Published online Sep 26, 2023. doi: 10.12998/wjcc.v11.i27.6551
Peer-review started: June 6, 2023
First decision: August 8, 2023
Revised: August 18, 2023
Accepted: August 31, 2023
Article in press: August 31, 2023
Published online: September 26, 2023
Processing time: 106 Days and 6.9 Hours
Undifferentiated pleomorphic sarcomas, also known as spindle cell sarcomas, are a relatively uncommon subtype of soft tissue sarcomas in clinical practice.
We present a case report of a 69-year-old female patient who was diagnosed with undifferentiated spindle cell soft tissue sarcoma on her left thigh. Surgical excision was initially performed, but the patient experienced a local recurrence following multiple surgeries and radioactive particle implantations. High-intensity focused ultrasound (HIFU) was subsequently administered, resulting in complete ablation of the sarcoma without any significant complications other than bone damage at the treated site. However, approximately four months later, the patient experienced a broken lesion at the original location. After further diagnostic workup, the patient underwent additional surgery and is currently stable with a good quality of life.
HIFU has shown positive outcomes in achieving local control of limb spindle cell sarcoma, making it an effective non-invasive treatment option.
Core Tip: High-intensity focused ultrasound (HIFU) has the potential to be a reliable, minimally invasive technique that could revolutionize cancer treatment. Although relatively rare, limb spindle cell sarcomas can be effectively controlled by non-invasive treatment, as demonstrated in this case report. Continued advancements in HIFU technology will improve its effectiveness and benefit patients.
- Citation: Zhu YQ, Zhao GC, Zheng CX, Yuan L, Yuan GB. Managing spindle cell sarcoma with surgery and high-intensity focused ultrasound: A case report. World J Clin Cases 2023; 11(27): 6551-6557
- URL: https://www.wjgnet.com/2307-8960/full/v11/i27/6551.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i27.6551
Limb spindle cell sarcomas are undifferentiated pleomorphic sarcomas, and they account for 12.18% of soft tissue sarcomas (STS) of the extremities[1]. STS in adults are a heterogeneous group of tumors of mesenchymal origin that share similar biological patterns of local tumor growth and metastasis. They can be carcinogenic or tumorigenic in their morphology[2]. This rare malignancy accounts for only up to 1% of all cancers[3]. Due to its rarity and complexity, there are few effective studies on its clinicopathological features and diagnosis. Here, we present a case involving a lower-extremity spindle cell sarcoma, detailing its treatment [including high-intensity focused ultrasound (HIFU)] and the evaluation of the treatment efficacy, in order to raise awareness about treatment options for this rarely detected tumor.
A 69-year-old female patient presented with a 3-cm-diameter firm mass that had gradually increased over the prior 6 years on the left thigh, with local pain.
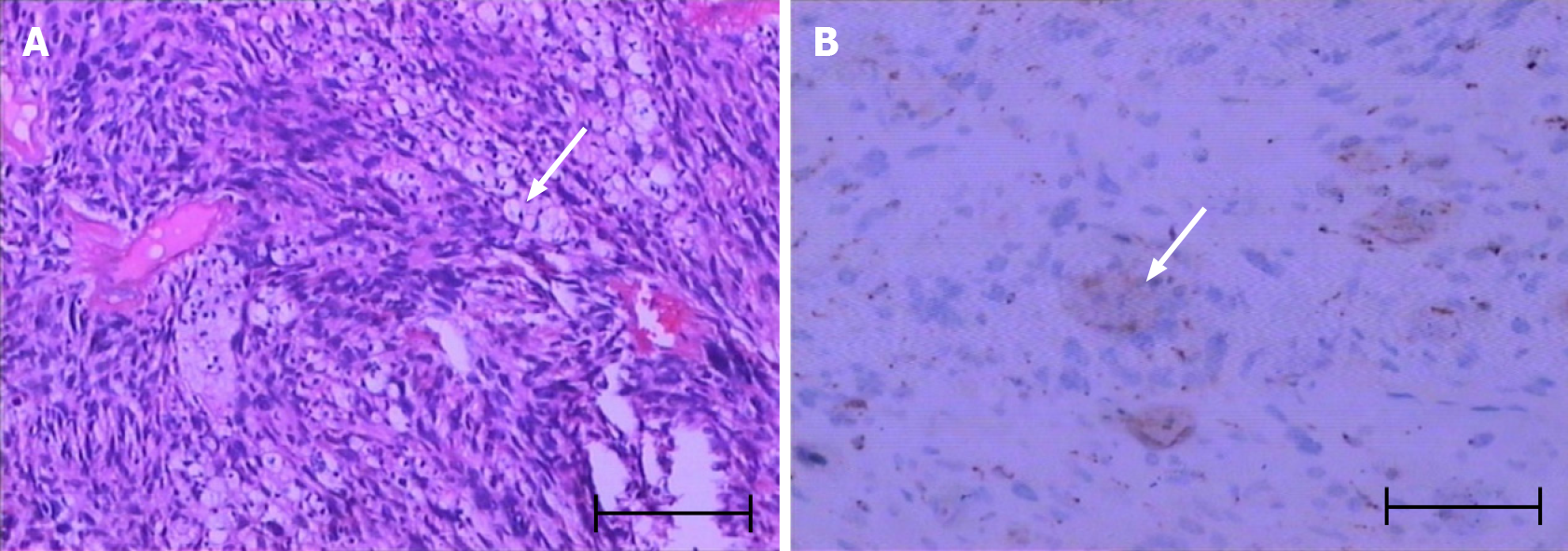
Lumpectomy was performed at The Second Affiliated Hospital of Chongqing Medical University. Postoperative pathology results confirmed the mass to be a spindle cell soft tissue sarcoma. Postoperative immunohistochemistry results indicated CK(-), EMA(-), Vim(+), S100(-), SMA(±), Act(-), CD34(+), BCL-2(-), CD9(±), Ki-67(+), 50% AB(+), MBP(-), NF(-), and CD68(+), confirming the diagnosis of spindle cell soft tissue sarcoma (Figure 1A and B). The patient was treated with an expanded resection.
However, after 2 years, a firmer mass with some tenderness was found at the surgical site. Therefore, the patient underwent another expanded resection, followed by radioactive particle implantation. Postoperative immunohistochemistry results indicated CK(-), EMA(±), DES(-), S100(-), SMA(-), CD34(+), SDX-10(-), CDK4(-), MDM2(-), CD68(-), CD99(±), BCL-2(+), Vim(+), and Ki-67(+) > 50%.
Nevertheless, after 16 mo, magnetic resonance imaging (MRI) revealed that the patient had relapsed. Subsequently, the patient underwent three lumpectomies and radioactive particle implantation.
Despite this, after 5 mo, the follow-up pathology results revealed another relapse. A new treatment plan was designed: five sessions of HIFU (which occurred on March 5, June 11, August 20, October 13, and November 24, 2021), using an integrated circuit -type HIFU tumor treatment system (Chongqing Haifu Medical Technology Co., Ltd., China), which mainly consists of an ultrasonic generator, a focused ultrasonic transducer, a motion system, a control system, and a B-ultra real-time guidance system. The vertical scanning mode with a slice thickness of 2 mm was used. The ultrasonic transmitter worked at frequencies of 0.85 and 1.5 MHz. The ultrasonic power was 150–238W. The duration of each treatment was 275–1325s. The focal length was 135 mm and the lesion had a diameter > 5 cm.
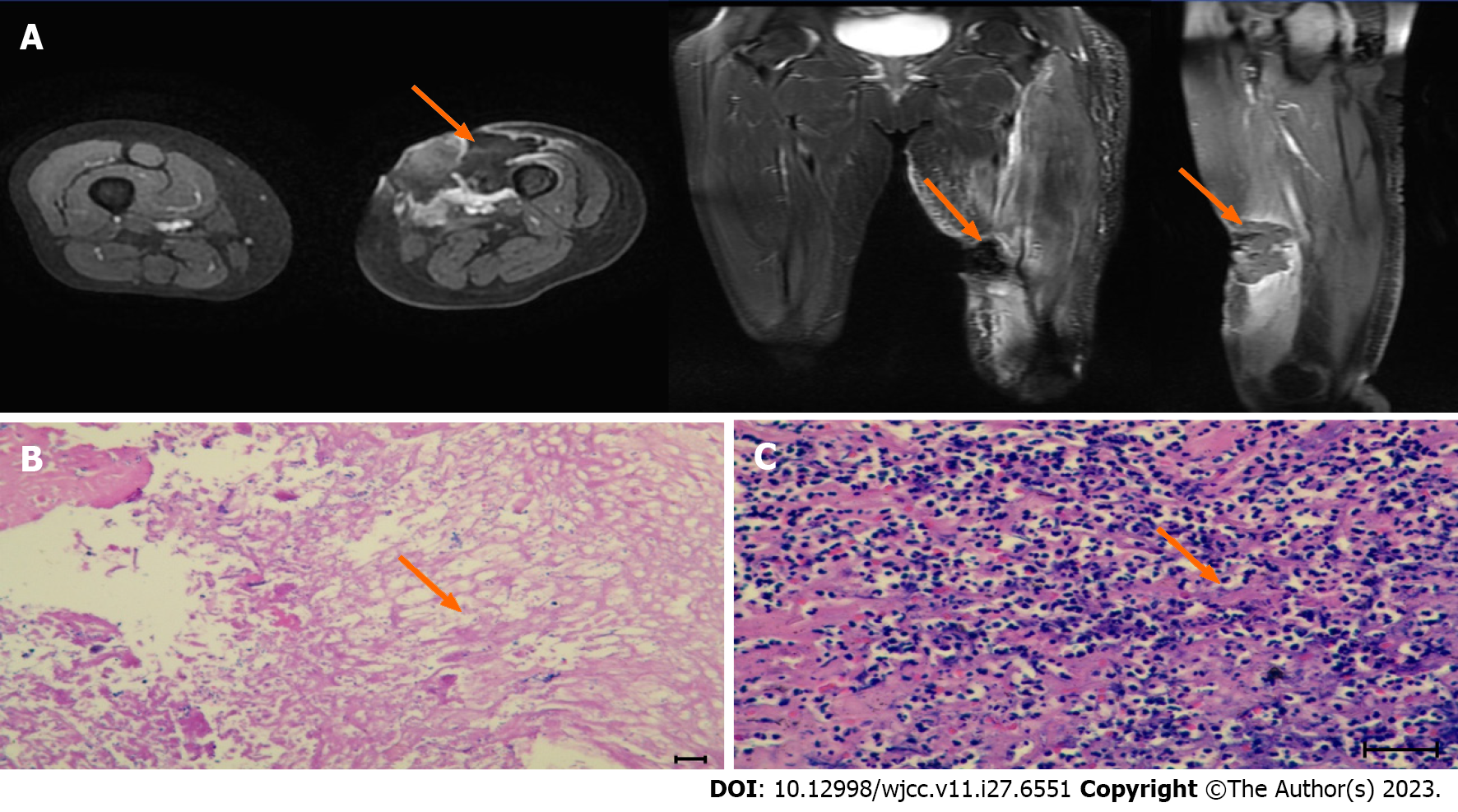
The ablation effect was assessed by MRI. After the first HIFU session, MRI indicated grayscale changes for the whole mass at the lesion site, mild skin edema, and orange peel-like changes, without induration. MRI indicated coagulative necrosis in the treated region, with homogeneous enhancement at the edge of the tumor (Figure 2A). Residual tumor cells were not found in repeated biopsies at 2 and 4 wk after 5 HIFU (Figure 2B and C).
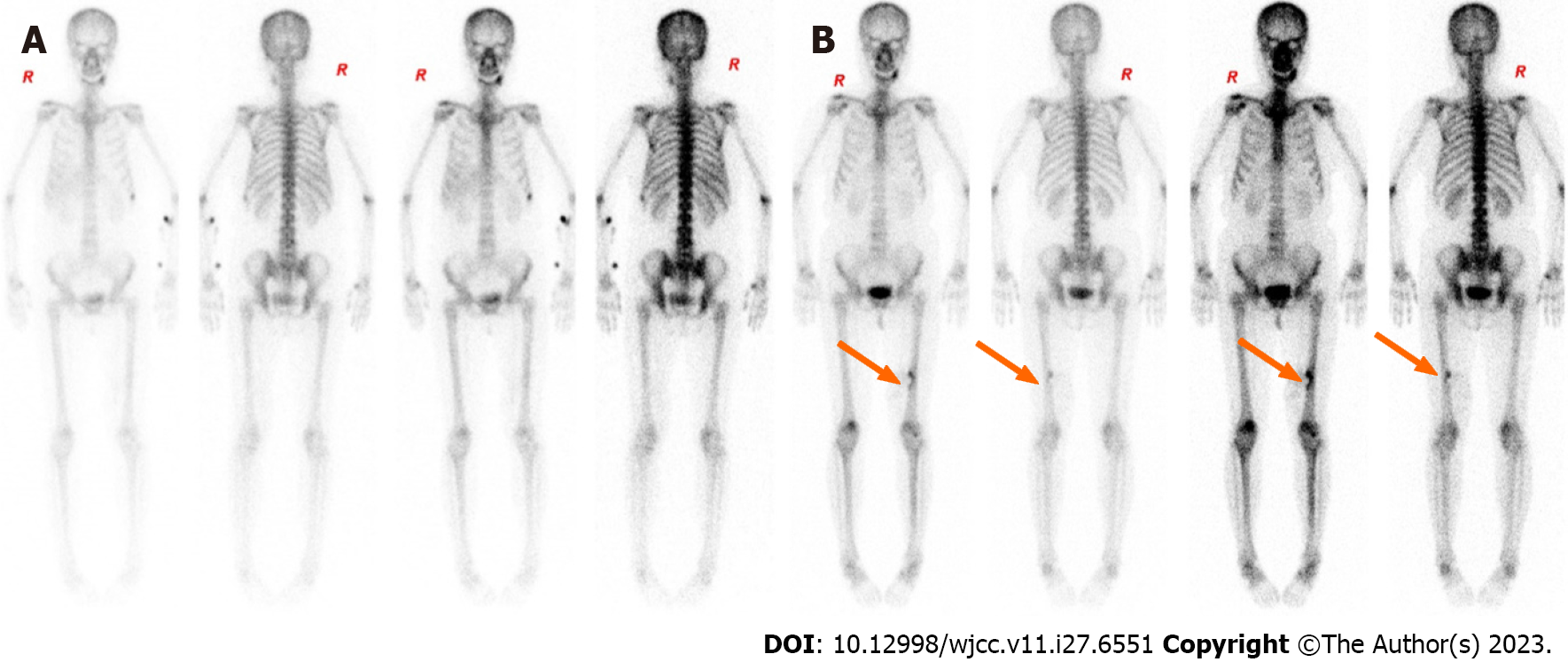
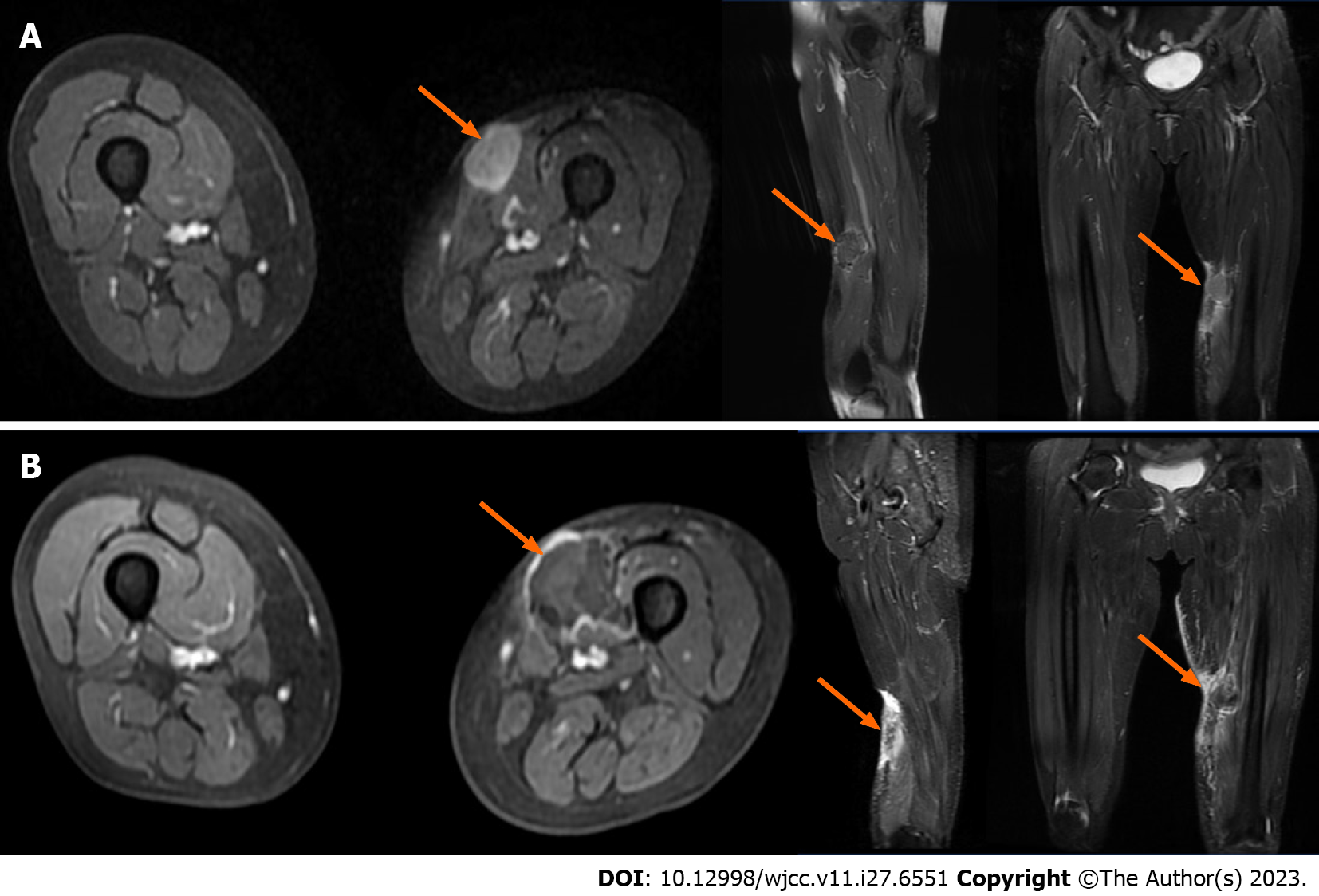
During the course of the disease (April 26, 2017 to April 2, 2022), the patient underwent seven chest computed tomography (CT) scans, all of which were free of lung metastases, four whole-body bone scans (whole-body scans before and after HIFU are shown in Figure 3A and B), all of which were free of bone metastases but showed localized bone damage, and ten MRI scans (MRI scans before and after HIFU are shown in Figure 4A and B). HIFU completely ablated the tumor without complications except for localized bone damage. No further chemotherapy, radiotherapy, or biological therapy was required for tumor control.
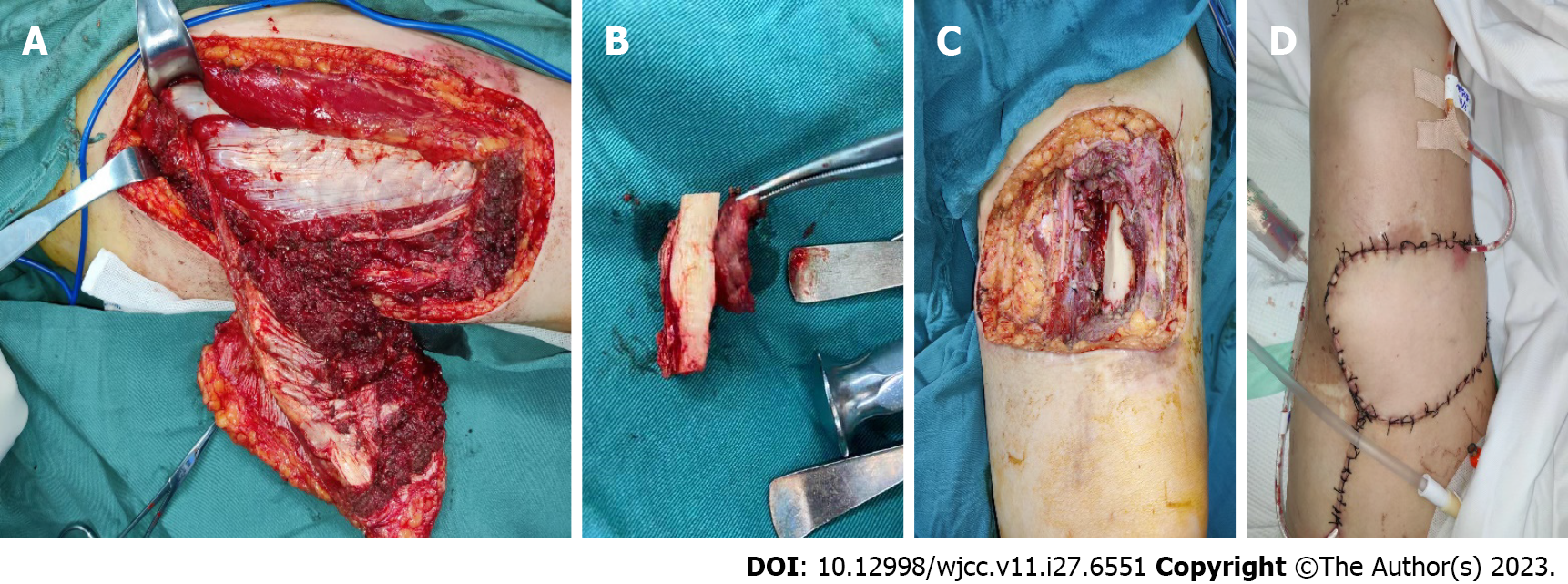
Unfortunately, the patient returned 4 mo later with a 2 cm × 4 cm tissue breakdown at the site of the original lesion.
The patient underwent a comprehensive evaluation followed by a successful surgery on March 3, 2022 (Figure 5).
The patient is now in a stable condition and her limb has been preserved. Further collection and analysis of follow-up data is underway.
Diagnosing spindle cell cancers can be challenging due to their rarity and complexity. Differentiating this condition from clear spindle cell tumor, spindle cell/sclerosing rhabdomyosarcoma, and pleomorphic rhabdomyosarcoma is crucial. Factors such as age of onset, gender, clinical manifestations, and imaging findings should be carefully considered to make an accurate diagnosis. Immunohistochemical tests, including β-catenin nuclear staining, Ki-67 staining, and anti-CD34 antibody tests, are critical in achieving a definitive diagnosis[4-6]. De Vita et al[7] suggested that immunohistochemistry for MyoD1, Myogenin, and base depletion can be used in the diagnosis. By combining clinical imaging and postoperative pathology, our medical team was able to reach a definitive diagnosis of spindle cell sarcomas for this patient.
The management of limb spindle cell sarcomas requires a comprehensive approach that considers the tumor’s behavior[8], individual patient factors, and the potential risks and benefits of various treatment options. Surgery is typically the primary treatment option[9]. Aggressive management is necessary for regional and distant metastases. However, despite the diligent efforts made, it is worth mentioning that limb spindle cell sarcomas still exhibit a notable recurrence rate[10-12], which, in certain instances, may necessitate the consideration of amputation as a treatment option. Additionally, there is a risk of radiation toxicity associated with radiotherapy[13]. Consequently, physicians have been actively seeking alternative treatments that are both safe and effective.
Recently, HIFU has gained popularity as a treatment options for solid tumors[14]. It utilizes a piezoelectric transducer with a fixed aperture and focal length to produce ultrasound waves (1-7 MHz). These waves have thermal and mechanical impacts on tissue. Thermal effects involve heating the targeted tissue, resulting in coagulative necrosis and cell death at higher energy doses (> 55 °C). CT or MRI guidance helps convert the ultrasound waves into heat energy, which fuses at the focal point, causing coagulation and necrosis[15]. HIFU accurately ablates lesions without affecting surrounding normal tissues[16]. It has been widely used for treating uterine fibroids[17], pancreatic cancer[18], prostate cancer[19], thyroid nodules[20], hepatocellular carcinoma[21], and even bone metastases[22,23]. HIFU is also utilized in the treatment of soft tissue sarcomas. The application of MRI-guided focused ultrasound (MR-HIFU) in myxofibrosarcoma (MFS) has shown promise. Studies by Vanni et al[24] and Zhao et al[25] have reported positive results in using MR-HIFU for recurrent MFS cases, both as a palliative treatment to alleviate pain and as a curative treatment for effective local disease control. While HIFU demonstrated effectiveness in our patient, it is important to note that there was bone damage observed at the treatment site. To assess tumor progression and bone damage during HIFU treatment, a combination of MRI and whole body bone imaging can be utilized[26,27].
Based on research findings, HIFU has been proven to be a safe, effective, reproducible, and minimally invasive treatment option. It offers the advantage of avoiding the potential side effects associated with radiotherapy and chemotherapy[28]. It achieves a delicate balance by effectively preventing bleeding, minimizing tissue damage, and enhancing the overall quality of life for patients[25]. In this case, the utilization of HIFU treatment proved to be a safe, effective, reliable, and noninvasive approach for addressing a soft tissue lesion in the left lower extremity of the patient. Nevertheless, it is important to note that the follow-up period in this study was relatively brief, and therefore, additional extensive clinical studies are required to validate and substantiate these findings.
In conclusion, limb spindle cell sarcomas are rare, but this case report highlights the effectiveness of non-invasive HIFU for local control of such tumors. Further studies on HIFU for treating spindle cell sarcomas are necessary to improve the management of this condition.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aydin S, Turkey S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Nakayama R, Mori T, Okita Y, Shiraishi Y, Endo M. A multidisciplinary approach to soft-tissue sarcoma of the extremities. Expert Rev Anticancer Ther. 2020;20:893-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 2. | Bickels J, Malawer MM. Adult Soft-Tissue Sarcomas of the Extremities. J Bone Joint Surg Am. 2022;104:379-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Naghavi AO, Fernandez DC, Mesko N, Juloori A, Martinez A, Scott JG, Shah C, Harrison LB. American Brachytherapy Society consensus statement for soft tissue sarcoma brachytherapy. Brachytherapy. 2017;16:466-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 4. | Hames-Fathi S, Nottley SWG, Pillay N. Unravelling undifferentiated soft tissue sarcomas: insights from genomics. Histopathology. 2022;80:109-121. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Abs D, Landman S, Osio A, Lepesant P, Schneider P, Obadia D, Moguelet P, Farges C, Poirot B, Lehmann-Che J, Lebbé C, Battistella M. Spindle cell tumor with CD34 and S100 co-expression and distinctive stromal and perivascular hyalinization showing EML4-ALK fusion. J Cutan Pathol. 2021;48:896-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Garcia del Muro X, de Alava E, Artigas V, Bague S, Braña A, Cubedo R, Cruz J, Mulet-Margalef N, Narvaez JA, Martinez Tirado O, Valverde C, Verges R, Viñals J, Martin-Broto J; Spanish Group for Research on Sarcoma. Clinical practice guidelines for the diagnosis and treatment of patients with soft tissue sarcoma by the Spanish group for research in sarcomas (GEIS). Cancer Chemother Pharmacol. 2016;77:133-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | De Vita A, Vanni S, Fausti V, Cocchi C, Recine F, Miserocchi G, Liverani C, Spadazzi C, Bassi M, Gessaroli M, Campobassi A, De Luca G, Pieri F, Farnedi A, Franchini E, Ferrari A, Domizio C, Cavagna E, Gurrieri L, Bongiovanni A, Riva N, Calpona S, Di Menna G, Debonis SA, Ibrahim T, Mercatali L. Deciphering the Genomic Landscape and Pharmacological Profile of Uncommon Entities of Adult Rhabdomyosarcomas. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Bleckman RF, Acem I, van Praag VM, Dorleijn DMJ, Verhoef C, Schrage YM, Haas RML, van de Sande MAJ; The Collaborative Persarc Research Group; collaborative PERSARC research group. Multimodality treatment of undifferentiated pleomorphic soft tissue sarcoma of the extremity (eUPS) in the elderly. Eur J Surg Oncol. 2022;48:985-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Hua H, He Z, Lei L, Xie H, Deng Z, Cheng Z, Zuo S, Sun C, Yu C. Retroperitoneal Spindle Cell Tumor: A Case Report. Front Surg. 2021;8:764901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Meyer M, Seetharam M. First-Line Therapy for Metastatic Soft Tissue Sarcoma. Curr Treat Options Oncol. 2019;20:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 11. | Salerno KE, Alektiar KM, Baldini EH, Bedi M, Bishop AJ, Bradfield L, Chung P, DeLaney TF, Folpe A, Kane JM, Li XA, Petersen I, Powell J, Stolten M, Thorpe S, Trent JC, Voermans M, Guadagnolo BA. Radiation Therapy for Treatment of Soft Tissue Sarcoma in Adults: Executive Summary of an ASTRO Clinical Practice Guideline. Pract Radiat Oncol. 2021;11:339-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 103] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 12. | Gamboa AC, Gronchi A, Cardona K. Soft-tissue sarcoma in adults: An update on the current state of histiotype-specific management in an era of personalized medicine. CA Cancer J Clin. 2020;70:200-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 345] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 13. | Hoefkens F, Dehandschutter C, Somville J, Meijnders P, Van Gestel D. Soft tissue sarcoma of the extremities: pending questions on surgery and radiotherapy. Radiat Oncol. 2016;11:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 14. | Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer. 2005;5:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1029] [Cited by in RCA: 810] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 15. | Bachu VS, Kedda J, Suk I, Green JJ, Tyler B. High-Intensity Focused Ultrasound: A Review of Mechanisms and Clinical Applications. Ann Biomed Eng. 2021;49:1975-1991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 119] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 16. | Tempany CM, McDannold NJ, Hynynen K, Jolesz FA. Focused ultrasound surgery in oncology: overview and principles. Radiology. 2011;259:39-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 172] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 17. | Liu L, Wang T, Lei B. High-intensity focused ultrasound (HIFU) ablation versus surgical interventions for the treatment of symptomatic uterine fibroids: a meta-analysis. Eur Radiol. 2022;32:1195-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 18. | Sofuni A, Asai Y, Tsuchiya T, Ishii K, Tanaka R, Tonozuka R, Honjo M, Mukai S, Nagai K, Yamamoto K, Matsunami Y, Kurosawa T, Kojima H, Homma T, Minami H, Nakatsubo R, Hirakawa N, Miyazawa H, Nagakawa Y, Tsuchida A, Itoi T. Novel Therapeutic Method for Unresectable Pancreatic Cancer-The Impact of the Long-Term Research in Therapeutic Effect of High-Intensity Focused Ultrasound (HIFU) Therapy. Curr Oncol. 2021;28:4845-4861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Napoli A, Alfieri G, Scipione R, Leonardi A, Fierro D, Panebianco V, De Nunzio C, Leonardo C, Catalano C. High-intensity focused ultrasound for prostate cancer. Expert Rev Med Devices. 2020;17:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | Lang BHH, Woo YC, Chiu KW. Combining high-intensity focused ultrasound (HIFU) ablation with percutaneous ethanol injection (PEI) in the treatment of benign thyroid nodules. Eur Radiol. 2021;31:2384-2391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 21. | Sehmbi AS, Froghi S, Oliveira de Andrade M, Saffari N, Fuller B, Quaglia A, Davidson B. Systematic review of the role of high intensity focused ultrasound (HIFU) in treating malignant lesions of the hepatobiliary system. HPB (Oxford). 2021;23:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Lin X, Chen W, Wei F. Technique Success, Technique Efficacy and Complications of HIFU Ablation for Palliation of Pain in Patients With Bone Lesions: A Meta-Analysis of 28 Feasibility Studies. Ultrasound Med Biol. 2021;47:1182-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Bongiovanni A, Foca F, Oboldi D, Diano D, Bazzocchi A, Fabbri L, Mercatali L, Vanni S, Maltoni M, Bianchini D, Casadei C, Matteucci F, Nanni O, Rossi B, Sintuzzi E, Martoni ME, Zavoiu V, Barone D, Altini M, Ibrahim T. 3-T magnetic resonance-guided high-intensity focused ultrasound (3 T-MR-HIFU) for the treatment of pain from bone metastases of solid tumors. Support Care Cancer. 2022;30:5737-5745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Vanni S, De Vita A, Gurrieri L, Fausti V, Miserocchi G, Spadazzi C, Liverani C, Cocchi C, Calabrese C, Bongiovanni A, Riva N, Mercatali L, Pieri F, Casadei R, Lucarelli E, Ibrahim T. Myxofibrosarcoma landscape: diagnostic pitfalls, clinical management and future perspectives. Ther Adv Med Oncol. 2022;14:17588359221093973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 25. | Zhao Y, Hu X, Zhong X, Shen H, Yuan Y. High-intensity focused ultrasound treatment as an alternative regimen for myxofibrosarcoma. Dermatologic Therapy. 2021;34. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Siedek F, Yeo SY, Heijman E, Grinstein O, Bratke G, Heneweer C, Puesken M, Persigehl T, Maintz D, Grüll H. Magnetic Resonance-Guided High-Intensity Focused Ultrasound (MR-HIFU): Overview of Emerging Applications (Part 2). Rofo. 2019;191:531-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Siedek F, Yeo SY, Heijman E, Grinstein O, Bratke G, Heneweer C, Puesken M, Persigehl T, Maintz D, Grüll H. Magnetic Resonance-Guided High-Intensity Focused Ultrasound (MR-HIFU): Technical Background and Overview of Current Clinical Applications (Part 1). Rofo. 2019;191:522-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 28. | Elhelf IAS, Albahar H, Shah U, Oto A, Cressman E, Almekkawy M. High intensity focused ultrasound: The fundamentals, clinical applications and research trends. Diagn Interv Imaging. 2018;99:349-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |