Published online Aug 16, 2023. doi: 10.12998/wjcc.v11.i23.5580
Peer-review started: May 9, 2023
First decision: June 13, 2023
Revised: June 22, 2023
Accepted: July 18, 2023
Article in press: July 18, 2023
Published online: August 16, 2023
Processing time: 98 Days and 17.2 Hours
Clinically amyopathic deramatomyositis was manifested as the various cutaneous dermatomyositis (DM) manifestations without muscle weakness. Anti-melanoma differentiation-associated gene 5 (anti-MDA5) and anti-Ro52 antibody-dual positive clinically amyopathic DM patients are at a high risk of developing rapidly progressive interstitial lung disease, and they exhibit an immensely high half-year mortality.
We presented three patients with anti-MDA5 and anti-Ro52 antibody-dual positive DM patients and we reviewed the previous studies on the link between anti-MDA5 and anti-Ro52 antibody-dual positive DM. Although we aggressively treated these patients similarly, but they all exhibited different prognoses. We reviewed the importance of clinical cutaneous rashes as well as the pathogenesis and treatment in the dual positive anti-MDA5 and anti-Ro52 associated DM.
Patients with anti-MDA5 anti-Ro52 antibody-dual positive DM should be accurately diagnosed at an early stage and should be treated aggressively, thus, the patient’s prognosis can be significantly modified.
Core Tip: In this study, we presented three rare cases of anti-melanoma differentiation-associated gene 5 (Anti-MDA5) and anti-Ro52 dual positive clinically amyopathic dermatomyositis (DM) accompanied by rapidly interstitial lung disease (ILD). Moreover, we reviewed the clinical manifesetations, pathogenesis and therapy about it. More imterestingly, there was a similarity between anti-MDA5 associated DM complicated rapidly progressive ILD and severe coronavirus disease 2019 pneumomia.
- Citation: Ye WZ, Peng SS, Hu YH, Fang MP, Xiao Y. Anti-melanoma differentiation-associated gene 5 and anti-Ro52 antibody-dual positive dermatomyositis accompanied by rapidly lung disease: Three case reports. World J Clin Cases 2023; 11(23): 5580-5588
- URL: https://www.wjgnet.com/2307-8960/full/v11/i23/5580.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i23.5580
Clinically amyopathic dermatomyositis (DM) (CADM) is a subset of DM and presents as the various cutaneous manifestations of DM without muscle weakness. The anti-melanoma differentiation-associated gene 5 (anti-MDA5) antibody, previously known as anti-CADM 140 antibody, is associated with life-threatening rapidly progressive interstitial lung disease (ILD) (RP-ILD)[1-3]. The incidence of ILD in anti-MDA5-associated DM is about 42% to 100% and those who test positive for anti-MDA5 antibody positive have a 20-fold higher risk of developing RP-ILD compared with those who test negative[2]. Moreover, in a retrospective cohort study, the result of anti-Ro52 antibody was positive in 74.7% of patients with anti-MDA5-associated DM ILD patients, this correlated with an increased risk of RP-ILD and cutaneous ulcerations[4]. The study demonstrated that the coexistence of anti-Ro52 antibody and anti-MDA5 was linked to a subset of patients with more aggressive phenotypes[4]. On the other hand, several studies described that early management of the disease leads to a good prognosis. Therefore, it is essential for us to recognize the anti-MDA5-associated DM early. We present three rare cases of dual-positive anti-MDA5- and anti-Ro52 associated DM that to characterizes RP-ILD, and reviewed previous studies that can facilitate the early recognition and treat timely treatment of CADM patients that exhibit RP-ILD complications.
Case 1: A 40-year-old woman presenting with a two-week history of the hand lesions and weakness of limbs was admitted to our dermatology clinic.
Case 2: A 43-year-old woman, with a relevant medical history, was admitted to our dermatology clinic for the following complaint of two-month history of weakness, fatigue, shortness of breathe and skin lesions.
Case 3: A 40-year-old woman presented to the dermatology clinic featured with mucocutaneous lesions, shortness of breath, and weakness.
Case 1: She initially complained of arthralgia and new-onset papules on the hand, and after two days, she progressively developed mild myalgia, weakness and shortness of breathe.
Case 2: She was initially diagnosed with sjogren’s syndrome that did not respond to hydroxychloroquine.
Case 3: Symptoms began two weeks earlier and were associated with new-onset muscle weakness on climbing stairs, progressively worsening shortness of breath, oral mucous ulceration and lesions on the head, back, hand and leg.
None of these three patients had obvious diseases in the past.
They all exhibited no personal and family history in the past.
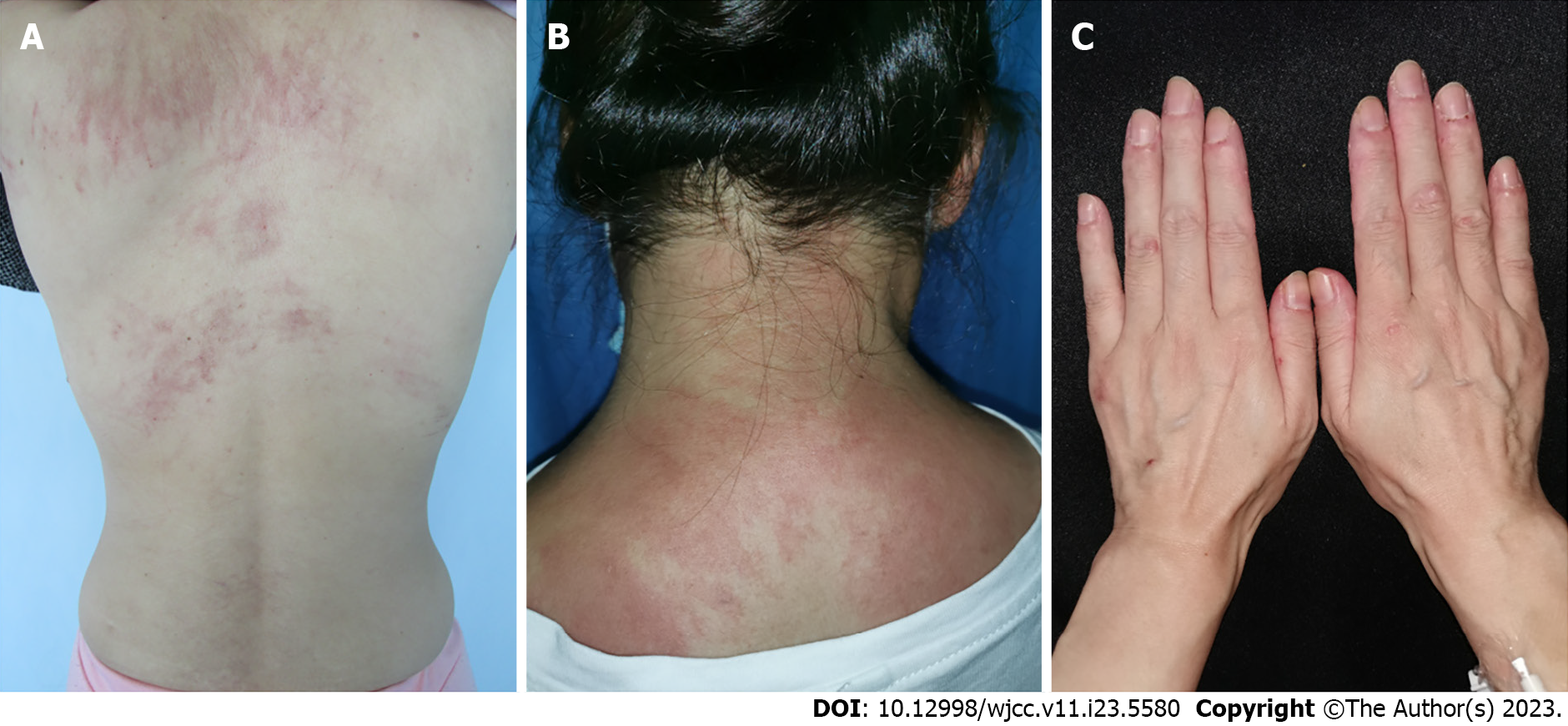
Case 1: The physical examination revealed gottron’s papules, gottron’s sign, shawl sign and flagellate erythema (Figure 1A).
Case 2: Physical examination indicated eyelid edema with pink patches (heliotrope sign), shawl sign, photosensitivity and mechanic’s hands. She exhibited mild weakness of proximal muscles (Figure 1B).
Case 3: A physical examination revealed gottron’s papules, gottron’s sign, shawl sign, holster sign, oral ulcerations and subcutaneous nodules (Figure 1C).
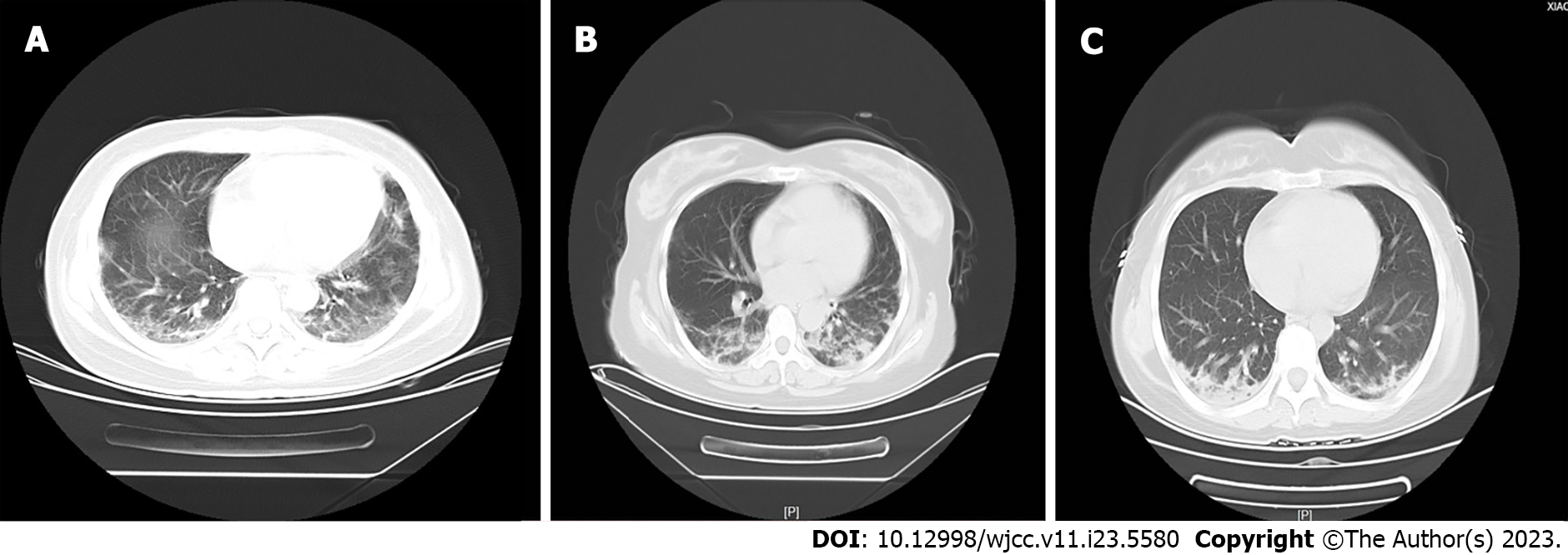
Case 1: Laboratory findings revealed significantly raised liver function tests and the muscle enzymes (Table 1). Abdominal computed tomography (CT) scan and cancer-specific antigen were normal. Chest CT scan showed diffuse bilateral ground-glass opacities in the lung fields (Figure 2A), and the lung function test revealed a restrictive ventilatory impairment.
| Laboratory parameter | Case 1 | Case 2 | Case 3 |
| Age | 40 | 43 | 40 |
| Gender | F | F | F |
| White cell counts (× 109/L) (3.5-9.5) | 9.82 | 7.72 | 6.26 |
| Haemoglobin (115-150) | 139 | 97 | 100 |
| Platelets (× 109/L) (126-350) | 143 | 192 | 235 |
| CRP (mg/L) (0-3) | 3.46 | 6.07 | 1.84 |
| ESR (mm/hour) (0-15) | 7.6 | 47 | 58 |
| AST (IU/L) (13-35) | 430 | 206 | 414 |
| ALT (IU/L) (7-40) | 215 | 134 | 282 |
| LDH (IU/L) (120-250) | 482 | 380 | 470 |
| CK (IU/L) (40-200) | 1484 | 17 | 108 |
| Albumin (g/L) (40-55) | 27.5 | 32.9 | 27 |
| Ferritin (ng/mL) (12-135) | 1640.38 | 87.56 | 458.49 |
| Procalcitonin (ng/mL) (0-0.5) | |||
| Basline level at admission | 0.11 | 0.13 | 0.1 |
| On the day of worsening | 0.24 | 0.32 | 0.1 |
| C3 (mg/dL) (0.75-1.35) | 0.46 | 1.04 | 0.77 |
| C4 (mg/dL) (0.09-0.36) | 0.27 | 0.18 | 0.28 |
| ANA screening | 1/1000 | 1/1000 | 1/320 |
| Fluorescent pattern of ANA | Cytoplasmic | Cytoplasmic | Cytoplasmic |
| Anti-MDA5 | Positive | Positive | Positive |
| Anti-Ro52 | Positive | Positive | Positive |
Case 2: Her temperature, blood pressure, the abdomen CT scan and the cancer-specific antigens, were within normal limits (Table 1). Moreover, skin and muscle biopsies were performed and findings were similar to DM. Her CT scan revealed worse than last time, which showed peri-bronchovascular and sub-pleural patchy ground glass opacities and nodules (Figure 2B). Lung function tests showed moderate restrictive ventilatory impairment.
Case 3: Laboratory findings are shown in Table 1. Chest CT scan revealed diffuse bilateral ground-glass opacity in the lung field, and the lung function test was normal (Figure 2C). Auto-antibody and extractable nuclear antigen testing were performed and revealed positive antinuclear antibody and anti-Ro52 antibody (Table 1).
Case 1: Abdominal CT scan and cancer-specific antigen were normal. Chest CT scan showed diffuse bilateral ground-glass opacities in the lung fields (Figure 2A).
Case 2: Her CT scan revealed worse than last time, which showed peri-bronchovascular and sub-pleural patchy ground glass opacities and nodules (Figure 2B).
Case 3: Chest CT scan revealed diffuse bilateral ground-glass opacity in the lung field, and the lung function test was normal (Figure 2C).
All three patients were diagnosed with CADM, moreover, anti-MDA5 and anti-Ro52-antibody patients were dual positive.
Case 1: She was placed on methylprednisolone and supportive therapy.
Case 2: Triple therapy with methylprednisolone, cyclophosphamide and intravenous immunoglobulins (IVIG) was administrated.
Case 3: The combination of methylprednisolone, cyclophosphamide and IVIG conbination was initiated. Moreover, jak inhibitor was also administered.
Case 1: The shortness of breath progressively worsened and the oxygen partial pressure dropped significantly. She was then transferred to an intensive care unit and her parameters gradually increased. Finally, she died of respiratory failure.
Case 2: Although the lesions gradually regressed, but her respiratory symptoms were worsened. Consequently, after multidisciplinary meeting, plasma exchange was recommended after multidisciplinary meeting. However, she died of respiratory failure.
Case 3: After one month, the skin and lung symptoms improved remarkably, and the chest CT scan indicated noticeable remission.
Anti-MDA5-associated DM, which is a rare subtype of DM that presents with unique mucocutaneous features, including cutaneous ulceration, painful palmar papules, pannuiculitis, arthritis, alopecia and a lower incidence of myositis, was initially reported in 2005[2,3]. Meanwhile, several rare skin manifestations, including unilateral heliotrope rash, pharyngeal ulceration and perforation and psoriasiform rashes, were considered as an initial symptoms of anti-MDA5-associated DM[1-5]. The incidence of anti-MDA5-associated DM varies in different regions. For instance, around 82% of CADM Japanese patients were anti-MDA5-antibody positive, and 42%-50% CADM non-Japanese patients were positive[6]. However, the prevalence of anti-MDA5-antibody associated ILD was 11%-57% in Europeans and United States patients, and it was 92%-100% in Asians[1]. In addition, various MDA-5-associated DM cases have been reported in previous studies, underlying the connection between anti-MDA5 antibody and high risk of RP-ILD in these patients[1,4,7]. More importantly, patients with anti-MDA5 antibody-associated DM complicated with RP-ILD exhibited an immensely poor prognosis due to the progressive course and a poor response to the treatment. More than 70% of the patients died of respiratory failure in the following one to six months, which is quite dangerous without timely recognition and management[8]. Additionally, the researcher stated that the coexistence of anti-MDA5 and anti-Ro52 antibodies, which manifests in multiple systemic autoimmune disorders and acts as a common antibodies in the pathogenesis of these diseases, led to a significantly lower survival rate in these patients compared to those positive for only anti-MDA5 antibody[4]. Therefore, it is crucial to identify the disease early, and to treat it aggressively.
In the present three cases, the patients exhibited anti-MDA5 antibody and classical traditional characteristics of anti-MDA5-associated DM with RP-ILD, but with varying prognoses. The first patient manifested traditional lesions and clinical manifestations of anti-MDA5 associated DM with RP-ILD. She initially manifested ILD followed by the onset of traditional lesions, such as cutaneous papules and plaques (gottron’s plaques), ulcerated in gottron’s plaques, eyelid edema with pink patches (heliotrope sign), lesion over the neck and back (shawl sign), photosensitivity and mechanic’s hands. Although we immediately effected the CADM diagnosis due to the clinical traditional lesions and identified the co-occurrence of anti-MDA5 and anti-Ro52 antibodies, we failed to determine the relationship between CADM and RP-ILD in the early phase, which led to the dismal course of RP-ILD. For the second patient, we diagnosed the CADM complicated with RP-ILD, and dual positive anti-MDA5 and anti-Ro52 antibodies, earlier than the first one. She exhibited a satisfactory response to methylprednisolone, and she manifested a remarkable recovery with the complete resolution of lung inflammation, clinical lesions and shortness of breathe. However, the disease of CADM complicated RP-ILD flared after one month, furthermore, she manifested cutaneous lesions relapse, and her shortness of breath worsened[8]. The patient exhibited a poor response to the triple treatment and plasma exchange, and she succumbed to the diseases. We hypothesized that, starting the triple treatment early, might result in better survival. Moreover, the disease recurrence could be rationalized as follows: Firstly, the drug eruption stimulant could induce the disease relapse; and second, although multiple inflammatory indicators were normal, but the disease could not be in complete control.
In the three cases, the coexistence of anti-MDA5 and anti-Ro52 antibodies attracted our more attention, which was similar with the results reported in previous studies[4,5,9-12]. However, the exact mechanism through which the co-occurrence of anti-Ro52 and anti-MDA5 results in RP-ILD is unclear. Anti-MDA5 antibody recognizes double-strands RNA viruses and activates type I interferon (IFN) production, which is regarded as an intracellular pattern recognition receptor[13]. Both anti-MDA5 and anti-Ro52 antibodies are significantly induced by IFN, and, perhaps, the two antibodies form a novel complex, which particularly responds to a viral infection in the innate immune system[10,13]. The correlation of anti-Ro52 antibodies and anti-MDA5-associated RP-ILD patients should warrant close monitoring.
In regard to the reviewed studies, we observed that anti-MDA5 and anti-Ro52 antibody-dual positive DM was common in the 27-69-year-old age group and more often in women (Table 2). The cutaneous features, including heliotrope sign, gottron’s, periungual erythema, painful palmar papules, mechanic’s hand, facial rash, and cutaneous ulceration, were observed in these patients. However, several particular cutaneous manifestations, such as cutaneous ulceration, painful palmar papules and panniculitis, were supposedly secondary to the development of ILD, especially in the early stage of the disease. The clinical manifestations of anti-MDA5 DM differs substantially from the other forms of DM, with three distinct clinical phenotypes, according to the predominance of pulmonary, skin or vascular symptoms, however, the pathogenesis of these three forms of anti-MDA5 DM is largely unknown.
| Ref. | Age | Sex | Cutaneous | Pulmonary | Serologies | Treatment | Outcome |
| Huang et al[10] | 52 | M | Heliotrope, gottron’s periungual erythema | RP-ILD | MDA5 + Ro52 + | GC, CYC, RTX, ECMO, and MMF + Tac | Recurrent |
| 54 | F | Heliotrope, gottron’s cutaneous ulcers | RP-ILD | MDA5 + Ro52 + | GC, IVIG, CYC, RTX, ECMO/lung Tx and now MMF + Tac | Improved | |
| 59 | F | Palmar papules, periungual erythema | RP-ILD | MDA5 + Ro52 + | GC, CYC, ECMO/lung Tx and now MMF + Tac | Improved | |
| 43 | M | Gottron’s, palmar papules, diffuse violaceous rash cataneous ulcers | RP-ILD | MDA5 + Ro52 + | GC, CYC, rituximab | Dead | |
| 69 | M | Heliotrope, gottron’s | RP-ILD | MDA5 + Ro52 + | GC, CYC | Dead | |
| 58 | F | Gottron’s, mechanic’s hands, skin Bx | RP-ILD | MDA5 + Ro52 + | MMF, GC, CYC, CsA, RTX | Dead | |
| 44 | F | Gottron’s ulcerations | RP-ILD | MDA5 + Ro52 + | GC, MMF and RTX | Dead | |
| 65 | M | Heliotrope, gottron’s diffuse erytheatous rash, skin Bx, ulceration | RP-ILD | MDA5 + Ro52 + | GC, IVIG, CYC, imuran | Recurrent | |
| 31 | M | Gottron’s ulcerations, helitrope, skin Bx | RP-ILD | MDA5 + Ro52 + | GC, MTX, IVIG, MMF, AZA, CYC + Tac | Improved | |
| 27 | F | Heliotrope, gottron’s cutaneous ulcers periungual erythema | ILD | MDA5 + Ro52 + | GC, AZA, MMF, IVIG, RTX + Tac | Recurrent | |
| 59 | F | Helitrope, gottton’s cutaneous ulcers, periungualerythema, mechanic’s hand | ILD | MDA5 + Ro52 + | GC, HCQ, AZA, CsA, MMF, RTX | Improved | |
| 51 | F | Periorbitaledema, heliotrope, Gottron’s, palmar papules, periungual erythema | ILD | MDA5 + Ro52 + | GC, MTX, AZA, IVIG, MMF, PLEX, IV epoprostenol | Improved | |
| 59 | M | Facial rash, skin ulcers, palmar papules | ILD | MDA5 + Ro52 + | GC, IVIG, MMF | Ongoing | |
| Gupta et al[9] | 27 | F | Upper eyelids, extensor surface of hands and digit | ILD | MDA5 + Ro52 + | GC, CsA, IVIG | Improved |
| Mehta et al[12] | 37 | F | Unkown | ILD | MDA5 + Ro52 + | CYC, IVIG | Dead |
| 42 | F | Unkown | ILD | MDA5 + Ro52 + | GC, rituximab | Dead | |
| Chen et al[24] | 48 | F | Facial rash | ILD | MDA5 + Ro52 + | GC, MMF hydroxychloroquine | Improved |
| Huang et al[11] | 50 | F | Unkown | RP-ILD | MDA5 + Ro52 + | GC, TAc, CYC, IVIG | Dead |
| 42 | F | Unkown | RP-ILD | MDA5 + Ro52 + | GC, CYC | Improved | |
| 51 | F | Unkown | RP-ILD | MDA5 + Ro52 + | GC, CYC, IVIG | Dead | |
| 30 | F | Unkown | RP-ILD | MDA5 + Ro52 + | GC, CYC, IVIG | Improved | |
| 46 | M | Unkown | RP-ILD | MDA5 + Ro52 + | GC, CsA, CYC | Improved | |
| 58 | M | Unkown | RP-ILD | MDA5 + Ro52 + | GC, CYC, MTX | Improved | |
| Xu et al[17] | 28 | F | Gottron’s ulceration | RP-ILD | MDA5 + Ro52 + | GC, IVIG | Improved |
| Current | 40 | M | Heliotrope, gottron’s | RP-ILD | MDA5 + Ro52 + | GC, CYC, IVIG | Dead |
| 43 | M | Heliotrope, gottron’s periungual erythema | RP-ILD | MDA5 + Ro52 + | GC, CYC, IVIG | Dead | |
| 40 | M | Heliotrope, gottron’s cutaneous ulcers mechanic’s hand | RP-ILD | MDA5 + Ro52 + | GC, CYC, IVIG | Improved |
Currently, an increasing number of cases pertaining to anti-MDA5-associated DM were reported. The inflammatory storm, the elevated levels of multiple cytokines including IFN-γ, monocyte chemoattractant protein-1, monocyte chemoattractant protein-3, stem cell growth factor-β, interleukin (IL)-8, and cutaneous T-cell attracting chemokine, were observed in anti-MDA5 antibody-associated RP-ILD, which were linked with poor outcome. Multiple inflammatory indicators such as ferritin IL-18, IL-6, albumin, anti-MDA5 antibody titer, KL-6 and anti-Ro52 antibody, were associated with patient prognosis[14,15]. The current study indicated diffuse cytoplasmic staining of the MDA5 pattern, it was observed that the specific cytoplasmic MDA5 pattern was related to a higher ILD risk, which was consistent with the reviewed studies. Perhaps the antibodies lead to the development of pathways which drive pathogenesis (for example, MDA5 and Ro52 are both IFN-induced proteins)[16]. Therefore, those results indicate that the anti-MDA5 antibody profile differs based on the clinical phenotype of the disease. Interestingly, there was a similarity between anti-MDA5 associated DM complicated with RP-ILD and severe coronavirus disease 2019 (COVID-19) pneumonia[17]. Moreover, a recent study indicated that the incidence of positive anti-MDA5 antibody was 48.2% in COVID-19 patients[18]. I think that these results should be considered carefully. Indeed, although the titers of anti-MDA5 Abs are statistically higher in the non-survivals infected syndrome coronavirus 2 patients vs the survivals, the orders of magnitude are very low (5.95 ± 5.16 U/mL vs 8.22 ± 6.64 U/mL, P = 0.030)[19]. However, the pathogenesis of anti-MDA5 antibody-associated DM was not comprehensively described, and there was no evidence-based consensus for the treatment of anti-MDA5-associated DM. Several studies have emphasized that the importance of early diagnosis and aggressive treatment of anti-MDA5-associated RP-ILD was emphasized, which led to a remarkable decrease in its mortality[20]. For the second patient, the triple treatment was started early, we would have prevented the onset of inflammatory storm.
In regard to the reviewed literature, the triple treatment exhibited prominent effect compared with the other combination therapy. Nevertheless, the study proposed that the combination of high-dose glucocorticoids and calcineurin antagonists with or without cyclophosphamide as the first-line treatment, and extracorporeal membrane oxygenation were considered in life-threatening conditions while waiting for a clinical response to lung transplant[21]. Meanwhile, the other study suggested that a combination of glucocorticoids, cyclophosphamide and mycophenolate mofetil was the first choice. This notwithstanding, numerous studies revealed that triple treatment (e.g., high-dose glucocorticoids, immunosuppressive agents and IVIG and rare treatment (such as jak inhibitor, infliximab and plasma exchange) indicated a satisfactory disease respond[22-24], which was observed in 34 patients treated in previous studies[5,9-11,24]. Several researchers announced that triple treatment, particularly in China, was intensively supported to perform at the onset of the disease, thus, with regard to patients, a satisfactory outcome could be actualized. However, the combination of multiple immunosuppressing agents predisposes patients to viral, bacterial, and rare fungal infection. In particular, the attention of cytomegalovirus reactivation was affected by the previous study[20]. Consequently, it is imperative that dermatologists recommend multidisciplinary treatment proposals for patients with anti-MDA5-associated RP-ILD patient.
The final young female patient exhibited a short disease course, distinct clinical symptoms and minimal ILD, and as well as the coexistence of anti-MDA5 and anti-Ro52 antibodies, high ferritin and low oxygen pressure, which implied a worse prognosis than the anti-MDA5-associated DM. However, the early and aggressive therapy led to a significant remission.
Hererin, the case series first described that the anti-MDA5 and anti-Ro52 antibody-dual positive DM patient should receive accurate and early diagnosis and aggressive treatment. Moreover, the current study noted that early recognition of CADM cutaneous eruptions, especially anti-MDA5 antibody-associated DM, should be considered. However, several limitations exist. First, due to the small sample size, researchers cannot develop a strong conclusion that is applicable to the general population. Additionally, more detailed data such as clinical features, family history, laboratory results, and long-term outcomes are unavailable. The cases are expected to increase awareness on the rising number of cutaneous eruptions related to anti-MDA5 and anti-Ro52 antibody-dual positive DM, however, to explore characteristic cutaneous lesions and effective treatment, it is necessary to expand the sample size of CAMD patients.
The case indicates that anti-MDA5 antibody-associated DM patients should receive accurate and early diagnosis and aggressive treatment. Dermatologists should consider monitoring MDA5 and Ro52 Abs while they monitor characteristic cutaneous lesions.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Coutant F, France; Mahmoud MZ, Saudi Arabia S-Editor: Qu XL L-Editor: A P-Editor: Yu HG
| 1. | DeWane ME, Waldman R, Lu J. Dermatomyositis: Clinical features and pathogenesis. J Am Acad Dermatol. 2020;82:267-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 234] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 2. | Kurtzman DJB, Vleugels RA. Anti-melanoma differentiation-associated gene 5 (MDA5) dermatomyositis: A concise review with an emphasis on distinctive clinical features. J Am Acad Dermatol. 2018;78:776-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 138] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 3. | Sato S, Hirakata M, Kuwana M, Suwa A, Inada S, Mimori T, Nishikawa T, Oddis CV, Ikeda Y. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum. 2005;52:1571-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 527] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 4. | Xu A, Ye Y, Fu Q, Lian X, Chen S, Guo Q, Lu LJ, Dai M, Lv X, Bao C. Prognostic values of anti-Ro52 antibodies in anti-MDA5-positive clinically amyopathic dermatomyositis associated with interstitial lung disease. Rheumatology (Oxford). 2021;60:3343-3351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 5. | Luo L, Huang W, Ren F, Zhou J, Huang D, Tang L. Pharyngeal ulceration and perforation: a rare manifestation in anti-MDA5 dermatomyositis. Rheumatology (Oxford). 2021;60:e132-e133. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Borges IBP, Silva MG, Shinjo SK. Prevalence and reactivity of anti-melanoma differentiation-associated gene 5 (anti-MDA-5) autoantibody in Brazilian patients with dermatomyositis. An Bras Dermatol. 2018;93:517-523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Muramatsu T, Tono T, Kanayama Y, Hasegawa Y, Kondo J, Hoshiyama T, Wada T, Arinuma Y, Tanaka S, Yamaoka K. A case of anti-MDA5 antibody-positive dermatomyositis developing reversible cerebral vasospasm syndrome successfully treated by multi-immunosuppressant combination including mycophenolate mofetil. Mod Rheumatol Case Rep. 2021;5:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 8. | Cao H, Zheng J. [Clinical features, diagnosis and prognosis of patients with anti-MDA5 antibody positive dermatomyositis]. Zhenduanxue Lilun Yu Shijian. 2021;20:8-14. [DOI] [Full Text] |
| 9. | Gupta R, Kumar S, Gow P, Hsien-Cheng Chang L, Yen L. Anti-MDA5-associated dermatomyositis. Intern Med J. 2020;50:484-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Huang K, Vinik O, Shojania K, Yeung J, Shupak R, Nimmo M, Avina-Zubieta JA. Clinical spectrum and therapeutics in Canadian patients with anti-melanoma differentiation-associated gene 5 (MDA5)-positive dermatomyositis: a case-based review. Rheumatol Int. 2019;39:1971-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 11. | Huang W, Ren F, Wang Q, Luo L, Zhou J, Huang D, Pan Z, Tang L. Clinical features of thirty-two patients with anti-melanoma differentiation-associated gene 5 antibodies. Clin Exp Rheumatol. 2019;37:803-807. [PubMed] |
| 12. | Mehta AA, Paul T, Cb M, Haridas N. Anti-MDA5 antibody-positive dermatomyositis with rapidly progressive interstitial lung disease: report of two cases. BMJ Case Rep. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Hall JC, Casciola-Rosen L, Samedy LA, Werner J, Owoyemi K, Danoff SK, Christopher-Stine L. Anti-melanoma differentiation-associated protein 5-associated dermatomyositis: expanding the clinical spectrum. Arthritis Care Res (Hoboken). 2013;65:1307-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 220] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 14. | Nishioka A, Tsunoda S, Abe T, Yoshikawa T, Takata M, Kitano M, Matsui K, Nakashima R, Hosono Y, Ohmura K, Mimori T, Sano H. Serum neopterin as well as ferritin, soluble interleukin-2 receptor, KL-6 and anti-MDA5 antibody titer provide markers of the response to therapy in patients with interstitial lung disease complicating anti-MDA5 antibody-positive dermatomyositis. Mod Rheumatol. 2019;29:814-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Giannini M, Ohana M, Nespola B, Zanframundo G, Geny B, Meyer A. Similarities between COVID-19 and anti-MDA5 syndrome: what can we learn for better care? Eur Respir J. 2020;56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Nombel A, Fabien N, Coutant F. Dermatomyositis With Anti-MDA5 Antibodies: Bioclinical Features, Pathogenesis and Emerging Therapies. Front Immunol. 2021;12:773352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 160] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 17. | Xu L, Wang L, Lv C, Tan W. Anti-MDA-5-positive dermatomyositis associated rapidly progressive interstitial lung disease, a virus-triggered autoimmune-like symptom? Rheumatology (Oxford). 2021;60:4428-4429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Wang G, Wang Q, Wang Y, Liu C, Wang L, Chen H, Jiao T, Hu C, Lei X, Guo L, Ren L, Li M, Zhao Y, Zeng X, Zhang D, Cao B, Wang J. Presence of Anti-MDA5 Antibody and Its Value for the Clinical Assessment in Patients With COVID-19: A Retrospective Cohort Study. Front Immunol. 2021;12:791348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 19. | Coutant F, Bachet R, Pin JJ, Alonzo M, Miossec P. Monoclonal antibodies from B cells of patients with anti-MDA5 antibody-positive dermatomyositis directly stimulate interferon gamma production. J Autoimmun. 2022;130:102831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Matsuda KM, Yoshizaki A, Kuzumi A, Fukasawa T, Ebata S, Yoshizaki-Ogawa A, Sato S. Combined immunosuppressive therapy provides favorable prognosis and increased risk of cytomegalovirus reactivation in anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis. J Dermatol. 2020;47:483-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Romero-Bueno F, Diaz Del Campo P, Trallero-Araguás E, Ruiz-Rodríguez JC, Castellvi I, Rodriguez-Nieto MJ, Martínez-Becerra MJ, Sanchez-Pernaute O, Pinal-Fernandez I, Solanich X, Gono T, Gonzalez-Gay MA, Plana MN, Selva-O'Callaghan A; MEDRA5 (Spanish MDA5 Register) group (listed contributors at the end of the article). Recommendations for the treatment of anti-melanoma differentiation-associated gene 5-positive dermatomyositis-associated rapidly progressive interstitial lung disease. Semin Arthritis Rheum. 2020;50:776-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 22. | Mao MM, Xia S, Guo BP, Qian WP, Zheng ZX, Peng XM, Chen RC, Luo Q, Han Q. Ultra-low dose rituximab as add-on therapy in anti-MDA5-positive patients with polymyositis /dermatomyositis associated ILD. Respir Med. 2020;172:105983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Endo Y, Koga T, Suzuki T, Hara K, Ishida M, Fujita Y, Tsuji S, Takatani A, Shimizu T, Sumiyoshi R, Igawa T, Umeda M, Fukui S, Nishino A, Kawashiri SY, Iwamoto N, Ichinose K, Tamai M, Nakamura H, Origuchi T, Kuwana M, Kawakami A. Successful treatment of plasma exchange for rapidly progressive interstitial lung disease with anti-MDA5 antibody-positive dermatomyositis: A case report. Medicine (Baltimore). 2018;97:e0436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Chen L, Owens K, Murina A. Anti-MDA5 antibody-positive dermatomyositis presenting as unilateral eyelid edema. JAAD Case Rep. 2020;6:909-911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |