Published online Aug 26, 2023. doi: 10.12998/wjcc.v11.i24.5823
Peer-review started: June 13, 2023
First decision: July 7, 2023
Revised: July 16, 2023
Accepted: July 28, 2023
Article in press: July 28, 2023
Published online: August 26, 2023
Processing time: 73 Days and 0.2 Hours
This is the first report of an ROS1-CENPW fusion gene in pancreatic malignancies.
A 77-year-old woman with a pancreatic tumor and multiple liver metastases was admitted to our hospital. Genetic testing revealed the presence of the ROS1-CENPW fusion gene, a rare fusion gene that has not been previously reported in the field of pancreatic cancer. The patient received crizotinib plus AG (albumin paclitaxel plus gemcitabine) chemotherapy. After treatment, the patient’s condi
The ROS1-CENPW gene treatment regimen used in this case is an excellent treatment option that provides new hope for patients with advanced pancreatic cancer and similar genetic mutations. To date, owing to the rarity of the ROS1-CENPW fusion gene, our team has encountered only a single case. Therefore, the efficacy of crizotinib plus AG chemotherapy in patients with pancreatic acinar cell carcinoma harboring the ROS1-CENPW fusion gene requires further validation.
Core Tip:ROS1-CENPW is a novel fusion gene locus in pancreatic acinar cell carcinoma that has not been previously reported. We detected this rare fusion gene in a 77-year-old female patient with pancreatic acinar cell carcinoma. We adopted the treatment regimen of crizotinib combined with AG chemotherapy (gemcitabine 1000 mg/m2 plus albumin paclitaxel 125 mg/m2). A good therapeutic effect was achieved.
- Citation: Wang T, Shen YY. Rare ROS1-CENPW gene in pancreatic acinar cell carcinoma and the effect of crizotinib plus AG chemotherapy: A case report. World J Clin Cases 2023; 11(24): 5823-5829
- URL: https://www.wjgnet.com/2307-8960/full/v11/i24/5823.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i24.5823
Pancreatic cancer is a highly aggressive disease with insidious onset, rapid progression, and poor prognosis[1]. Its molecular features include genomic instability and a high oncogene and tumor suppressor gene mutation rate[2]. Pancreatic cancer has a unique tumor cell microenvironment and immunosuppressive properties[3,4]; therefore, chemotherapy alone is of little benefit due to cytotoxicity and drug resistance[5]. Personalized medical treatments targeting specific genes have attracted considerable attention due to their high efficacy and unique status in pancreatic cancer treatment[6]. Unfortunately, KRAS, CDKN2A, TP53, and SMAD4, highly expressed in pancreatic cancer, have not shown satisfactory results as clinical therapeutic targets[7,8].
Pancreatic acinar cell carcinoma (PACC) is a rare pancreatic tumor that secretes various exocrine enzymes such as trypsin and lipase. Compared to pancreatic tumors arising from the ductal epithelium, PACC accounts for less than 2% of all pancreatic tumors. Metastatic PACC is generally incurable[9]. Therefore, developing personalized treatment plans for individual patients has become a priority. The discovery and reporting of the ROS1-CENPW locus may present a new unique approach for the targeted therapy of pancreatic cancer.
Appetite loss, weight loss, fatigue, and abdominal pain for more than 1 year.
A 77-year-old woman presented to our hospital complaining of appetite loss, weight loss, fatigue, and abdominal pain accompanied by radiating shoulder pain. These symptoms had persisted for more than 1 year.
No previous history of hypertension, diabetes, cardiovascular and cerebrovascular diseases; No history of hepatitis, tuberculosis, malaria and other infectious diseases; No history of blood transfusion, trauma, surgery; No history of food and drug allergy.
The patient was born and raised in Jiaxing, Zhejiang; No history of smoking or drinking; No history of exposure to radioactive substances. No familial genetic history.
Physical examination revealed the following: height and weight, 150 cm and 42 kg, respectively; blood pressure, 139/69 mmHg; and pulse rate, 84 bpm. The abdomen was soft with tenderness in the upper part; no rebound tenderness, jaundice, or other abnormal signs were observed.
Tumor indicators (Table 1) showed that carbohydrate antigen (CA19-9) was 233.70 U/mL, alpha-fetoprotein was 0.97 ng/mL, carcinoembryonic antigen (CEA) was 58.81 ng/mL, and cancer antigen was 125 4.45 U/mL.
| Date | CEA (ng/mL) normal range: 0-5.00 | CA199 (U/mL) normal range: 0-37.00 | CA125 (U/mL) normal range: 0-35.00 | AFP (ng/mL) normal range: 0-20.00 |
| 3.30 | 58.81 | 233.70 | 4.45 | 0.97 |
| 5.8 | 48.69 | 133.80 | 9.24 | 3.18 |
| 5.24 | 38.16 | 245.10 | 5.15 | 1.01 |
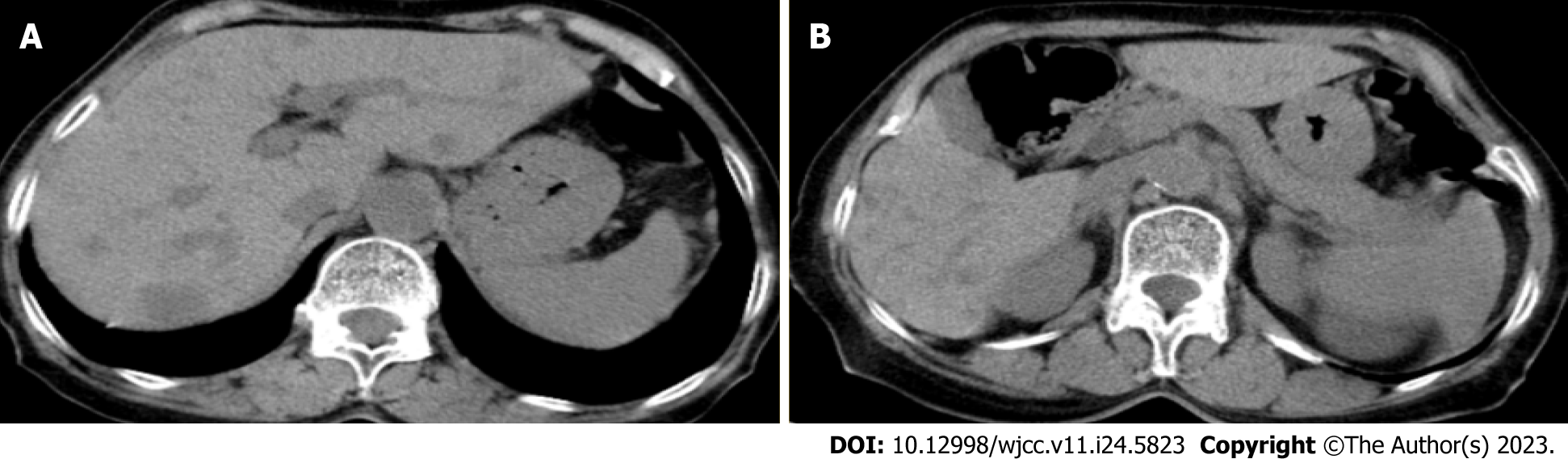
Imaging results from Jiaxing No. 1 Hospital indicated multiple lesions in the liver and a tumor in the pancreatic tail (Figure 1).
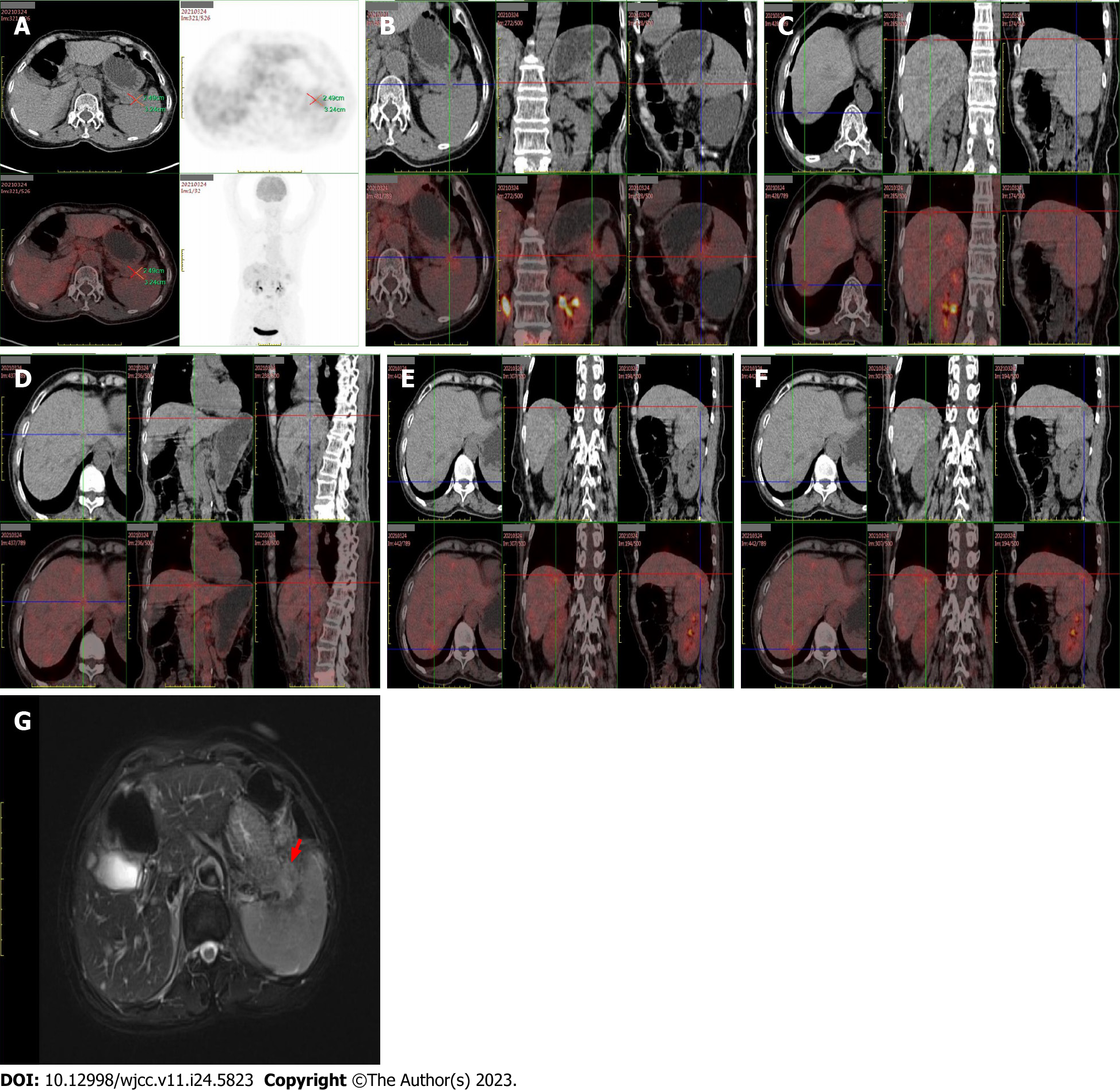
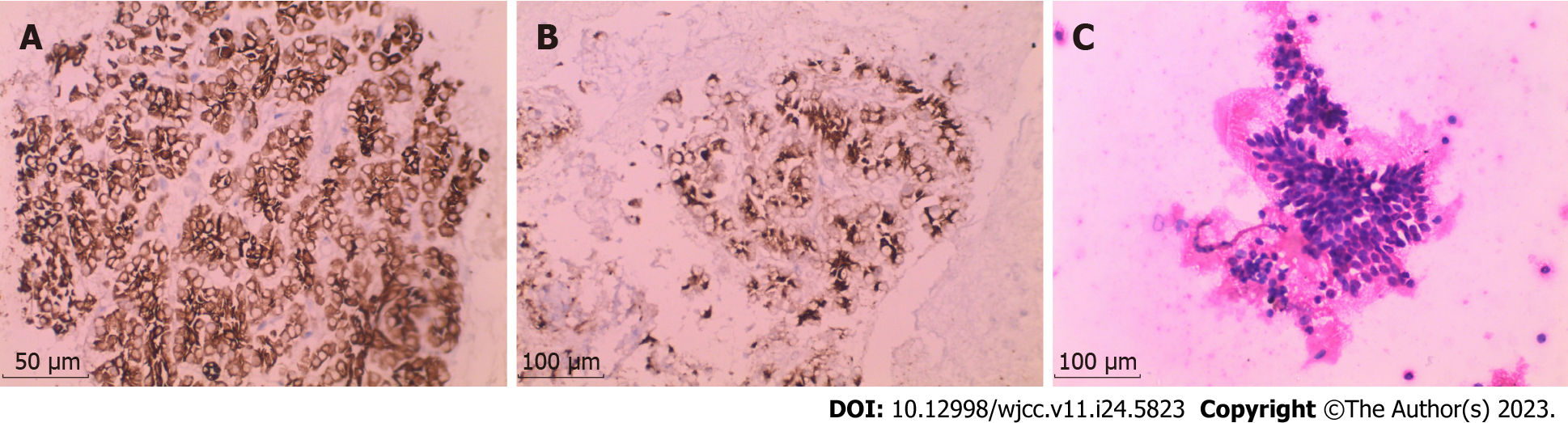
Several additional examinations were performed to confirm this diagnosis. Positron emission tomography–computed tomography (Figure 2) revealed irregular soft-tissue density at the pancreatic tail approximately 2.49 cm × 3.24 cm with an indistinct boundary. The tumor had an uneven mass density, with a plain computed tomography (CT) value of approximately 26 Hounsfield units and no widening of the pancreatic duct. Elevated metabolism of fluorodeoxyglucose (FDG), with a standardized uptake value (SUVmax) of 2.5, was noted. Diffuse thickening and turbidity were observed in the peritoneum, reticulum, and mesentery, with increased density and multiple nodules, the larger of which was approximately 2.17 cm. The FDG metabolism was unevenly elevated (SUVmax, 2.5). Multiple enlarged lymph nodes were detected in the retroperitoneal and pelvic mesangial area, of which the largest was about 1.87 cm × 1.38 cm; the FDG metabolism increased to a SUVmax of 3.9. Magnetic resonance imaging revealed a low signal at T1, a slightly high signal at T2, and limited diffusion on diffusion-weighted imaging. Overall, there were no obvious abnormalities in liver morphology or size, with smooth liver margins and no widening of the liver fissure. Multiple patchy low-density shadows with unclear boundaries were observed in the liver parenchyma, with a low signal on T1, a slightly high signal on T2, and a high signal on diffusion images as well as increased FDG metabolism (SUVmax, 2.6). The hilar region was well structured. No dilatation of the intra-or extrahepatic bile ducts was observed. Needle biopsy and immunohistochemistry results (Figure 3) suggested that the histological type was acinar cell carcinoma.
Chemical and immunotherapies were considered the first-choice treatments considering that the tumor had metastasized to multiple organs. To enhance treatment strategy accuracy, the patient was enrolled in a gene sequencing project, the data of which showed that her tumor mutation load was low with microsatellite stability, which enabled a genetic analysis. Based on second-generation sequencing technology, four types of mutations (including point mutation, insertion-deletion of small fragments, copy number variation, and currently known fusion genes) of 1,016 genes related to tumorigenesis and development, were detected (Tables 2-4), including mutations in KRAS, PIK3R2, TP53, TGFBR2, and CDKN2A. ROS1-CENPW (intergenic) gene fusion has not been previously reported in the literature. The ROS1-CENPW fusion protein does not retain the intact kinase domain of ROS1, and its effect on gene function is unknown.
| Gene | Transcript | Nucleobase alternation | Amino acid alternation | Functional domain | Mutation frequency |
| KRAS | NM_033360.2 | c.34G>C | p.G12R | EX2 | 52.7% |
| PIK3R2 | NM_005027.2 | c.443C>T | p.P148L | EX4 | 18.0% |
| TP53 | NM_000546.5 | c.797G>T | p.G266V | EX8 | 15.1% |
| TGFBR2 | NM_001024847.2 | c.1528_1532delGAAAG | p.E510Hfs*29 | EX7 | 15.0% |
| CDKN2A | NM_000077.4 | c.35C>A | p.S12* | EX1 | 13.9% |
| Gene | Transcript | Mutation type | Functional domain | Copy coefficient |
| KRAS | NM_033360.2 | Amplification | All exon | 1.7 |
| Gene | Transcript | Mutation type | Functional domain | Mutation frequency |
| ROS1-CENPW (intergenic) | NM_002944.2; NM_001012507.2 | Fusion | EX41:intergenic | 21.6% |
Pancreatic acinar cell carcinoma.
To target this mutation, we selected crizotinib, a specific tyrosine kinase inhibitor (TKI), as the drug of choice for targeted therapy. Crizotinib was administered orally at a dose of 200 mg twice daily. AG chemotherapy (gemcitabine 1000 mg/m2 plus albumin paclitaxel 125 mg/m2) was administered on the first and eighth days, followed by a week’s rest.
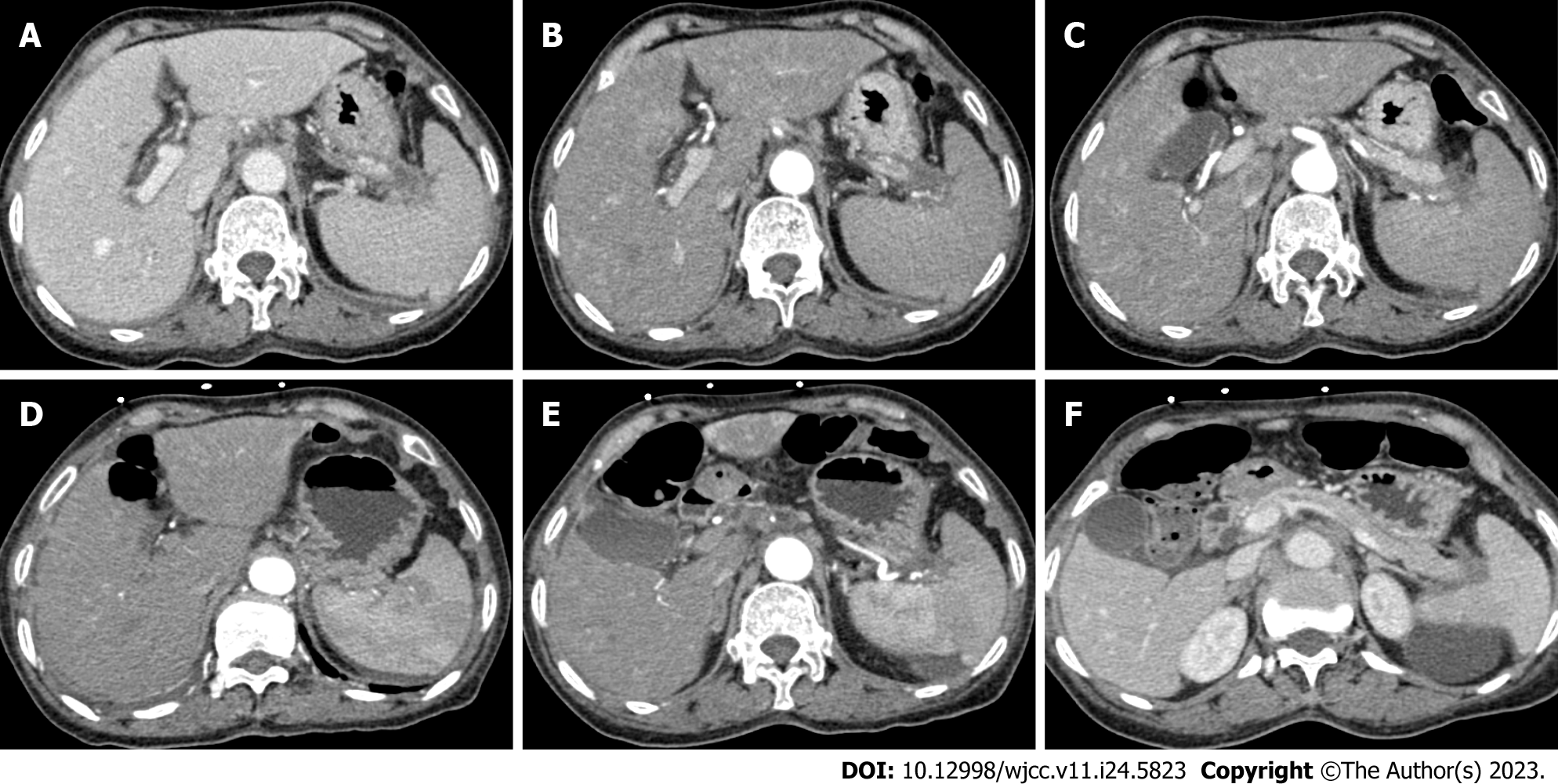
The patient cooperated without adverse reactions or intolerance; therefore, the treatment regimen remained unchanged, and she received regular treatment. Laboratory examination data (Table 1) showed that the CEA levels had declined by degrees. The CA19-9 levels remained stable and were not significantly elevated. Detailed data and other examination parameters are listed in Table 1. An enhanced CT scan (Figure 4) indicated that until the examination on June 21, the liver metastasis had diminished, and the pancreatic mass had shrunk.
Herein we report the case of a patient with pancreatic cancer harboring a specific fusion gene, the ROS1-CENPW mutation. ROS1-CENPW is a fusion gene that has not been previously used for diagnosing and treating pancreatic cancer. ROS1 is a proto-oncogene with various physiological functions. Twenty-six genes have been found to fuse with ROS1 and drive a variety of cancers in adult and pediatric patients when ROS1 produces chimeric oncoproteins[10,11]. Different N-terminal fusion partners generate different cell signaling and oncogenic properties. ROS1 fusion oncoproteins such as SDC4-ROS1 and CD74-ROS1 regulate mitogen-activated protein kinase signaling pathways[12]. The CENPW locus, a novel biomarker primarily expressed in hepatocellular and breast cancers, is closely associated with occurrence, development, and poor prognosis of cancer[13,14]. However, since the ROS1-CENPW fusion protein does not retain the complete kinase domain of ROS1, its effect on gene function remains unknown.
PACC is a rare malignant acinar-differentiated tumor with poor clinical prognosis and molecular heterogeneity[9,15]. Hypersecretion syndrome can result from the excessive release of lipases, and surgical resection remains the treatment of choice for PACC[16]. In this case, the patient missed the best time for surgical treatment due to her age and distant tumor metastasis. Fortunately, PACC often features multiple somatic gene mutations; therefore, mapping targeted treatment sites using genetic testing facilitates personalizing the treatment of locus genes[17]. Crizotinib, a specific TKI, is a competitive small-molecule inhibitor of ATP molecular implantation at the ROS1 site[18]. Oral TKI resistance often develops[19]. However, it inhibits ROS1, MET, ALK, and other sites, is considered the gold standard for first-line treatment, and has been approved by the United States Food and Drug Administration (FDA) in the United States[20]. In addition to crizotinib, lorlatinib and entrectinib have been approved by the FDA for targeted therapy of ROS1, with an overall response rate of > 60% for all four targeted drugs[21].
In summary, ROS1-CENPW is a novel fusion gene locus in PACC that has not been previously reported. We searched the PubMed (https://pubmed.ncbi.nlm.nih.gov/), Reference Citation Analysis (https://www.referencecitationanalysis.com/), and MedlinePlus (https://medlineplus.gov/) databases using ROS1-CENPW as a keyword. However, no relevant reports have been published regarding this rare fusion gene. This is a unique finding. Gene sequencing was performed in patients with PACC. Both ROS1 and CENPW have been reported only in breast cancer and hepatocellular carcinoma. However, neither has been reported for pancreatic-related cancers. However, effective targeting sites for the treatment of PACC are lacking. Thus, discovering the ROS1-CENPW complex may open new avenues for targeted therapy. In this case, the treatment regimen resulted in a favorable outcome. CEA levels decreased significantly, liver metastases decreased significantly, CA19-9 levels stabilized, and CT showed shrinkage of the pancreatic mass. In this case, the treatment plan of crizotinib plus AG chemotherapy achieved satisfactory therapeutic effects. Therefore, it may be a good treatment option for patients with PACC harboring the ROS1-CENPW fusion mutation. Owing to the possible specificity in individual cases, it is worth exploring whether the treatment plan of crizotinib plus AG chemotherapy is universally applicable for treating patients with PACC who are carrying the ROS1-CENPW fusion gene.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Doval D, India; Zeng C, United States S-Editor: Liu JH L-Editor: Webster JR P-Editor: Yuan YY
| 1. | Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008-2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1674] [Article Influence: 334.8] [Reference Citation Analysis (1)] |
| 2. | Yang H, Zhang W, Ding J, Hu J, Sun Y, Peng W, Chu Y, Xie L, Mei Z, Shao Z, Xiao Y. A novel genomic instability-derived lncRNA signature to predict prognosis and immune characteristics of pancreatic ductal adenocarcinoma. Front Immunol. 2022;13:970588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Ho WJ, Jaffee EM, Zheng L. The tumour microenvironment in pancreatic cancer - clinical challenges and opportunities. Nat Rev Clin Oncol. 2020;17:527-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 854] [Cited by in RCA: 792] [Article Influence: 158.4] [Reference Citation Analysis (0)] |
| 4. | Nevala-Plagemann C, Hidalgo M, Garrido-Laguna I. From state-of-the-art treatments to novel therapies for advanced-stage pancreatic cancer. Nat Rev Clin Oncol. 2020;17:108-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 267] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 5. | Sarvepalli D, Rashid MU, Rahman AU, Ullah W, Hussain I, Hasan B, Jehanzeb S, Khan AK, Jain AG, Khetpal N, Ahmad S. Gemcitabine: A Review of Chemoresistance in Pancreatic Cancer. Crit Rev Oncog. 2019;24:199-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 6. | Casolino R, Braconi C, Malleo G, Paiella S, Bassi C, Milella M, Dreyer SB, Froeling FEM, Chang DK, Biankin AV, Golan T. Reshaping preoperative treatment of pancreatic cancer in the era of precision medicine. Ann Oncol. 2021;32:183-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 7. | Petersen GM. Familial pancreatic cancer. Semin Oncol. 2016;43:548-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 118] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 8. | Luo J. KRAS mutation in pancreatic cancer. Semin Oncol. 2021;48:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 204] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 9. | Wood LD, Klimstra DS. Pathology and genetics of pancreatic neoplasms with acinar differentiation. Semin Diagn Pathol. 2014;31:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Uguen A, De Braekeleer M. ROS1 fusions in cancer: a review. Future Oncol. 2016;12:1911-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Drilon A, Jenkins C, Iyer S, Schoenfeld A, Keddy C, Davare MA. ROS1-dependent cancers - biology, diagnostics and therapeutics. Nat Rev Clin Oncol. 2021;18:35-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 186] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 12. | Neel DS, Allegakoen DV, Olivas V, Mayekar MK, Hemmati G, Chatterjee N, Blakely CM, McCoach CE, Rotow JK, Le A, Karachaliou N, Rosell R, Riess JW, Nichols R, Doebele RC, Bivona TG. Differential Subcellular Localization Regulates Oncogenic Signaling by ROS1 Kinase Fusion Proteins. Cancer Res. 2019;79:546-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 13. | Zhou Y, Chai H, Guo L, Dai Z, Lai J, Duan J, Liu Y, Ding Q. Knockdown of CENPW Inhibits Hepatocellular Carcinoma Progression by Inactivating E2F Signaling. Technol Cancer Res Treat. 2021;20:15330338211007253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Wang L, Wang H, Yang C, Wu Y, Lei G, Yu Y, Gao Y, Du J, Tong X, Zhou F, Li Y, Wang Y. Investigating CENPW as a Novel Biomarker Correlated With the Development and Poor Prognosis of Breast Carcinoma. Front Genet. 2022;13:900111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 15. | Thompson ED, Wood LD. Pancreatic Neoplasms With Acinar Differentiation: A Review of Pathologic and Molecular Features. Arch Pathol Lab Med. 2020;144:808-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 16. | Mustafa S, Hruban RH, Ali SZ. Acinar cell carcinoma of the pancreas: a clinicopathologic and cytomorphologic review. J Am Soc Cytopathol. 2020;9:586-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Al-Hader A, Al-Rohil RN, Han H, Von Hoff D. Pancreatic acinar cell carcinoma: A review on molecular profiling of patient tumors. World J Gastroenterol. 2017;23:7945-7951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Heigener DF, Reck M. Crizotinib. Recent Results Cancer Res. 2018;211:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Roys A, Chang X, Liu Y, Xu X, Wu Y, Zuo D. Resistance mechanisms and potent-targeted therapies of ROS1-positive lung cancer. Cancer Chemother Pharmacol. 2019;84:679-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | D'Angelo A, Sobhani N, Chapman R, Bagby S, Bortoletti C, Traversini M, Ferrari K, Voltolini L, Darlow J, Roviello G. Focus on ROS1-Positive Non-Small Cell Lung Cancer (NSCLC): Crizotinib, Resistance Mechanisms and the Newer Generation of Targeted Therapies. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 21. | Azelby CM, Sakamoto MR, Bowles DW. ROS1 Targeted Therapies: Current Status. Curr Oncol Rep. 2021;23:94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |