Published online Sep 16, 2023. doi: 10.12998/wjcc.v11.i26.6289
Peer-review started: June 17, 2023
First decision: August 4, 2023
Revised: August 15, 2023
Accepted: August 21, 2023
Article in press: August 21, 2023
Published online: September 16, 2023
Processing time: 83 Days and 0.3 Hours
Collision tumors of primary malignant lymphoma and adenocarcinoma in the colon are rare. Primary diffuse large B-cell lymphoma (DLBCL)–adenocarcinoma collision tumors are especially rare.
A 74-year-old woman presented with abdominal pain of 1 mo duration. Biopsy under colonoscopy revealed adenocarcinoma of the ascending colon. Subsequently, the patient underwent laparoscopic radical resection of right colon cancer with lymph node dissection. A collision tumor was found incidentally through postoperative pathological sampling. Genetic analysis showed a collision tumor of DLBCL with germinal center B-cell subtype and TP53 mutation, and adenocarcinoma arising in a tubulovillous adenoma in the colon, with BRAF mutation and mutL homolog 1 promoter methylation. The patient died 3 mo after surgery. To our knowledge, this is the 23rd reported case of collision tumor of colorectal adenocarcinoma and lymphoma. The mean age of the 23 patients was 73 years. The most common site was the cecum. There were 15 cases with follow-up data including 11 living and four dead with a 3-year overall survival rate of 71.5%.
Based on pathological and genetic analysis, surgery combined with chemotherapy or chemoradiotherapy may have good therapeutic effects for collision tumor.
Core Tip: Coexisting of primary diffuse large B-cell lymphoma (DLBCL) and adenocarcinoma in the colon are extremely rare. Here, we report a case of collision tumor of primary DLBCL, not otherwise specified with germinal center B-cell subtype and TP53 mutation, and adenocarcinoma arising in a tubulovillous adenoma in the colon, with BRAF mutation and mutL homolog 1 promoter methylation. Definite diagnosis is usually difficult until pathological confirmation. Based on pathological examination and genetic analysis, surgery combined with dose-adjusted chemotherapy or chemoradiotherapy may have good therapeutic effects.
- Citation: Jiang M, Yuan XP. Collision tumor of primary malignant lymphoma and adenocarcinoma in the colon diagnosed by molecular pathology: A case report and literature review. World J Clin Cases 2023; 11(26): 6289-6297
- URL: https://www.wjgnet.com/2307-8960/full/v11/i26/6289.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i26.6289
Colorectal cancers (CRCs) are malignant epithelial tumors originating in the large bowel with glandular or mucinous differentiation, and are the second most common cancer in women and the third most common in men[1]. Adenocarcinoma is the most common malignant tumor of the colon, while primary malignant lymphoma is relatively rare. Two or more distinct tumors of different cell lineages that independently occur in the same space or organ and combine to form one mass are defined as collision tumors[1,2]. Collision tumors of primary malignant lymphoma and adenocarcinoma in the colon are rare. To the best of our knowledge, only 22 cases have been described in the literature[3-24]. Here, we report a case of collision tumor of primary diffuse large B-cell lymphoma (DLBCL), not otherwise specified (NOS) with germinal center B-cell (GCB) subtype and TP53 mutation, and adenocarcinoma arising in a tubulovillous adenoma in the colon, with BRAF mutation and mutL homolog 1 (MLH1) promoter methylation. We also present a review of the literature.
A 74-year-old woman presented with abdominal pain of 1 mo duration and was referred to Sun Yat-Sen Memorial Hospital, in March 2018.
She presented with abdominal pain of 1 mo duration.
She mentioned a loss of 8 kg over the previous 6 mo and had anemia. Hypertension was confirmed for many years.
The patient denied any family history of malignant tumors.
Upon physical examination, no peripheral lymphadenopathy or hepatosplenomegaly was found. A mass was noted in the right lower quadrant.
Blood examination showed an increase in carbohydrate antigen 125, carbohydrate antigen 19-9 level, and a decrease in lymphocyte proportion and count, hemoglobin, and total protein (Table 1). Serum lactate dehydrogenase was elevated (538 U/L, normal 108-252 U/L). Blood detection for syphilis, hepatitis B virus, hepatitis C virus and human immunodeficiency virus was negative or normal.
| Item | Detection value | Unit | Normal range |
| White blood cell count | 8.25 | ×109/L | 3.50-9.50 |
| Red cell count | 3.80 | ×1012/L | 3.80-5.10 |
| Hemoglobin | 98 | g/L | 115-150 |
| Platelet count | 163 | ×109/L | 125-350 |
| Lymphocyte proportion | 5.2 | % | 20.0-50.0 |
| Lymphocyte count | 0.43 | ×109/L | 1.10-3.20 |
| Alanine aminotransferase | 5 | U/L | 7-40 |
| Total bilirubin | 30.8 | µmol/L | 3.4-22.2 |
| Direct bilirubin | 10.8 | µmol/L | 0.0-3.4 |
| Indirect bilirubin | 20.0 | µmol/L | 3.4-18.8 |
| Serum sodium | 133.1 | mmol/L | 137.0-147.0 |
| Serum calcium | 1.88 | mmol/L | 2.10-2.60 |
| Cystatin C | 1.40 | mg/L | 0.51-1.09 |
| β-Hydroxybutyric acid | 0.56 | mmol/L | 0.02-0.30 |
| High-density lipoprotein cholesterol | 0.65 | mmol/L | 0.80-1.96 |
| Apolipoprotein A1 | 0.74 | g/L | 1.00-1.60 |
| Apolipoprotein E | 51.7 | mg/L | 27.0-49.0 |
| Prealbumin | 0.06 | g/L | 0.18-0.40 |
| Total protein | 48.2 | g/L | 65.0-85.0 |
| Albumin | 23.8 | g/L | 40.0-55.0 |
| Albumin–globulin ratio | 1.0 | 1.2-2.4 | |
| Phosphocreatine kinase | 20 | U/L | 26-174 |
| Lactate dehydrogenase | 538 | U/L | 108-252 |
| Highly sensitive C-reactive protein | 104.30 | mg/L | 0.00-3.00 |
| Cholinesterase | 2730 | U/L | 5300-12900 |
| Leucylaminopeptidase | 17.5 | U/L | 20.0-60.0 |
| Retinol binding protein | 15.0 | mg/L | 25.0-70.0 |
| Serum iron | 4.1 | µmol/L | 7.0-32.0 |
| Total iron binding capacity | 23.0 | µmol/L | 45.0-75.0 |
| Unsaturated iron binding capacity | 18.9 | µmol/L | 31.0-51.0 |
| Transferrin | 1.27 | g/L | 1.90-3.80 |
| Adenosine deaminase | 21.1 | U/L | 0.0-15.0 |
| Superoxide dismutase | 94 | U/mL | 129-216 |
| Carcinoembryonic antigen | 1.5 | ng/mL | ≤ 5 |
| Alpha-fetoprotein | 1.6 | ng/mL | ≤ 25 |
| Carbohydrate antigen 72-4 | 2.8 | U/mL | ≤ 7 |
| Carbohydrate antigen 125 | 109.3 | U/mL | ≤ 35 |
| Carbohydrate antigen 19-9 | 40.5 | U/mL | ≤ 34 |
Hypertensive heart disease was diagnosed by color sonography. Chest radiography showed no evidence of metastasis in the lung. Contrast-enhanced computed tomography revealed bowel wall thickening with contrast enhancement at the cecum, but no lymph node or organ metastasis and a negative scan of the liver and spleen. Colonoscopy revealed a tumor of the ascending colon, which was confirmed as adenocarcinoma on biopsy.
The patient underwent laparoscopic radical resection of right colon cancer with lymph node dissection.
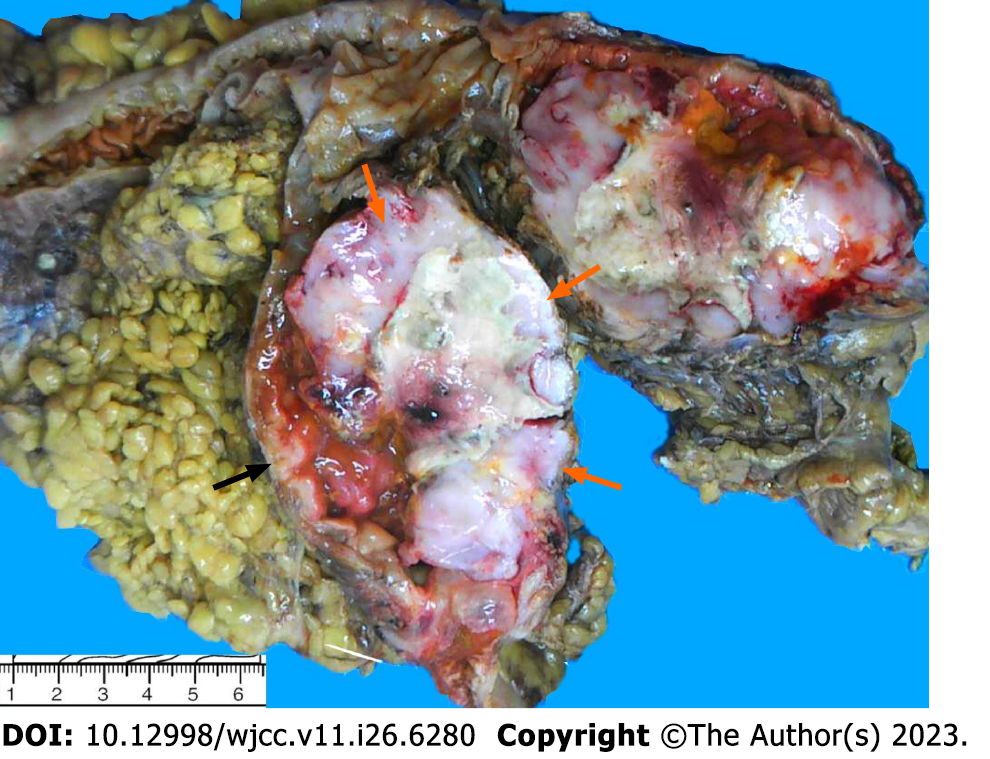
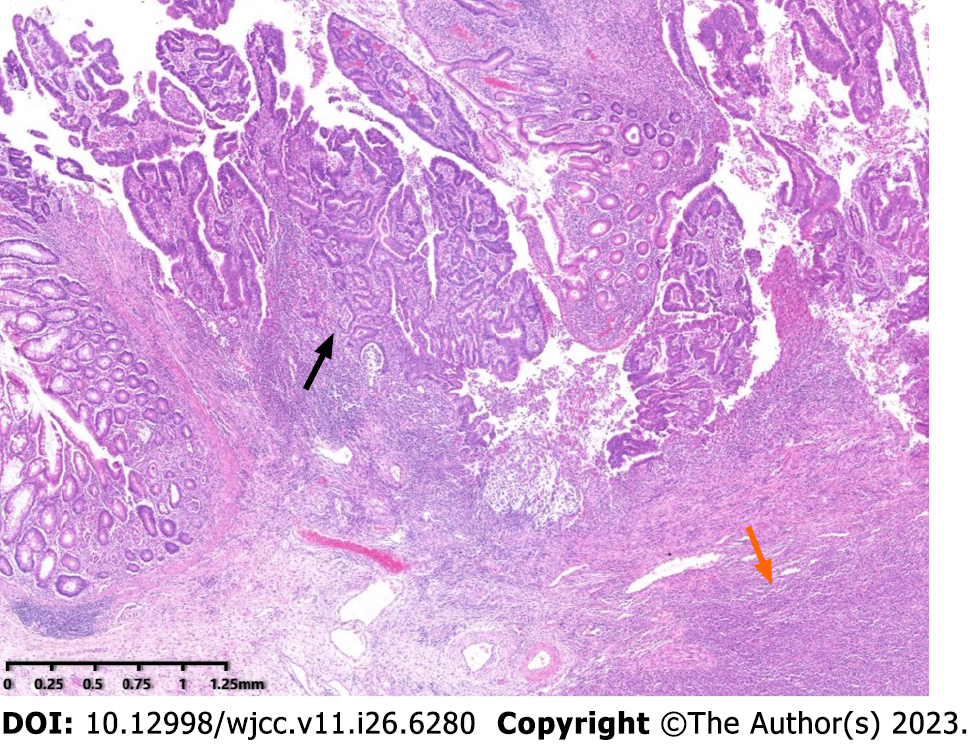
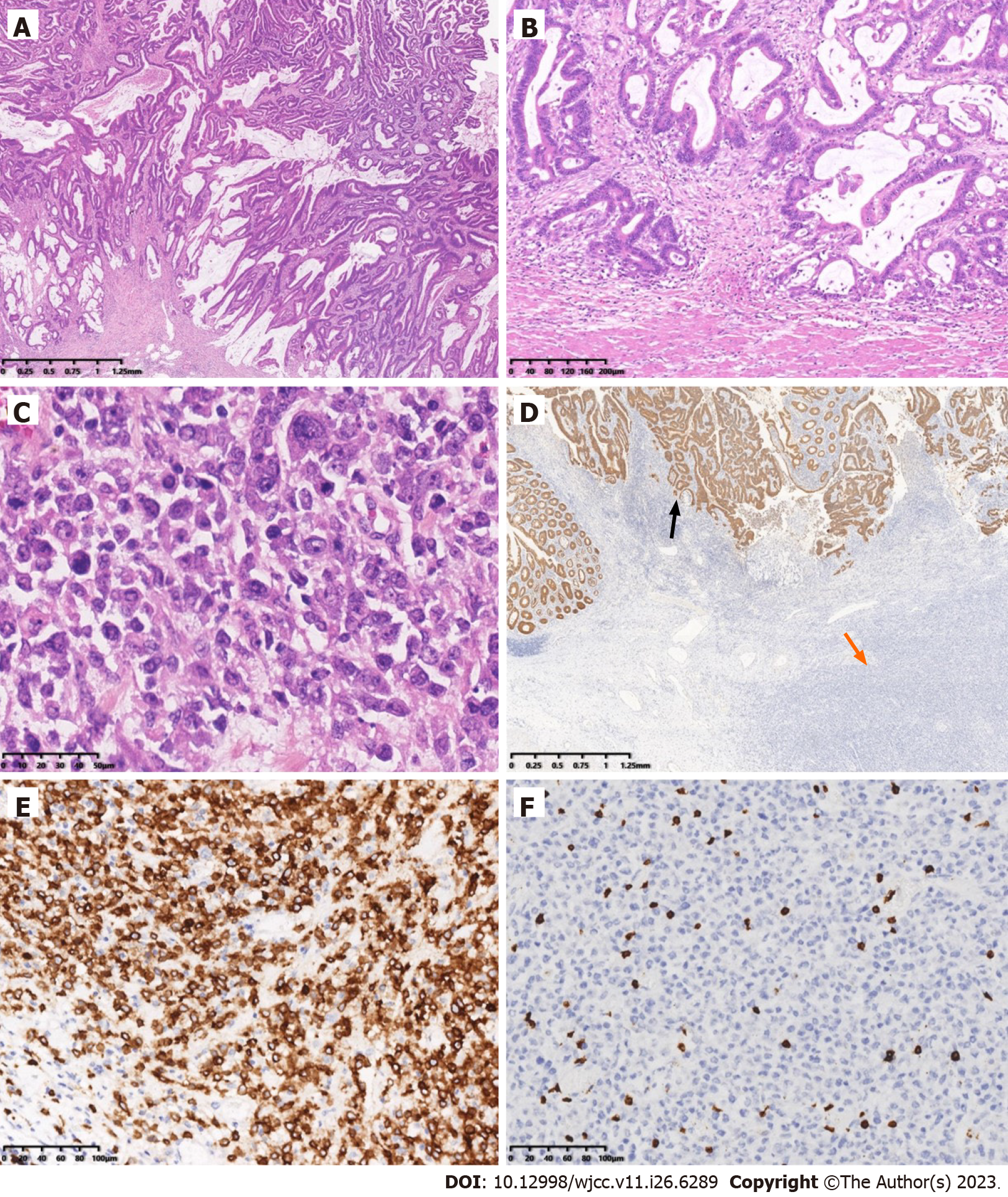
Pathological analysis was performed on a formalin-fixed section of the intestine with omentum (including ileum, cecum and ascending colon). The lengths of the three portions were 6 cm, 11 cm and 22 cm, respectively; and the circumferences were 2.5 cm, 8 cm and 6 cm. A circumferential ulcerative mass a size of 11 cm × 7.5 cm × 10 cm was seen on the cecal mucosa adjacent to the ileocecal valve (Figure 1). Macroscopically, the mass appeared to consist of two tumors. The cut surface of the mass with crater-like appearance was gray, soft, fish-like, with a necrotic-appearing central area, whereas the remaining polypoid part of the mass was hard and grayish-white. A cross-section demonstrated that these two tumors formed a union, and it was impossible to determine grossly the borderline of the two tumors. Adjacent to the tumor, a 0.6-cm polypoid mass was found in the ascending colon. The appendix was 5 cm long, 0.8 cm in diameter, and the volume of the omentum was 15 cm × 15 cm × 1.5 cm. Nine lymph nodes varying in size from 0.1 cm to 1.2 cm were isolated from the specimen. Microscopy showed that the tumor had two components that were adjacent to each other but independent (Figure 2). One component was a moderately differentiated adenocarcinoma with a mucinous component arising in a tubulovillous adenoma (Figure 3A), which invaded the muscularis propria with pushing borders (Figure 3B). The other component was distributed from the mucosal lamina to the subserous layer. The medium-to-large lymphocytes grew diffusely, with uniform morphology, frequent mitosis, and obvious atypia (Figure 3C). There was no intramural and extramural vascular, lymphatic or perineural invasion. The surgical cut margins and the appendix were free of tumors and all lymph nodes lacked tumor dissemination. Histological examination of the 0.6-cm polypoid mass in the ascending colon showed a tubular adenoma.
Immunohistochemistry revealed that the adenocarcinoma strongly expressed cytokeratin (CK) (Figure 3D), CK20, caudalrelated homeobox transcription factor 2 and mutS homolog 6, but was negative for mutS homolog 2, MLH1 and postmeiotic segregation increased 2. Immunohistochemistry of the atypical lymphocytes was strongly and diffusely positive for paired-box 5, cluster of differentiation 20 (CD20) (Figure 3E), CD79, CD10, B-cell lymphoma (Bcl)-6, multiple myeloma oncogene-1 and lambda, and negative for kappa, Bcl2, cyclinD1, CD5, CD21, CD23, CD38, CD138, CD3 (Figure 3F), CD56, anaplastic lymphoma kinase, terminal deoxynucleotidyl transferase and CK. The MIB-1 labeling index was approximately 80%. Expression of C-myc was detected in 30% of these atypical lymphocytes. P53-positive immunoreactivity was 90%. In situ hybridization was negative for Epstein–Barr virus (EBV)-encoded small RNA.
Mutational analysis of BRAF was restricted to the V600E alteration. BRAF mutations were detected from formalin-fixed paraffin-embedded sections using the Snapshot mutation detection platform. MLH1 promoter methylation was performed by quantitative real-time polymerase chain reaction. Adenocarcinoma had BRAF mutation and MLH1 promoter methylation. IgH gene rearrangement study showed that the B-cell rearrangement was positive (polymerase chain reaction + fragment analysis). Fluorescence in situ hybridization of the atypical lymphocytes identified no translocations of IGH/BCL2, IGH/MYC, IGH/CCND1 and no break-apart rearrangement of BCL6. TP53 mutations were found by the AmpliChip p53 Research Test (Roche Molecular Systems), which is a microarray-based assay that detects mutations in exons 2–11.
The morphological, immunohistochemical and molecular pathological findings confirmed the diagnosis of collision tumor of primary DLBCL, NOS with GCB subtype and TP53 mutation, and adenocarcinoma arising in a tubulovillous adenoma in the colon, with BRAF mutation and MLH1 promoter methylation. The colon adenocarcinoma was staged as T2N0M0 according to the American Joint Committee on Cancer Tumor–Node–Metastasis staging system[25]. Postoperative positron emission tomography–computed tomography scan revealed that there was no metastasis to the lymphoid system or bone marrow, which was confirmed by a bone marrow biopsy. DLBCL was stage IE with bulky disease, according to the revised staging system for malignant lymphoma based on the Lugano classification[26,27]. The Eastern Cooperative Oncology Group performance status[28] of the patient was 2. The International Prognostic Index (IPI) of the National Comprehensive Cancer Network[29] score was 5, which indicated that the patient was in the high–intermediate risk of survival group.
The patient underwent laparoscopic radical resection of right colon cancer with lymph node dissection.
The symptoms rapidly progressed and the patient died 3 mo after the initial diagnosis of collision tumor of primary malignant lymphoma and adenocarcinoma in the colon.
There are few reports of colonic adenocarcinoma in collision with primary colorectal lymphoma[3-24]. The clinicopathological characteristics of the present case and 22 previous cases with collision tumors of primary colorectal lymphoma and adenocarcinoma are reviewed.
This type of tumor mostly occurs in older people. The age of the patients ranged from 43 to 86 years, with a mean of 73 years. Most of the patients were > 60 years old (21/22) and the male-to-female ratio was 1.3 to 1. In all cases, anemia, multiple colon polyps, and inflammatory bowel disease were the most common diseases in past medical history. Most cases had symptoms associated with colorectal adenocarcinoma (20/23). The most common site of lesions was the cecum (7/23). The histological morphology of tumors could be roughly divided into three types[14]. Thirteen cases were in the most common type, which was carcinoma and lymphoma are in the same tumor and the two types of tumor cells are mixed and grow crosswise. Seven cases and our case were in the second type, with carcinoma and lymphoma in the same tumor and independently. Third, the two tumor components are in separate tumors, and the two tumors are adjacent or close to each other.
Most of the adenocarcinoma components were moderately differentiated (18/21), and there were two well-differentiated and one poorly differentiated adenocarcinoma. Among 16 patients with available histopathological staging of adenocarcinoma, there were seven, four, three, one and one in stages I, IIA, IIIB, IIIC and IV, respectively. Three cases showed adenocarcinoma arising in a villous adenoma. There was a large tubulovillous adenoma containing invasive adenocarcinoma in two cases. The most common histological subtypes of the lymphoma were B-cell lymphomas, such as DLBCL (9 cases), and only one case was peripheral T-cell lymphoma. Among 20 patients with an available histopathological staging of lymphoma, there were seven, three and 10 in stages I, II and IV, respectively. Sixteen of 19 patients had an intermediate or high-risk IPI score, and three had a low or intermediate-risk IPI score[28-31]. Thus, > 50% of cases were stage IV and 84.2% of cases had intermediate or high-risk IPI scores in lymphoma components.
There were 15 cases with follow-up data, including 11 alive and four dead (3 died of malignant lymphoma and 1 of nontumor causes), and survival time ranged from 1 to 48 mo. Three-year overall survival rate was 71.5%. According to Kaplan–Meier survival analysis, the overall survival rate of patients who underwent surgery combined with chemotherapy or radiotherapy was higher than that of surgery alone, although the difference was not significant due to the small sample size (0.8333 vs 0.514). This suggested that adjuvant radiotherapy or chemotherapy combined with surgery had survival benefits. Seven patients underwent surgery alone and six underwent surgery in combination with chemotherapy [4 R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone); 1 FOLFOX (folinic acid, fluorouracil and oxaliplatin) to R-CHOP; 1 FOLFOX]; two received surgery in combination with chemotherapy and radiotherapy (1 R-CHOP; 1 R-CHOP to FOLFOX); and five received R-CHOP chemotherapy only, among eight patients who received chemotherapy. Among three patients who died from malignant lymphoma progression, two underwent surgery alone and one received surgery combined with chemotherapy (FOLOFX and R-CHOP). Whether lymphoma was a preference factor in the patient’s prognosis remains unclear, due to the lack of large sample data[6].
Collision colorectal adenocarcinoma and lymphoma are rare and only a few cases have been reported in the literature[3-24]. Although rectal magnetic resonance imaging could detect different morphology and signal intensity in each tumor, pathological identification of the two components is the only way to make the correct diagnosis[4,32]. Pathologists should be aware of the existence of collision tumors for two reasons[4,8]. The first reason is that the presence of lymphocytes in the vicinity of colonic carcinomas is common, but these cells are not always reactive. A different appearance from that of the other on gross resection specimen and/or an extensive or monotonous infiltrate should alert pathologists to carefully assess its morphology, immunophenotype and clonality to confirm or exclude a coexisting lymphoma. The second reason is any effacement of the architecture of lymph nodes from a resection specimen with a known colonic carcinoma needs a closer look to exclude the possibility of an underlying lymphoma.
Primary colonic lymphoma and adenocarcinoma might be attributed to the advanced age of the patient[8]. The development of DLBCL in one case with a medical history of rheumatoid arthritis was determined to be driven by EBV, with immunosuppression being the underlying cause[24].
Microsatellite instability (MSI) drives one of the key mechanisms of oncogenesis in CRC and the presence of MSI is important in two main scenarios[33]. Firstly, in BRAF wild-type cases, MSI confers a good prognosis, and CRCs that are microsatellite stable in the context of BRAF mutation usually have poor prognosis. The present case with BRAF mutation and mismatch repair (MMR) had survival time of only 3 mo. Drugs targeting the BRAF gene may bring hope for the treatment of such collision tumors. Secondly, the presence of MSI is important in the context of cancer immunotherapy. More recently, studies have reported significant responses of MSI cancers (CRCs and others) to programmed death ligand 1 inhibitor in patients who failed conventional therapy[34,35]. In the large intestine, the most common type of lymphoma is DLBCL (> 50%), followed by extranodal marginal zone lymphoma, follicular lymphoma, mantle cell lymphoma and Burkitt’s lymphoma. The incidence of large intestinal lymphoma has increased over the years, attributable to acquired or iatrogenic immunodeficiency[36]. Patients with immunodeficiency are more prone to develop lymphoma. Although the influence of one cancer on the other is largely unknown, certain proneoplastic cytokines that are released by tumor cells have paracrine activities[18]. One could envision a situation whereby one tumor type is secreting transforming growth factor b, not only cloaking the source tumors but also any adjacent disparate tumors. One tumor type might benefit from its collision counterpart via the stimulation of angiogenesis[18]. The loss of MMR and resulting MSI are contributing factors to this association between carcinoma and lymphoid infiltration. The presence of lymphoma may help developing adenocarcinoma evade the immune system[20]. Therefore, immunotherapy with MMR benefits both adenocarcinoma and lymphoma. EBV is an established risk factor for lymphoma and may also increase the risk of adenocarcinoma[7,24].
A therapeutic dilemma exists in deciding the course of treatment in patients with collision adenocarcinoma and lymphoma[5]. There is agreement that surgical treatment alone is effective for localized disease, while combined chemotherapy is the mainstay for disseminated disease. The treatment of collision tumors requires simultaneous treatment of the two tumor components; mainly surgical resection combined with radiotherapy and chemotherapy[15]. For the adenocarcinoma component, if it is well-differentiated, there is no need to perform radiotherapy and chemo
Based on pathological examination and genetic analysis, surgery combined with dose-adjusted chemotherapy or chemoradiotherapy may have good therapeutic effects for collision tumor of lymphoma–adenocarcinoma, but additional case studies are needed for confirmation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alkhatib AJ, Jordan; Arumugam VA, India S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73:233-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1532] [Reference Citation Analysis (3)] |
| 2. | WHO Classification of Tumours Editorial Board. Digestive system tumours. WHO Classification of Tumors. 5th ed, vol. 1. Lyon: IARC press; 2019. |
| 3. | Mannweiler S, Dinges HP, Beham-Schmid C, Hauser H, Starlinger M, Regauer S. Colliding / concomitant tumors of the intestine: report of 3 cases. Pathol Oncol Res. 2003;9:188-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Sahasrabudhe N, Khirwadkar N, Prescott R. Synchronous adenocarcinoma and marginal zone B-cell lymphoma of the colon: A case report. Diagnostic Histopathology. 2009;15:318-322. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Devi P, Pattanayak L, Samantaray S. Synchronous adenocarcinoma and mucosa-associated lymphoid tissue lymphoma of the colon. Saudi J Gastroenterol. 2011;17:69-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Shigeno T, Fujimori K, Tsuruta F, Nozawa Y, Nagaya T, Maejima T. Ileocecal collision tumor composed of adenocarcinoma and primary malignant lymphoma. Clin J Gastroenterol. 2011;4:79-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Chang H, Chuang WY, Shih LY, Tang TC. Collision in the colon: concurrent adenocarcinoma and diffuse large B-cell lymphoma in the same tumour. Acta Clin Belg. 2011;66:302-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 8. | Argyropoulos T, Foukas P, Kefala M, Xylardistos P, Papageorgiou S, Machairas N, Boltetsou E, Machairas A, Panayiotides IG. Simultaneous occurrence of colonic adenocarcinoma and MALT lymphoma: A series of three cases. World J Gastrointest Oncol. 2012;4:89-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Lin HH, Jiang JK, Lin JK. Collision tumor of low-grade B-cell lymphoma and adenocarcinoma with tuberculosis in the colon: a case report and literature review. World J Surg Oncol. 2014;12:147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Sathya G, Ramkumar G, Ganesh P, Jeevan Kumar S. Collision tumour of sigmoid colon--a rare presentation. Trop Gastroenterol. 2014;35:120-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Velu AR, Srinivasamurthy BC, Nagarajan K, Sinduja I. Colonic adenocarcinoma, mucosa associated lymphoid tissue lymphoma and tuberculosis in a segment of colon: A case report. World J Gastrointest Oncol. 2014;6:377-380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Kus T, Aktas G, Kalender ME, Sari I, Ulker E, Camci C. Collision tumor consisting of primary follicular lymphoma and adenocarcinoma in the cecum: A case report and literature review. Oncol Lett. 2016;11:2801-2805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Soto AR, Vazquez EG, Grigg-Gutierrez NM, Magno-Pagatzaurtundua P, Cáceres W, Toro DH. Conundrum of a Large Bowel Neoplasm: Collision Tumor. ACG Case Rep J. 2018;5:e13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Kim SH. Collsion Tumor of Adenocarcinoma and Diffuse Large B-cell Lymphoma in the Rectum: a Case Report and Literature Review. Investing Magn Reson Imaging. 2019;23:374-380. [DOI] [Full Text] |
| 15. | Bao L, Feng X, Wang T, Xing F. Collision tumor of carcinoma and lymphoma in the cecum: case report and review of literature. Int J Clin Exp Pathol. 2020;13:2907-2915. [PubMed] |
| 16. | Kataoka J, Nitta T, Ota M, Fujii K, Ishii M, Senpuku S, Ueda Y, Tsuchimoto Y, Takeshita A, Miyatake J, Ishibashi T. Collision Tumor Comprising Primary Malignant Lymphoma and Adenocarcinoma in the Ascending Colon. Case Rep Gastroenterol. 2021;15:379-388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Lin YS, Hamilton AER, Henderson C, Farzin M. Tiny but Mighty: Collision Tumor of a Superficial Adenocarcinoma Arising From a Tubulovillous Adenoma with Associated Low-Grade Follicular Lymphoma. Int J Surg Pathol. 2021;29:759-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Schep D, Van Koughnett JA, Velker V, Correa RJM. Synchronous colonic B cell lymphoma and adenocarcinoma in an elderly patient treated with R-mini-CHOP followed by resection. BMJ Case Rep. 2022;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 19. | Zapata Palomino M, Caicedo-Holguín I, Pardo S, Mera AT, Gómez AP, Zorrilla JO. Case report: Tumor collision in the colon, adenocarcinoma - lymphoma. Int J Surg Case Rep. 2022;98:107573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Hopster D, Smith PA, Nash JR, Elders K, Poston GJ. Synchronous multiple lymphomatous polyposis and adenocarcinomata in the large bowel. Postgrad Med J. 1995;71:443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Padmanabhan V, Trainer TD. Synchronous adenocarcinoma and mantle cell lymphoma of the colon. Arch Pathol Lab Med. 2003;127:E64-E66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Eshra A, Al-Hendal A, Al Enezi M, Al-Mishaan M, Abo Dief W. One patient, two lymphomas, three primaries. Gulf J Oncolog. 2010;39-43. [PubMed] |
| 23. | Sasaki S, Hatanaka K, Sahara N, Uekusa T, Hirayama K, Shirahata A, Ishimaru M. Collision tumor of primary malignant lymphoma and adenocarcinoma in the colon: report of a case. Surg Today. 2010;40:975-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Yu JR, Huang Q, Hou P, Lai JP. Collision tumor of colonic adenocarcinoma and EBV-driven large B-cell lymphoma: a case report and review of literature. Cancer Treatment Communications. 2015;3:7-12. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. American Joint Committee on Cancer (AJCC) Cancer Staging Manual. 7th edition. Springer, New York, NY, 010 pp143. |
| 26. | Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA; Alliance, Australasian Leukaemia and Lymphoma Group; Eastern Cooperative Oncology Group; European Mantle Cell Lymphoma Consortium; Italian Lymphoma Foundation; European Organisation for Research; Treatment of Cancer/Dutch Hemato-Oncology Group; Grupo Español de Médula Ósea; German High-Grade Lymphoma Study Group; German Hodgkin's Study Group; Japanese Lymphorra Study Group; Lymphoma Study Association; NCIC Clinical Trials Group; Nordic Lymphoma Study Group; Southwest Oncology Group; United Kingdom National Cancer Research Institute. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059-3068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2850] [Cited by in RCA: 3852] [Article Influence: 350.2] [Reference Citation Analysis (0)] |
| 27. | Ricard F, Cheson B, Barrington S, Trotman J, Schmid A, Brueggenwerth G, Salles G, Schwartz L, Goldmacher G, Jarecha R, Narang J, Broussais F, Galette P, Liu M, Bajpai S, Perlman E, Gillis J, Smalberg I, Terve P, Zahlmann G, Korn R. Application of the Lugano Classification for Initial Evaluation, Staging, and Response Assessment of Hodgkin and Non-Hodgkin Lymphoma: The PRoLoG Consensus Initiative (Part 1-Clinical). J Nucl Med. 2023;64:102-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 28. | International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993;329:987-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3971] [Cited by in RCA: 4196] [Article Influence: 131.1] [Reference Citation Analysis (0)] |
| 29. | Zhou Z, Sehn LH, Rademaker AW, Gordon LI, Lacasce AS, Crosby-Thompson A, Vanderplas A, Zelenetz AD, Abel GA, Rodriguez MA, Nademanee A, Kaminski MS, Czuczman MS, Millenson M, Niland J, Gascoyne RD, Connors JM, Friedberg JW, Winter JN. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014;123:837-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 654] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 30. | Thieblemont C, Cascione L, Conconi A, Kiesewetter B, Raderer M, Gaidano G, Martelli M, Laszlo D, Coiffier B, Lopez Guillermo A, Torri V, Cavalli F, Johnson PW, Zucca E. A MALT lymphoma prognostic index. Blood. 2017;130:1409-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 136] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 31. | Solal-Céligny P, Roy P, Colombat P, White J, Armitage JO, Arranz-Saez R, Au WY, Bellei M, Brice P, Caballero D, Coiffier B, Conde-Garcia E, Doyen C, Federico M, Fisher RI, Garcia-Conde JF, Guglielmi C, Hagenbeek A, Haïoun C, LeBlanc M, Lister AT, Lopez-Guillermo A, McLaughlin P, Milpied N, Morel P, Mounier N, Proctor SJ, Rohatiner A, Smith P, Soubeyran P, Tilly H, Vitolo U, Zinzani PL, Zucca E, Montserrat E. Follicular lymphoma international prognostic index. Blood. 2004;104:1258-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1289] [Cited by in RCA: 1294] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 32. | Zhao X, Zhang G, Li CH. Collision carcinoma of the rectum involving neuroendocrine carcinoma and adenocarcinoma: A case report. World J Clin Cases. 2021;9:4789-4796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 33. | Dudley JC, Lin MT, Le DT, Eshleman JR. Microsatellite Instability as a Biomarker for PD-1 Blockade. Clin Cancer Res. 2016;22:813-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 671] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 34. | Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA Jr. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3799] [Cited by in RCA: 4925] [Article Influence: 615.6] [Reference Citation Analysis (0)] |
| 35. | Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509-2520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6096] [Cited by in RCA: 7218] [Article Influence: 721.8] [Reference Citation Analysis (0)] |
| 36. | Gustafsson BI, Siddique L, Chan A, Dong M, Drozdov I, Kidd M, Modlin IM. Uncommon cancers of the small intestine, appendix and colon: an analysis of SEER 1973-2004, and current diagnosis and therapy. Int J Oncol. 2008;33:1121-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |