Published online Sep 16, 2023. doi: 10.12998/wjcc.v11.i26.6159
Peer-review started: April 7, 2023
First decision: July 3, 2023
Revised: July 12, 2023
Accepted: July 25, 2023
Article in press: July 25, 2023
Published online: September 16, 2023
Processing time: 153 Days and 20 Hours
Villous adenoma is a rare tumor in the urinary system that usually occurs in the bladder. It is extremely uncommon in the renal pelvis. Most of the previously reported cases have been diagnosed with severe hydronephrosis associated with renal parenchyma atrophy prior to surgery. Because of its rarity, available information on the pathogenesis, diagnosis, treatment and prognosis of the disease is limited. We reported a case of kidney stones with hydronephrosis. During percutaneous nephroscopic lithotripsy, a renal pelvis tumor was found. Biopsy confirmed that the tumor was a villous adenoma of the renal pelvis.
A 68-year-old female was admitted to the hospital due to right kidney stones with right hydronephrosis. After admission, a urinary system plain computed tomography scan was performed, which revealed right kidney stones with right hydronephrosis and right upper ureteral dilatation. Multiple new cauliflower-like papillary masses were then discovered in the renal pelvis and calyces during right percutaneous nephroscopic lithotripsy. Biopsy results indicated villous adenoma with high-grade glandular intraepithelial neoplasia. The patient underwent laparoscopic radical resection of the right kidney and ureter. Based on histopathological and immunohistochemical examination, the patient was diagnosed with villous adenoma without adenocarcinoma.
Villous adenoma is rare in the urinary system. We reported a case of renal pelvis villous adenoma, which may provide useful information for the early diagnosis and treatment of this tumor.
Core Tip: Villous adenoma is a rare tumor in the urinary system. We reported a patient who was admitted to the hospital due to right kidney stones with right hydronephrosis. Biopsy indicated a villous adenoma with high-grade glandular intraepithelial neoplasia after right percutaneous nephroscopic lithotripsy. The patient underwent laparoscopic radical resection of the right kidney and ureter. Based on histopathological and immunohistochemical results, the patient was diagnosed with villous adenoma without adenocarcinoma.
- Citation: Li LL, Song PX, Xing DF, Liu K. Early diagnosis of renal pelvis villous adenoma: A case report. World J Clin Cases 2023; 11(26): 6159-6164
- URL: https://www.wjgnet.com/2307-8960/full/v11/i26/6159.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i26.6159
Villous adenoma is characterized by mucous glandular neoplastic cells appearing in a sessile papillary arrangement. It is commonly seen in the gastrointestinal tract, especially in the colon and rectum. It is worth noting that villous adenoma is uncommon in the urinary tract. They can occur in the bladder, urachus, ureter and urethra. The typical clinical presentations are hematuria, irritative voiding symptoms and mucinous urine[1,2]. Villous adenoma is rare in the renal pelvis[3]. We here report the early diagnosis of a case of renal pelvis villous adenoma, which has not previously been reported.
A 68-year-old female complained of pain and discomfort in her right back for 10 d beginning on December 2, 2020.
Her symptoms were aggravated after anti-inflammatory treatment at a local hospital.
The patient had right kidney stones with hydronephrosis for 5 years.
The patient denied any family history of related conditions.
Physical examination revealed no obvious percussion pain in bilateral renal regions or obvious tenderness in bilateral ureteral regions.
A radionuclide renogram showed that the left and right renal glomerular filtration rates were 57.8 and 15.6 mL/min, respectively. Serum carcinoembryonic antigen (CEA) was 25.43 ng/mL and serum carbohydrate antigen 19-9 (CA 19-9) was 69.29 U/mL.
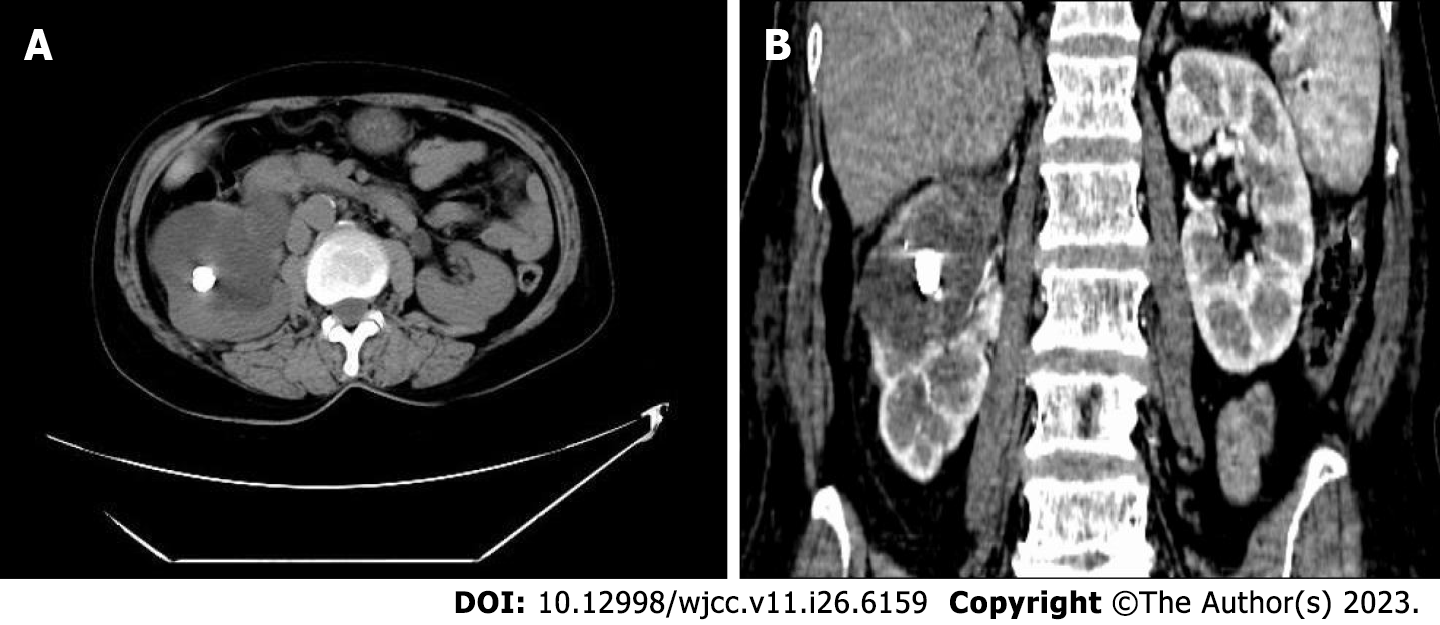
Urinary system computed tomography (CT) scan revealed right kidney stones with right hydronephrosis and right upper ureteral dilatation (Figure 1). Contrast-enhanced CT scan of the entire abdominal pelvic cavity showed no obvious suspicious space-occupying lesions in the right renal pelvis or obvious abnormalities in the gastrointestinal tract.
After right ureteroscopy, a right percutaneous nephroscopic lithotripsy (PNL) was performed. A large amount of turbid gelatinous effusion was found in the upper ureter and pelvis, and multiple new cauliflower-like papillary masses were discovered in the renal pelvis and calyces. A biopsy was performed. The biopsy results indicated a villous adenoma with high-grade glandular intraepithelial neoplasia (Figure 2).
Differential diagnoses showed a urothelial carcinoma of the renal pelvis, squamous cell carcinoma of the renal pelvis, and inflammatory polyp of the renal pelvis.
Based on the results of the biopsy and pathological examination, the final diagnosis was renal pelvis villous adenoma.
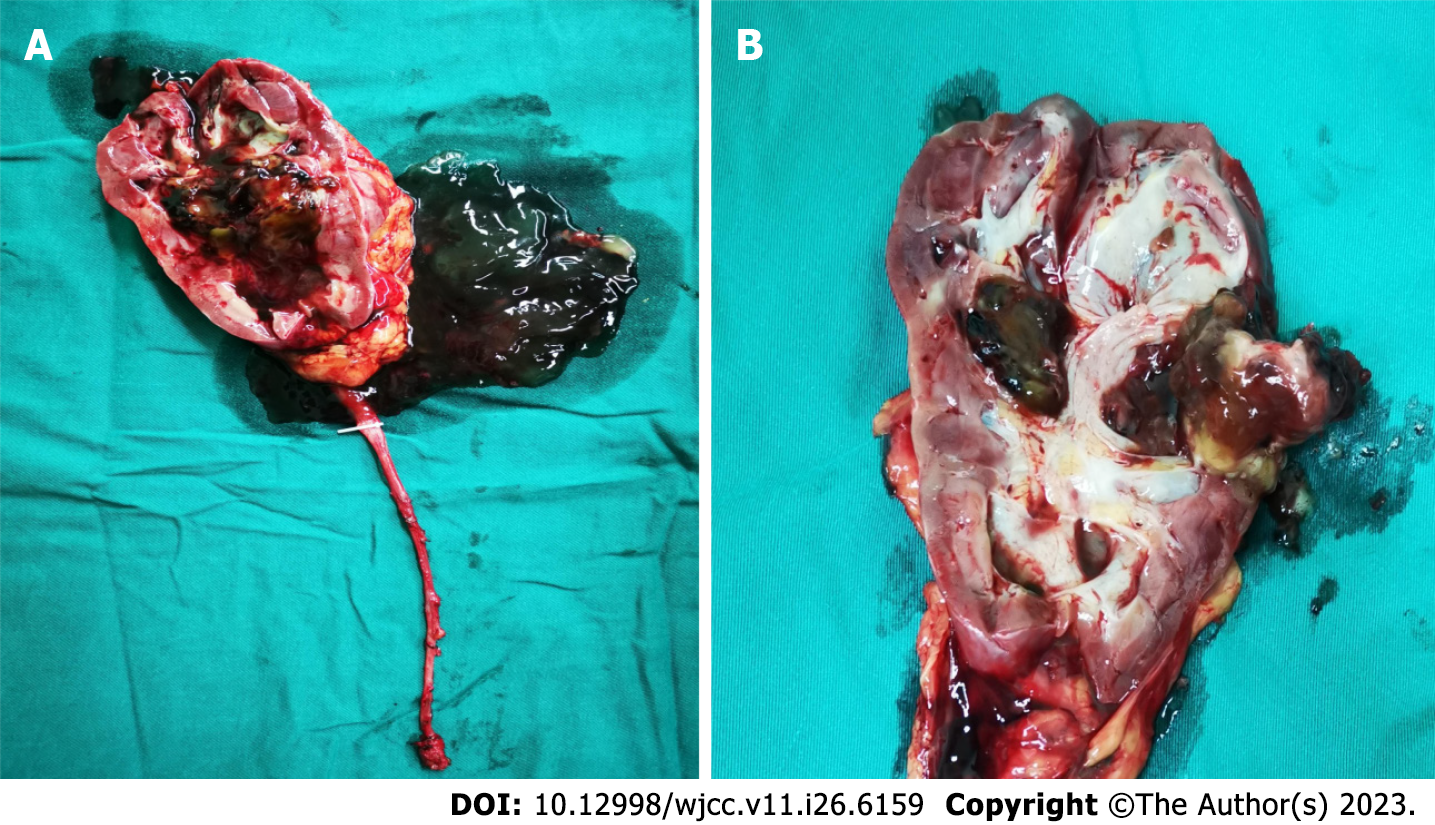
The patient underwent laparoscopic radical resection of the right kidney and ureter. In brief, the ureter was clamped in advance to prevent tumor dissemination, and an enlarged lymph node at the renal hilus was removed. After surgery, we found that the right ureter and pelvis were enlarged and filled with a large amount of mucinous effusion. Obvious enlargement was observed in the upper segment, and a large amount of gelatinous mucinous effusion was found in the dissected specimen. A solid brown mass approximately 2.5 cm 2.0 cm in size was found on the dorsal side of the kidney, and a stone was wrapped inside. In addition, the mass, which was convex to the renal pelvis, was covered with necrotic tissue, but this was not obvious in the papillary mass, residual renal parenchyma, calyx or ureter (Figure 3). According to the histopathological and immunohistochemical results, the patient was diagnosed with villous adenoma without adenocarcinoma (Figure 4).
The patient recovered well without any discomfort. At the 1-mo follow-up, serum CEA and CA 19-9 decreased to 2.05 ng/mL and 21.23 U/mL, respectively. At the 1-year follow-up, serum CEA and CA 19-9 were 1.80 ng/mL and 19.85 U/mL, respectively, and no significant abnormalities were observed on urinary system CT.
The renal pelvis villous adenoma in our patient was found relatively early. The CT scan showed dilation of the renal pelvis and upper ureter, with no obvious obstruction in the ureter. All previously reported patients initially underwent non-functional nephrectomy, and villous adenoma was found in the postoperative pathological examination. Possible tumors cannot be identified by preoperative CT or other related examinations[4,5]. After admission, the CT for our case indicated favorable function in the right kidney; therefore PNL was scheduled. During the operation, a large number of new papillary masses were discovered in the renal collecting system and the upper ureter, which were similar in appearance to common urothelial tumors. Notably, we first discovered the appearance of renal pelvic villous adenoma under endoscopy and successfully diagnosed it early through biopsy, which has not been reported before.
Urinary calculi are frequently seen, but villous adenoma in the urinary system is very uncommon. Furthermore, the incidence of renal pelvis adenoma is extremely low. As in our patient, previously reported cases showed that most of the kidneys were complicated with stones, which had been present for a few years. Consequently, we considered that the development of renal pelvis villous adenoma might be associated with long-term stone stimulation. It is generally believed that renal pelvis villous adenoma is caused by long-term stimulation of stones and chronic inflammation[6]. The long-term stimulation of stones and associated infections will cause injury to the urinary tract epithelium, which stimulates the regeneration and repair of urinary tract epithelium. Intestinal metaplasia occurs during the process of repeated repair, and later the heterogeneity increases step by step. These processes may gradually result in villous adenoma and advanced intraepithelial neoplasia or even further malignant development of mucinous adenocarcinoma[7].
Due to the nonspecific clinical and imaging findings, the diagnosis of renal pelvis villous adenoma before surgical treatment is challenging. All previously reported cases were diagnosed by pathological examination after nephrectomy[8-12]. Unfortunately, our patient also required a right nephrectomy due to concerns about the complicating mucinous adenocarcinoma. We envision that percutaneous laser resection of renal pelvis villous adenoma may be attempted in future cases, especially in isolated kidney patients, which would preserve good renal function.
According to previous reports, most patients have urinary tract infections and kidney stones. Moreover, some patients may also have fever, low back pain, abdominal discomfort and weight loss, and a small portion may have mucinous urine[10,11]. However, no obvious specificity was found in these patients. Plain or enhanced CT scans usually only show renal calculi with renal effusion, but the exact tumor tissue cannot be identified. There is no obvious causality of the effusion and obstruction caused by renal calculi. Therefore, constant vigilance is necessary in patients whose stones do not easily cause severe obstruction or hydronephrosis.
In our case, ureteroscopy was initially attempted. During the operation, a large amount of mucinous urine was found in the upper segment of the ureter, but no obvious stenosis or other obstruction was found. This situation might be interpreted as the upper ureter and pelvis calyces were narrowed due to mucinous urine with high viscosity that was not eliminated, resulting in hydronephrosis. Subsequently, PNL was performed, during which a large number of papillary space-occupying tissues were found around the stone. They were similar in appearance to common urothelial tumors. Pathological examination showed that it was villous adenoma. After the exclusion of metastatic villous adenoma, no suspicious tumor tissue was found on abdominal and pelvic cavity CT scanning or gastroenteroscopy. All the results indicated that it was a primary renal pelvis villous adenoma. Therefore, ureteroscopy and percutaneous nephroscopy play important roles in the early diagnosis of suspicious masses.
Moreover, relevant tumor indicators in the patient were reduced after the operation. Serum CEA was 25.43 ng/mL and serum CA 19-9 was 69.29 U/mL before surgery, which decreased to 2.67 ng/mL and 30.63 U/mL, respectively, 1 wk after the operation. According to the literature, CEA and CA 19-9 levels also increase in patients with renal pelvis mucinous tumors[13]. Therefore, for patients without related primary tumors, the levels of CEA and CA 19-9 have certain reference significance for preoperative diagnosis and can be used as reference indices during postoperative follow-up.
The incidence of villous adenoma of the renal pelvis is low, and early diagnosis and treatment are difficult. We reported the appearance and characteristics of a renal pelvis villous adenoma for the first time through PNL biopsy, which provides valuable data for the early diagnosis and treatment of this tumor. The relevant serum tumor markers have certain reference value for the diagnosis and follow-up of renal pelvis villous adenoma.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cabezuelo AS, Spain; Hasan A, Egypt S-Editor: Lin C L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Wang J, Manucha V. Villous Adenoma of the Urinary Bladder: A Brief Review of the Literature. Arch Pathol Lab Med. 2016;140:91-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Val-Bernal JF, Mayorga M, Garijo MF. Villous adenoma of the urinary tract: a lesion frequently associated with malignancy. Hum Pathol. 2002;33:1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Hudson J, Arnason T, Merrimen JL, Lawen J. Intestinal type villous adenoma of the renal pelvis. Can Urol Assoc J. 2013;7:E138-E142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Bhat S, Chandran V. Villous adenoma of the renal pelvis and ureter. Indian J Urol. 2010;26:598-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Shih CM, Wu SC, Lee CC, Pan CC. Villous adenoma of the ureter with manifestation of mucus hydroureteronephrosis. J Chin Med Assoc. 2007;70:33-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Sagnotta A, Dente M, Socciarelli F, Cacchi C, Stoppacciaro A, Balducci G. Primary adenocarcinoma of the renal pelvis: histologic features of a stepwise process from intestinal hyperplasia to dysplasia in a patient with chronic renal abscess. Int J Surg Pathol. 2014;22:182-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Kaur G, Naik VR, Rahman MN. Mucinous adenocarcinoma of the renal pelvis associated with lithiasis and chronic gout. Singapore Med J. 2004;45:125-126. [PubMed] |
| 8. | Stoykov B, Kolev N, Dunev V, Genov P, Atanasov J, Mateva S. A rare case of huge villous adenoma of the renal pelvis deforming the abdominal wall. Urol Case Rep. 2020;31:101183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Dong C, Yang Y, Wu S, Chen G. Clinicopathological analysis of two cases with pelvis villous adenoma and review of relevant literature. J Cancer Res Ther. 2015;11:663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 10. | Rochio-Tafoya A, García-Muñiz JA, Flores-Gutiérrez JP, Gutiérrez-González A, Barboza-Quintana O, Garza-Guajardo R. Intestinal-type adenocarcinoma arising from a villous adenoma in the renal pelvis in the context of a kidney abscess: Case report. Urol Case Rep. 2023;46:102325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Karnjanawanichkul W, Tanthanuch M, Mitarnun W, Pripatnanont C. Renal pelvic villous adenoma presented with mucusuria: report of a case and literature review. Int J Urol. 2013;20:247-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Park S, Meng MV, Greenberg MS, Deng DY, Stoller ML. Muconephrosis. Urology. 2002;60:344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Ye YL, Bian J, Huang YP, Guo Y, Li ZX, Deng CH, Dai YP, Sun XZ. Primary mucinous adenocarcinoma of the renal pelvis with elevated CEA and CA19-9. Urol Int. 2011;87:484-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |