Published online Sep 26, 2023. doi: 10.12998/wjcc.v11.i27.6407
Peer-review started: August 1, 2023
First decision: August 16, 2023
Revised: August 22, 2023
Accepted: August 29, 2023
Article in press: August 29, 2023
Published online: September 26, 2023
Processing time: 50 Days and 15.7 Hours
Thyroglobulin (Tg) is one of the markers of thyroid cancer, and its concentration may be elevated in patients with malignant thyroid tumors. Thyroid-stimulating hormone (TSH) is secreted by the pituitary gland, which has a significant impact on thyroid gland function. Excessively high or low TSH levels may be associated with an increased risk of thyroid cancer. Thus, in-depth studies on the association of serum Tg and TSH levels with thyroid cancer risk in patients with thyroid nodules are warranted. This can help determine whether Tg and TSH levels can predict the degree of malignancy of thyroid nodules, which can in turn guide doctors in making accurate diagnoses and treatment decisions. Furthermore, such studies can provide more accurate diagnostic methods for thyroid nodules and help patients become aware of the presence of thyroid cancer as early as possible, thereby improving the success rate of treatment and prognosis.
To investigate the association of serum Tg and TSH levels with the risk of thyroid cancer in patients undergoing thyroid nodule surgery.
The clinical data and laboratory examination results of 130 patients who underwent thyroid nodule surgery were retrospectively analyzed. Furthermore, their preoperative serum Tg and TSH levels were recorded. Histopathological examination conducted during follow-up revealed the presence of thyroid cancer. Correlation analysis were used to analyze the association of Tg and TSH levels with the risk of thyroid cancer.
Of the 130 patients, 60 were diagnosed with thyroid cancer. Statistical analysis revealed a significant positive correlation between serum Tg levels and the risk of thyroid cancer (P < 0.05). This suggests that high serum Tg levels are associated with an increased risk of thyroid cancer. However, no significant correlation was observed between serum TSH levels and the risk of thyroid cancer (P > 0.05).
In patients who underwent thyroid nodule surgery, serum Tg levels exhibited a significant correlation with the risk of thyroid cancer but serum TSH levels did not. These findings suggest that serum Tg can serve as an important biomarker for assessing the risk of thyroid cancer in these patients.
Core Tip: This study aimed to investigate the potential association of serum thyroglobulin (Tg) and thyroid-stimulating hormone (TSH) levels with the risk of thyroid cancer among patients undergoing thyroid nodule surgery. Moreover, we retrospectively analyzed the clinical data and laboratory results of 130 patients who underwent thyroid nodule surgery. Their preoperative Tg and TSH levels were recorded, and subsequent histopathological examinations were conducted during follow-up to determine the presence of thyroid cancer. These results indicated that serum Tg levels were significantly correlated with the risk of thyroid cancer (P < 0.05), suggesting that high Tg levels are associated with an increased likelihood of developing thyroid cancer. However, no significant correlation was observed between serum TSH levels and thyroid cancer risk (P > 0.05). In conclusion, this study highlighted that in patients undergoing thyroid nodule surgery, serum Tg can serve as an important biomarker for assessing the risk of thyroid cancer, whereas serum TSH does not exhibit a significant predictive relationship.
- Citation: Shuai JH, Leng ZF, Wang P, Ji YC. Correlation analysis of serum thyroglobulin, thyroid-stimulating hormone levels, and thyroid-cancer risk in thyroid nodule surgery. World J Clin Cases 2023; 11(27): 6407-6414
- URL: https://www.wjgnet.com/2307-8960/full/v11/i27/6407.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i27.6407
Thyroid nodules are among the most common clinical problems in thyroid diseases, and their incidence is increasing year by year worldwide[1,2]. While most thyroid nodules are benign, approximately 5%-15% of cases are ultimately diagnosed as thyroid cancer[3-5]. Therefore, when evaluating and managing them, early identification of malignant lesions is crucial to the patients’ treatment and prognosis[6]. Furthermore, early diagnosis of thyroid cancer is important and allows early administration of appropriate treatment. Thyroid cancer usually appears early as a small lump or nodule confined to the thyroid before the cancer spreads to nearby tissues or lymph nodes[7]. Early treatment improves the chances of successful treatment and reduces the risk of disease recurrence and death. Early diagnosis is usually associated with lesser tumor burden and local spread, which may lead to simpler treatment[8]. Patients with early-stage thyroid cancer can often achieve effective tumor control with local treatments, such as thyroidectomy surgery or radioactive iodine therapy without the need for complex treatment options, thus reducing the risk of side effects and complications. Early diagnosis is associated with better prognosis[9]. In general, patients with early-stage thyroid cancer have higher survival rates and lower rates of disease recurrence. Early detection and treatment can reduce the risk of disease progression and metastasis as well as improve patients’ quality of life and long-term survival rate[10].
In recent years, several studies have focused on identifying reliable predictors to facilitate the assessment of benign and malignant thyroid nodules. Among these studies, serum thyroglobulin (Tg) and thyroid-stimulating hormone (TSH) have gained significant attention and have been considered as potential markers associated with the risk of thyroid cancer[11,12]. Tg is a glycoprotein synthesized by thyroid follicular cells and mainly functions to store and transport thyroid hormones within the thyroid gland[13-16]. In patients with thyroid cancer, elevated level of Tg produced by malignant cells is observed[17,18]. Thus, measurement of serum Tg levels can provide valuable insights into the presence or absence of thyroid cancer, particularly for the initial screening of patients with thyroid nodules and prediction of cancer risk, which has significant clinical implications. Alternatively, TSH is a hormone secreted by the pituitary gland that stimulates the synthesis and release of thyroid hormones[19,20]. Research has demonstrated a complex association between TSH and thyroid carcinogenesis[21-23].
Many people with early-stage thyroid cancer have no noticeable symptoms. Thyroid cancer is often discovered incidentally during a physical exam or during tests for other diseases, or other tests that may be the result of a neck lump or neck discomfort. The most commonly employed screening method for thyroid cancer is ultrasonography; however, it does not determine whether a lump is cancer. Ultrasonography can only show lump size, shape, and structure; however, it cannot determine whether it is benign or malignant[24,25]. Therefore, further tests such as fine needle aspiration biopsy are usually conducted to determine the nature of the mass. With some types of thyroid cancer, particularly early-stage microscopic cancers, pathologists may have experienced difficulty in identifying the presence of cancer in a fine needle aspiration biopsy sample, leading to misdiagnosis of or failure to detect cancers. The multiple subtypes and variability of thyroid cancer make early diagnosis more difficult. Some subtypes may exhibit nonspecific morphological features, making accurate diagnosis on fine needle aspiration biopsy challenging. In economically backward areas, early diagnosis of thyroid cancer is limited by insufficient medical resources, as well as insufficient medical institutions, imperfect technical equipment, and lack of professionals. These will affect the feasibility and accuracy of early diagnosis. Although there are some difficulties in the early diagnosis of thyroid cancer, continuous research and technological advances are expected to improve diagnostic accuracy. The development of new imaging techniques, molecular markers, and biological indicators may facilitate earlier detection and diagnosis of thyroid cancer. This study aimed to delve deeper into the potential significance of serum Tg and TSH levels in the diagnosis and screening of thyroid cancer. This will be accomplished by analyzing the association between these indicators and the risk of thyroid cancer in patients undergoing thyroid nodule surgery. The findings from this research are expected to provide clinicians with a more precise basis for assessment, thereby improving their ability to guide treatment decisions and management strategies for patients with thyroid nodules.
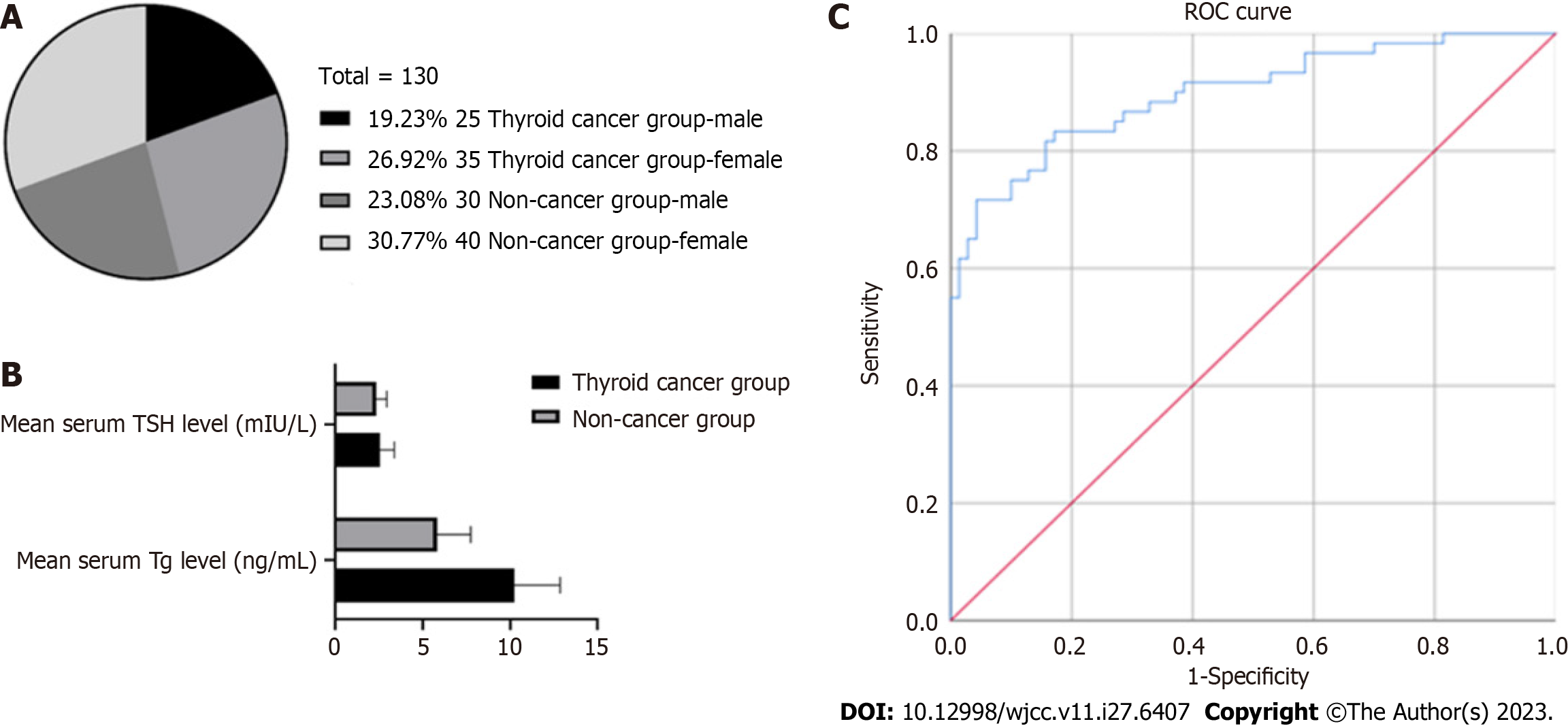
General information of the research hospital and patients: This study was conducted in Meishan City People's Hospital, and the clinical data and laboratory examination results of patients undergoing thyroid nodule surgery were retrospectively collected. Patients were enrolled from January 2021 to January 2023. The patients’ grouping information basic information (including age, gender, and medical history) is presented in Figure 1A, Table 1.
| Groups | Thyroid cancer group | Non-cancer group | P value |
| Average age (yr) | 45.21 ± 8.35 | 45.63 ± 7.94 | > 0.05 |
| Gender (Male/Female) | 25/35 | 30/40 |
The inclusion criteria were patients aged ≥ 18 years, with single or multiple thyroid nodules confirmed by ultrasonography, who received surgical treatment of thyroid nodules with serum Tg and TSH and pathological examination results, and who signed informed consent. The exclusion criteria were patients who received thyroid surgery or radiation therapy; who were diagnosed with thyroid cancer and underwent surgical treatment; with other obvious thyroid diseases, such as hyperthyroidism or thyroiditis; who were pregnant or breastfeeding; with serious heart disease, liver and kidney dysfunction, or other serious complications; and who refused to participate in the study.
The patients were divided into two groups according to their serum Tg and TSH levels: Cancer and noncancer groups. The cancer group consisted of 60 patients (25 men and 35 women). They were diagnosed with thyroid cancer by pathological examination. The noncancer group consisted of 70 patients (30 men and 40 women). The pathological examination results of the patients in the noncancer group indicated benign nodules or no malignant lesions. The purpose of this detailed grouping was better comparison and analyses of the differences between the two groups so as to deeply study the pathogenesis and related factors of thyroid cancer.
The main outcome measure was the association of serum Tg and TSH levels with the risk of thyroid cancer. The levels of serum Tg and TSH were measured using the following standard laboratory methods. Immunoradiometric assay uses antibodies labeled with a radioactive isotope to measure the serum Tg levels. The specific steps include combining the antibody labeled with a radioactive isotope with the Tg in the sample and then determining the Tg concentration by measuring the radioactive count of the label using a radiometer. Chemiluminescent immunoassay measures the serum TSH levels using antibodies labeled with a fluorescent substance. The specific steps include combining TSH in the sample with a fluorescently labeled antibody and determining the TSH concentration by optically measuring the luminous intensity of the fluorescent substance. The enzyme linked immunosorbant assay (ELISA) method combines Tg with a specific enzyme-labeled antibody and generates a detectable signal through the reaction of the enzyme substrate thereafter. Finally, the Tg concentration is determined by optical density measurement. ELISA can also be used to determine the TSH concentration. Similar to the Tg ELISA method, the TSH ELISA generates a measurable signal through the reaction of an enzyme-labeled antibody and a substrate, thereby determining the TSH concentration.
After data collection, data analysis was conducted using SPSS19.0 (https://www.ibm.com/cn-zh). First, descriptive statistical analysis is conducted to calculate the mean (mean) and standard deviation of serum Tg and TSH levels in the thyroid cancer and noncancer groups. Next, the independent sample t-test is employed to compare the differences in serum Tg and TSH levels between the two groups. This helps to determine whether there are significant differences between the groups. Furthermore, Pearson’s correlation coefficient is used to evaluate the association of serum Tg and TSH levels with the risk of thyroid cancer. The value of the correlation coefficient is between −1 and +1, in which close to −1 means negative correlation; close to +1, positive correlation; and close to 0, no correlation. Correlation coefficients and their corresponding P values are calculated to assess the strength and statistical significance of the linear relationship between these variables. In all statistical tests, P < 0.05 is used as the threshold of the significance level; P < 0.05 indicates that the difference or correlation is significant. These statistical methods can help in evaluating the association of serum Tg and TSH levels with the risk of thyroid cancer and in obtaining the correlation results to provide reference and guidance for further research.
Analysis of serum Tg levels revealed that the mean serum Tg level in the cancer group was 10.24 ± 2.63 ng/mL, whereas that in the noncancer group was 5.8 ± 1.91 ng/mL. Statistical analysis revealed that the difference between the two groups was statistically significant (P < 0.05) (Figure 1B), indicating that the serum Tg level of the cancer group was significantly higher than that of the noncancer group. These findings suggest that serum Tg levels are positively associated with the risk of thyroid cancer. High serum Tg levels may be an indicator of thyroid cancer, as serum Tg levels in the cancer group were significantly higher than those in the noncancer group. This means that when a patient’s serum Tg level is elevated, there may be a risk of thyroid cancer. However, it is noteworthy that an increase in serum Tg level cannot be used as the only diagnostic criterion for thyroid cancer because it may also be affected by other factors. Thus, further clinical evaluation and other detection methods, such as ultrasonography and tissue biopsy, are warranted to confirm the diagnosis and staging of thyroid cancer. Table 2 presents the comparison results of serum Tg levels between the two groups obtained in this experiment. These results provide important insights for further understanding of the pathogenesis and diagnosis of thyroid cancer as well as the treatment and prognostic evaluation of patients.
| Group | Thyroid cancer group | Non-cancer group | t | P value |
| Mean serum Tg level (ng/mL) | 10.24 ± 2.63 | 5.8 ± 1.91 | 1.863 | 0.013 |
This study compared a group of patients diagnosed with thyroid cancer with a healthy group to understand the relationship between serum TSH levels and thyroid cancer risk. The results indicated that the average serum TSH level in the cancer group was 2.54 ± 0.83 mIU/L, whereas that in the noncancer group was 2.3 ± 0.61 mIU/L. However, statistical analysis revealed no significant difference in serum TSH levels between the thyroid cancer and noncancer groups (P > 0.05) (Table 3), which showed that no significant correlation was observed between serum TSH levels and the risk of thyroid cancer. This suggests that TSH level is not a reliable indicator of thyroid cancer. It is noteworthy that the results of this study can only be applied to the study sample and study methodology. Other factors, such as thyroid cancer subtype, disease stage, and individual differences, may have an impact on the relationship between serum TSH levels and thyroid cancer risk. Therefore, when making clinical diagnosis and prediction, it is important to consider multiple factors and conduct a comprehensive evaluation of other clinical indicators to accurately assess the risk of thyroid cancer.
| Group | Thyroid cancer group | Non-cancer group | t | P value |
| Mean serum TSH level (mIU/L) | 2.54 ± 0.83 | 2.3 ± 0.61 | 3.483 | 0.373 |
Additional correlation analysis revealed a positive association between serum Tg levels and the risk of thyroid cancer. However, no significant correlation was observed between serum TSH levels and thyroid cancer risk. To further assess the relationship between serum Tg levels and cancer, a receiver operating characteristic analysis was conducted. The analysis showed a critical threshold of 6.7 ng/mL for serum Tg levels, with a sensitivity of 0.898. These findings suggest that serum Tg levels hold a significant diagnostic value (Table 4 and Figure 1C).
| Area | Standard error | 95%CI | P value | |
| 0.898 | 0.028 | Lower limit | Upper limit | < 0.01 |
| 0.844 | 0.953 | |||
The association between serum Tg and TSH levels and the risk of thyroid cancer is a trending topic in thyroid research. Many studies aimed to explore this topic to improve the early diagnosis and management of thyroid cancer. Early diagnosis can lead to better treatment outcomes and survival for patients with thyroid cancer. In the early stages, cancer is usually confined inside the thyroid and does not spread to other tissues or organs. This makes surgical removal of the thyroid and nearby lymph nodes possible and usually does not require radiation therapy or chemotherapy. In general, treatment of early-stage thyroid cancer is simple, and surgical removal of the thyroid gland in such cases is a common treatment alternative. Treatment of early-stage cancers often does not require major surgery or radiation therapy, thus reducing the risk of complications and side effects. Early diagnosis can help prevent thyroid cancer from progressing and metastasizing to other sites. If the cancer has spread to the lymph nodes or other organs, the treatment becomes more complicated, and the chances of a cure are significantly reduced.
Serum Tg levels are commonly used as a marker of thyroid cancer. In patients with thyroid cancer, malignant thyroid cells produce Tg with an elevated concentration. Therefore, serum Tg levels can be used as an indicator to assess the presence or absence of thyroid cancer[11]. However, the sensitivity and specificity of Tg are not perfect. Furthermore, elevated serum Tg levels can be observed in some benign conditions, such as thyroid nodules, inflammation, and abnormal thyroid function[12]. Consequently, while considering serum Tg levels as a screening tool for thyroid cancer, a comprehensive evaluation that combines other clinical and imaging features is necessary. TSH is a hormone secreted by the pituitary gland, which plays a central role in thyroid gland function and metabolism[26,27]. Some studies have demonstrated a potential correlation between TSH and the risk of thyroid cancer[27]. Elevated TSH levels are considered a risk factor for thyroid cancer as they can stimulate the proliferation and malignant transformation of thyroid cells[28]. However, not all studies have yielded consistent results, and some studies have failed to establish a conclusive association between TSH and thyroid cancer. Such an inconsistency may be attributed to study sample variations, study design differences, and the complex nature of thyroid functional status.
This study aimed to investigate the association of serum Tg and TSH levels with the risk of thyroid cancer in patients who underwent thyroid nodule surgery. In the retrospective analysis of the data of 130 patients, the following results and discussions were obtained. Our study exhibited a significant correlation between serum Tg levels and thyroid cancer[29]. Specifically, the cancer group had significantly higher Tg levels than the noncancer group, which is consistent with several previous studies. Malignant lesions in the thyroid often lead to increased production of Tg by cancer cells. Thus, serum Tg levels can serve as an effective marker of thyroid cancer. These findings suggest that evaluation of serum Tg levels holds potential clinical significance for the initial screening of patients with thyroid nodules and assessment of the risk of thyroid cancer.
However, this study showed no significant association between serum TSH levels and the risk of thyroid cancer. This finding is not entirely consistent with previous studies and could be influenced by various factors, such as the characteristics of the study participants, study design, and assay methods used. Further investigation is warranted to better understand the relationship between TSH and thyroid cancer risk. However, our correlation analysis revealed a positive association between serum Tg levels and the risk of thyroid cancer. This suggests that elevated serum Tg levels are associated with an increased risk of thyroid cancer. However, it is noteworthy that correlation does not imply causation but rather indicates the extent of the relationship between the two variables. Therefore, serum Tg level can only serve as an indicator of thyroid cancer and should not be used solely for diagnosing thyroid cancer.
In conclusion, this study showed that serum Tg level as an important biomarker for assessing the risk of thyroid cancer in patients with thyroid nodules. However, the role of serum TSH levels in thyroid cancer risk remains controversial to date, and further studies are warranted to elucidate its accuracy and reliability. Moreover, future studies can consider the incorporation of larger sample sizes and multicenter data to further verify and strengthen our findings.
This study aimed to explore the relationship between serum thyroglobulin (Tg) and thyroid-stimulating hormone (TSH) levels and the risk of thyroid cancer in individuals undergoing thyroid nodule surgery. The primary objective was to ascertain whether these biomarkers could serve as predictive tools for thyroid cancer.
A retrospective analysis was conducted on clinical data and laboratory results from 130 patients who had undergone thyroid nodule surgery. Preoperative serum Tg and TSH levels were recorded. Histopathological examination determined thyroid cancer status during follow-up. Statistical methods were employed to evaluate the potential correlations.
Among the 130 patients studied, 60 were diagnosed with thyroid cancer. Statistical analysis revealed a significant positive correlation between serum Tg levels and thyroid cancer risk (P < 0.05), indicating higher Tg levels were linked to increased risk. However, no significant correlation was found between serum TSH levels and thyroid cancer risk (P > 0.05).
In the context of patients undergoing thyroid nodule surgery, serum Tg levels were observed to have a notable correlation with the risk of thyroid cancer, while serum TSH levels did not exhibit such a correlation. These findings underscore the potential of serum Tg levels as a valuable biomarker for assessing thyroid cancer risk in patients with thyroid nodules.
The study's findings provide valuable insights into the clinical utility of serum Tg levels as a predictive tool for thyroid cancer risk. Further research is warranted to validate these findings across larger and more diverse patient populations. Additionally, exploring other potential biomarkers and refining predictive models could enhance the accuracy of thyroid cancer risk assessment.
Given the prevalence of thyroid nodules and the challenge of distinguishing between benign and malignant cases, understanding the correlation between Tg and TSH levels and thyroid cancer risk is essential. Identifying reliable biomarkers could enhance early detection and improve patient outcomes.
Thyroid nodules are a common clinical concern, with some harboring the potential for malignancy. Detecting thyroid cancer accurately is crucial. Serum Tg and TSH levels have been suggested as potential indicators of thyroid cancer risk, prompting the need for a comprehensive investigation.
We would like to express our gratitude to all medical research institutes and patients who participated in this study, as well as to all reviewers for their review of this article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Barber PA, New Zealand; Castagna MG, Italy S-Editor: Liu JH L-Editor: A P-Editor: Zhao S
| 1. | Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet. 2016;388:2783-2795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 741] [Cited by in RCA: 1025] [Article Influence: 113.9] [Reference Citation Analysis (1)] |
| 2. | Laha D, Nilubol N, Boufraqech M. New Therapies for Advanced Thyroid Cancer. Front Endocrinol (Lausanne). 2020;11:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 149] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 3. | Roman BR, Morris LG, Davies L. The thyroid cancer epidemic, 2017 perspective. Curr Opin Endocrinol Diabetes Obes. 2017;24:332-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 206] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 4. | Carling T, Udelsman R. Thyroid cancer. Annu Rev Med. 2014;65:125-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 248] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 5. | Seib CD, Sosa JA. Evolving Understanding of the Epidemiology of Thyroid Cancer. Endocrinol Metab Clin North Am. 2019;48:23-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 307] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 6. | Chen DW, Lang BHH, McLeod DSA, Newbold K, Haymart MR. Thyroid cancer. Lancet. 2023;401:1531-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 268] [Article Influence: 134.0] [Reference Citation Analysis (0)] |
| 7. | Haymart MR. Progress and Challenges in Thyroid Cancer Management. Endocr Pract. 2021;27:1260-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | Bauer AJ. Pediatric Thyroid Cancer: Genetics, Therapeutics and Outcome. Endocrinol Metab Clin North Am. 2020;49:589-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Grimm D. Recent Advances in Thyroid Cancer Research. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 10. | Maniakas A, Zafereo M, Cabanillas ME. Anaplastic Thyroid Cancer: New Horizons and Challenges. Endocrinol Metab Clin North Am. 2022;51:391-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 11. | Ciarallo A, Rivera J. Radioactive Iodine Therapy in Differentiated Thyroid Cancer: 2020 Update. AJR Am J Roentgenol. 2020;215:285-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 12. | Li S, Ren C, Gong Y, Ye F, Tang Y, Xu J, Guo C, Huang J. The Role of Thyroglobulin in Preoperative and Postoperative Evaluation of Patients With Differentiated Thyroid Cancer. Front Endocrinol (Lausanne). 2022;13:872527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Viola N, Agate L, Caprio S, Lorusso L, Brancatella A, Ricci D, Sgrò D, Ugolini C, Piaggi P, Vitti P, Elisei R, Santini F, Latrofa F. Thyroid autoimmunity, thyroglobulin autoantibodies, and thyroid cancer prognosis. Endocr Relat Cancer. 2023;30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 14. | Lin JD. Thyroglobulin and human thyroid cancer. Clin Chim Acta. 2008;388:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Knappe L, Giovanella L. Life after thyroid cancer: the role of thyroglobulin and thyroglobulin antibodies for postoperative follow-up. Expert Rev Endocrinol Metab. 2021;16:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Ritter A, Mizrachi A, Bachar G, Vainer I, Shimon I, Hirsch D, Diker-Cohen T, Duskin-Bitan H, Robenshtok E. Detecting Recurrence Following Lobectomy for Thyroid Cancer: Role of Thyroglobulin and Thyroglobulin Antibodies. J Clin Endocrinol Metab. 2020;105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 17. | Wassner AJ, Della Vecchia M, Jarolim P, Feldman HA, Huang SA. Prevalence and Significance of Thyroglobulin Antibodies in Pediatric Thyroid Cancer. J Clin Endocrinol Metab. 2017;102:3146-3153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Kim H, Park SY, Choe JH, Kim JS, Hahn SY, Kim SW, Chung JH, Jung J, Kim TH. Preoperative Serum Thyroglobulin and Its Correlation with the Burden and Extent of Differentiated Thyroid Cancer. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Okda TM, Atwa GMK, Eldehn AF, Dahran N, Alsharif KF, Elmahallawy EK. A Novel Role of Galectin-3 and Thyroglobulin in Prognosis and Differentiation of Different Stages of Thyroid Cancer and Elucidation of the Potential Contribution of Bcl-2, IL-8 and TNF-α. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 20. | Ku EJ, Yoo WS, Lee EK, Ahn HY, Woo SH, Hong JH, Chung HK, Park JW. Effect of TSH Suppression Therapy on Bone Mineral Density in Differentiated Thyroid Cancer: A Systematic Review and Meta-analysis. J Clin Endocrinol Metab. 2021;106:3655-3667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Yang X, Guo N, Gao X, Liang J, Fan X, Zhao Y. Meta-analysis of TSH suppression therapy and the risk of cardiovascular events after thyroid cancer surgery. Front Endocrinol (Lausanne). 2022;13:991876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Yavuz DG, Yazan CD, Hekimsoy Z, Aydin K, Gokkaya N, Ersoy C, Akalın A, Topaloglu O, Aydogan BI, Dilekci ENA, Alphan Uc Z, Cansu GB, Ozsari L, Iyidir OT, Olgun ME, Keskin L, Mert M, Can B, Gungor K, Galip T, Cantürk Z, Elbuken G, Pekkolay Z, Kutbay NO, Yorulmaz G, Kalkan AT, Unsal YA, Yay A, Karagun B, Bozkur E. Assesment of attainment of recommended TSH levels and levothyroxine compliance in differentiated thyroid cancer patients. Clin Endocrinol (Oxf). 2022;97:833-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Schiffmann L, Kostev K, Kalder M. Association between various thyroid gland diseases, TSH values and thyroid cancer: a case-control study. J Cancer Res Clin Oncol. 2020;146:2989-2994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Kaliszewski K, Diakowska D, Rzeszutko M, Nowak Ł, Wojtczak B, Sutkowski K, Ludwig M, Ludwig B, Mikuła A, Greniuk M, Tokarczyk U, Rudnicki J. Assessment of Preoperative TSH Serum Level and Thyroid Cancer Occurrence in Patients with AUS/FLUS Thyroid Nodule Diagnosis. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Díaz-Soto G, Fernández-Velasco P, Torres Torres B, López Gómez JJ, García Calvo S, de Luis Román D. Evolution of suppressing TSH therapy at diagnosis and in the long-term follow-up in a cohort of differentiated thyroid cancer. Endocrinol Diabetes Nutr (Engl Ed). 2022;69:844-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 26. | Rowe CW, Paul JW, Gedye C, Tolosa JM, Bendinelli C, McGrath S, Smith R. Targeting the TSH receptor in thyroid cancer. Endocr Relat Cancer. 2017;24:R191-R202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 27. | Al-Bader A, Zawawi F, Singer Z, Mlynarek A, Hier M, Tamilia M, Payne R. Preoperative TSH and thyroglobulin levels: would it predict thyroid cancer? Otolaryngol Pol. 2015;69:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Yoon JH, Choi W, Park JY, Hong AR, Kim SS, Kim HK, Kang HC. A challenging TSH/GH co-secreting pituitary adenoma with concomitant thyroid cancer; a case report and literature review. BMC Endocr Disord. 2021;21:177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Handelsman RS, Alvarez AL, Picado O, Farrá JC, Lew JI. Inverse Relationship of BMI to TSH and Risk of Papillary Thyroid Cancer in Surgical Patients. J Surg Res. 2019;244:96-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |