilateral tongue angioedema caused by angiotensin-converting enzyme inhibitor. J Laryngol Otol. 2010;124:1337-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Kuhlen JL Jr, Forcucci J. Angiotensin-converting enzyme inhibitor-induced unilateral tongue angioedema. Am J Med Sci. 2012;344:416-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Leung E, Hanna MY, Tehami N, Francombe J. Isolated unilateral tongue oedema: the adverse effect of Angiotensin converting enzyme inhibitors. Curr Drug Saf. 2012;7:382-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Amey G, Waidyasekara P, Kollengode R. Delayed presentation of ACE inhibitor-induced angio-oedema. BMJ Case Rep. 2013;2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Deepthi A, Shaheen, Kumar H, Ashraf S, Deepak JH. Images in Medicine - An Atypical Presentation of Unilateral Tongue Angioedema Caused by Acetaminophen. J Clin Diagn Res. 2017;11:ZJ01-ZJ02. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Arts L, van Bloemendaal L, Kooter AJ, Tuinman PR. Unilateral orolingual angioedema after thrombolysis in a patient with cerebrovascular ischemia. Intensive Care Med. 2018;44:1955-1956. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Imai T, Hyodo M, Uzawa N. Delayed angioedema of the unilateral tongue associated with angiotensin II receptor blocker in a patient with polypharmacy. Australas J Dermatol. 2019;60:e164-e165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Al-Hoqani ZK, Al-Kiyumi MH, Al-Tamemi SH, Al-Mahrezi AM. Unilateral Tongue Angioedema Induced by Angiotensin Converting Enzyme Inhibitor: A Case Report. Oman Med J. 2020;35:e92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Wollmach AD, Zehnder D, Schwendinger M, Tarnutzer AA. Unilateral orolingual angioedema in a patient with sarcoidosis after intravenous thrombolysis due to acute stroke without improvement after treatment with icatibant. BMJ Case Rep. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Lee JE, Bryant SM. Unilateral angioedema. Am J Emerg Med. 2021;49:302-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Gil Braga B, Cravo M, Neves P, Pinto F, Mendonça C. A Rare Case of Unilateral Tongue Edema with Angiotensin Converting Enzyme Inhibitors. Acta Med Port. 2022;35:588-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Tomeno W, Imajo K, Kuwada Y, Ogawa Y, Kikuchi M, Honda Y, Kato T, Kessoku T, Kirikoshi H, Yoneda M, Kitahora T, Saito S, Ozawa Y, Nakajima A. Distribution of liver stiffness in non-alcoholic fatty liver disease with higher fibrosis-4 index than low cut-off index. J Gastroenterol Hepatol. 2019;34:1411-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Hahn J, Hoffmann TK, Bock B, Nordmann-Kleiner M, Trainotti S, Greve J. Angioedema. Dtsch Arztebl Int. 2017;114:489-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Brown T, Gonzalez J, Monteleone C. Angiotensin-converting enzyme inhibitor-induced angioedema: A review of the literature. J Clin Hypertens (Greenwich). 2017;19:1377-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 20. | Stübgen JP. Unilateral macroglossia as sole presenting manifestation of internal carotid artery dissection. Ear Nose Throat J. 2011;90:434-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Ryan P, Rehman S, Prince S. Acute tongue swelling, the only initial manifestation of carotid artery dissection: a case report with differentiation of clinical picture. Ann Vasc Surg. 2015;29:365.e17-365.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7061] [Cited by in RCA: 8202] [Article Influence: 186.4] [Reference Citation Analysis (0)] |
| 23. | Naz S, Saleem MW, Haider AW. Angioedema; An Unreported Adverse Effect Of Pitavastatin. J Ayub Med Coll Abbottabad. 2018;30:603-604. [PubMed] |
| 24. | Al-Qaaneh AM, Obaid WT, Al-Mohammadi OS, Al-Qaaneh AM, Rabaan AA, Mustafa SM. Dose-dependent atorvastatin associated with angioedema. Int J Clin Pharmacol Ther. 2022;60:106-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Nettis E, Colanardi MC, Ferrannini A, Tursi A. Sodium benzoate-induced repeated episodes of acute urticaria/angio-oedema: randomized controlled trial. Br J Dermatol. 2004;151:898-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Kuruvilla ME, Sanan N. Amlodipine-induced angioedema: An unusual complication of a common medication. Allergy Rhinol (Providence). 2018;9:2152656718764139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Piller LB, Ford CE, Davis BR, Nwachuku C, Black HR, Oparil S, Retta TM, Probstfield JL; ALLHAT Collaborative Research Group. Incidence and predictors of angioedema in elderly hypertensive patients at high risk for cardiovascular disease: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). J Clin Hypertens (Greenwich). 2006;8:649-56; quiz 657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Horiuchi T, Hide M, Yamashita K, Ohsawa I. The use of tranexamic acid for on-demand and prophylactic treatment of hereditary angioedema-a systematic review. J Cutan Immunol Allergy. 2018;1:126-138. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |