Published online Aug 16, 2023. doi: 10.12998/wjcc.v11.i23.5468
Peer-review started: March 29, 2023
First decision: July 3, 2023
Revised: July 7, 2023
Accepted: July 17, 2023
Article in press: July 17, 2023
Published online: August 16, 2023
Processing time: 139 Days and 15.9 Hours
Many epidemiologic investigations have explored the relationship between viatmins and polycystic ovary syndrome (PCOS). However, the effectiveness of vitamin, vitamin-like nutrient, or mineral supplementation in reducing the risk of PCOS remains a subject of debate.
To investigate the impact of plasma levels of vitamins A, B12, D, E, and K on PCOS and key pathways implicated in its development, namely, insulin resis
Single nucleotide polymorphisms associated with vitamin levels were selected from genome-wide association studies. The primary analysis was performed using the random-effects inverse-variance-weighted approach. Complementary analyses were conducted using the weighted median, MR-Egger, MR-robust adjusted profile score, and MR-PRESSO approaches.
The results provided suggestive evidence of a decreased risk of PCOS with genetically predicted higher levels of vitamin E (odds ratio [OR] = 0.118; 95% confidence interval [CI]: 0.071–0.226; P < 0.001) and vitamin B12 (OR = 0.753, 95%CI: 0.568–0.998, P = 0.048). An association was observed between vitamin E levels and insulin resistance (OR = 0.977, 95%CI: 0.976–0.978, P < 0.001). Additionally, genetically predicted higher concentrations of vitamins E, D, and A were suggested to be associated with a decreased risk of hyperlipidemia. Increased vitamins K and B12 levels were linked to a lower obesity risk (OR = 0.917, 95%CI: 0.848–0.992, P = 0.031).
The findings of this MR study suggest a causal relationship between increased vitamins A, D, E, K, and B12 levels and a reduced risk of PCOS or primary pathways implicated in its development.
Core Tip: Higher vitamins A, D, E, K, and B12 levels were casually related to a reduced risk of polycystic ovary syndrome (PCOS) or main pathways implicated in its development, as suggested by our Mendelian randomization investigation. More prospective and functional in vivo and in vitro trials are required to clarify the role of vitamin supplements in the onset of PCOS and main pathways implicated in its development.
- Citation: Shen JY, Xu L, Ding Y, Wu XY. Effect of vitamin supplementation on polycystic ovary syndrome and key pathways implicated in its development: A Mendelian randomization study. World J Clin Cases 2023; 11(23): 5468-5478
- URL: https://www.wjgnet.com/2307-8960/full/v11/i23/5468.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i23.5468
Polycystic ovary syndrome (PCOS) is a widespread endocrine disease that affects a large number of sexually mature women globally[1,2]. The prevalence of PCOS according to the diagnostic criteria ranges from 6% to 10%[3]. Patients with PCOS are at an increased risk of diabetes mellitus, atherogenic dyslipidemia, systemic inflammation, hypertension, and coagulation disorders[4,5].
PCOS arises from a combination of hereditary and epigenetic vulnerability, insulin resistance, and adiposity-related mechanisms[6,7]. Modifying one’s lifestyle is among the recommended options for PCOS treatment and is highly advised for women seeking to improve their quality of life[8]. In recent years, there has been growing interest in nutritional supplements[9,10]. However, the potential of vitamin, vitamin-like nutrient, or mineral supplementation to reduce the risk of PCOS remains debatable. A meta-analysis found no evidence that vitamin D supplementation improved or alleviated metabolic and hormonal dysregulations in PCOS[11]. Nevertheless, convincing conclusions cannot be drawn at this point, and vitamin K may be a viable option for alleviating oxidative stress and improving glycemic control in PCOS[12].
The supplementation of specific nutrients and complementary treatments may improve the health conditions of women with PCOS by modulating critical pathways implicated in PCOS development, such as insulin signaling, insulin resistance, and lipid metabolism. However, observational studies largely constitute the primary evidence regarding the correlation between vitamin supplements and PCOS, which can be influenced by confounding or reverse causation. Mendelian randomization (MR) has emerged as an effective technique to identify the causal relationship of risk factors with diseases by using genetic variants as instrumental variables (IVs)[13]. MR enables stronger causal inferences than typical observational studies due to the random assignment of genetic variations during conception between parents and offspring.
To date, no MR analysis has explored the causal effect of vitamin supplements on PCOS. In this study, we aimed to conduct a two-sample MR analysis to assess the impact of plasma levels of vitamins A, B12, D, E, and K on PCOS and key pathways implicated in its development, namely, insulin resistance, hyperlipidemia, and obesity.
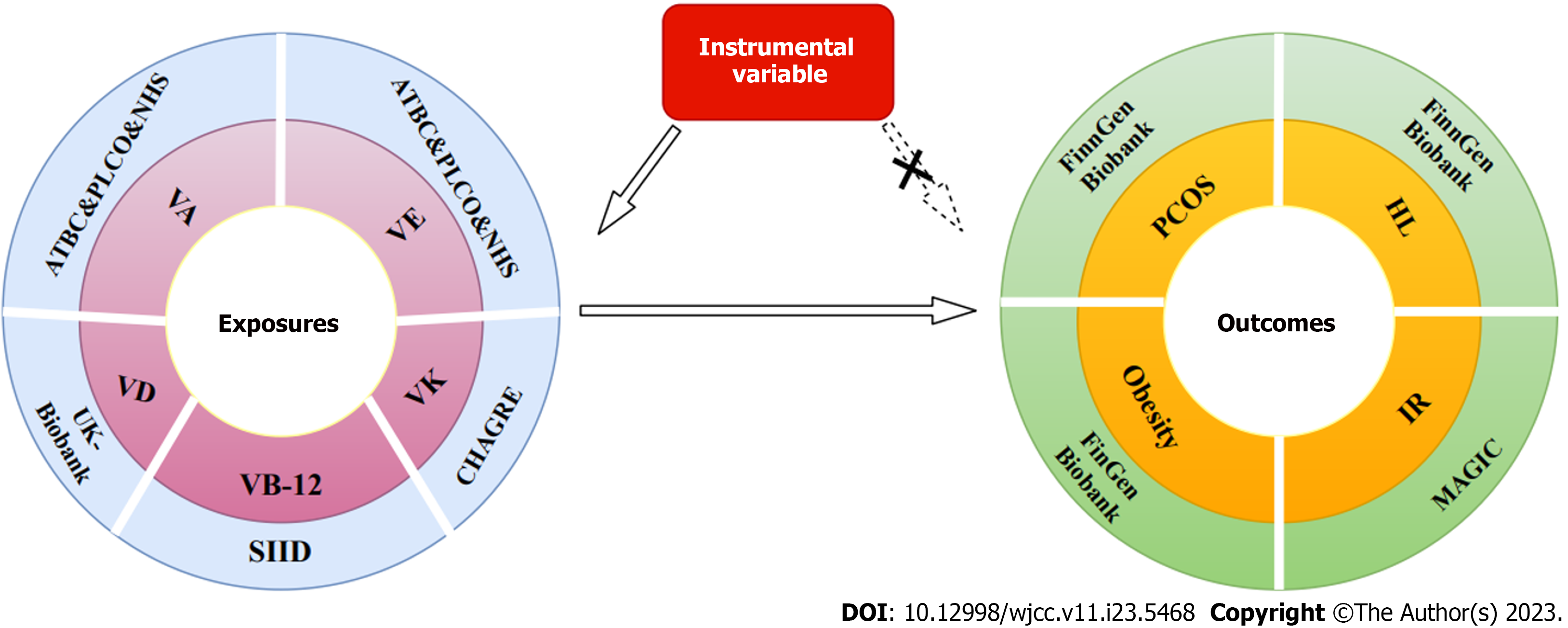
Vitamin supplementation has the potential to improve health outcomes in women with PCOS by influencing crucial pathways such as insulin resistance, lipid metabolism, and obesity. We conducted a two-sample MR analysis to identify the effect of plasma levels of vitamins A, B12, D, E, and K on PCOS and its associated pathways, including insulin resistance, hyperlipidemia, and obesity. Figure 1 provides a detailed overview of the study design.
The genetic association data for vitamin D were analyzed using blood samples obtained twice from the United Kingdom Biobank, a major population cohort[14] comprising volunteers aged 37-73 years from 22 evaluation centers across the United Kingdom, aiming to enhance disease prevention[15]. Genetic association data for vitamin B12 were obtained from sequencing initiatives in Iceland and Denmark involving European populations, explaining 5.1% of the variation in circulating vitamin B12 levels[16]. Genetic instruments for vitamins A and E concentration were obtained from three cohorts: the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study cohort; Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial Study; and the Nurses’ Health Study[17]. Data for vitamin K were obtained from the Cohorts for Heart and Aging Research in Genomic Epidemiology Consortium Nutrition Working Group, which involved 2138 individuals. Genetic correlations were examined using linear models adjusted for key components, sex, age, and study-specific factors[18]. To mitigate potential bias stemming from ancestry, participation was limited to individuals of European heritage. All analyses of this study were based on publicly accessible databases; thus, no additional ethical approval or informed consent was required. The genome-wide association studies (GWAS) included in the analysis are presented in Table 1.
| Exposures/Outcomes | Consortium | Ethnicity | Participants | Number of SNPs |
| Vitamin D | United Kingdom Biobank | European | 496946 | 6896093 |
| Vitamin K | CHAGRE | European | 2138 | / |
| Vitamin E | ATBC&PLCO&NHS | European | 7781 | / |
| Vitamin A | ATBC&PLCO&NHS | European | 7778 | / |
| Vitamin B12 | SIID | European | 45576 | / |
| Polycystic ovarian syndrome | FinnGen Biobank | European | 118870 | 16379676 |
| Hyperlipidaemia | FinnGen Biobank | European | 201497 | 16380389 |
| Obesity | FinnGen Biobank | European | 218735 | 16380465 |
| Insulin resistance | MAGIC | European | 37037 | 2435028 |
The primary measure in this MR study was PCOS. Table 1 provides a summary of the specific sources of outcome data. Summary statistics for PCOS in individuals of European ancestry were obtained from the FinnGen Biobank consortium, which includes 118870 participants. The FinnGen project is a unique research endeavor that integrates genetic data with digital healthcare information from over 500000 Finnish biobank participants[19]. Hyperlipidemia, obesity, and insulin resistance are key pathways related to PCOS. Hyperlipidemia and obesity data were also sourced from the FinnGen Biobank consortium. Insulin resistance data were retrieved from the Meta-Analyses of Glucose and Insulin-related traits Consortium, involving up to 37037 participants[20].
Single nucleotide polymorphisms (SNPs) associated with vitamins D, E, A, and B12 were defined at the genome-wide significance threshold (P < 5 × 10-8). Owing to the limited number of SNPs for vitamin K, SNPs at a level of genome-wide significance of P < 5 × 10−6 were chosen as IVs. To ensure instrument validity, SNPs were filtered within a 1000 kb window with an r2 < 0.01 threshold[21]. Through a search of the GWAS Catalog (https://www.ebi.ac.uk/gwas/), we identified pleiotropic IV SNPs associated with any confounding factor related to the outcome. Estimates of the effects of these vitamin-related genetic variations on outcome datasets were collected. Additionally, SNP harmonization was conducted to restore allele orientation. The final selection of SNPs used in this MR is presented in Supplemen
The primary analyses were performed using the random-effects inverse-variance-weighted (IVW) approach, assuming all SNPs as valid IVs. The IVW method is considered highly reliable when there is no evidence of directional pleiotropy among the selected IVs (P value for MR-Egger intercept > 0.05)[22]. Complementary analyses were conducted using the weighted median[23] and MR-Egger[23] methods as supplements to the IVW approach. The weighted median model generates consistent causal findings when over 50% of the weights are derived from valid SNPs. The MR-Egger regression method can detect and adjust for directional pleiotropy[24].
Furthermore, we performed MR-robust adjusted profile score (MR-RAPS) using the “Huber” loss function to model a random-effects distribution of the pleiotropic effects of genetic variants[25]. Additionally, the MR-PRESSO approach was used to identify outlier SNPs and provide causal estimations after removing probable outliers, assuming that the employed SNPs are valid[26].
We used Cochran’s Q test to analyze the heterogeneity of the estimations from each SNP. When there was no statistically significant heterogeneity (P > 0.05), we used the fixed-effects model; however, the random-effects model was used to produce highly conservative estimations. Pleiotropy analysis was conducted using the MR-Egger intercept test. A zero intercept for MR-Egger (P > 0.05) indicates no presence of pleiotropic bias[27].
All tests were performed using the statistical program “R” v3.5 with the “TwoSampleMR,” “MR-PRESSO,” “Mr.raps,” and “Forestplot” packages. All analyses were two-sided, and statistical significance was set at P < 0.05.
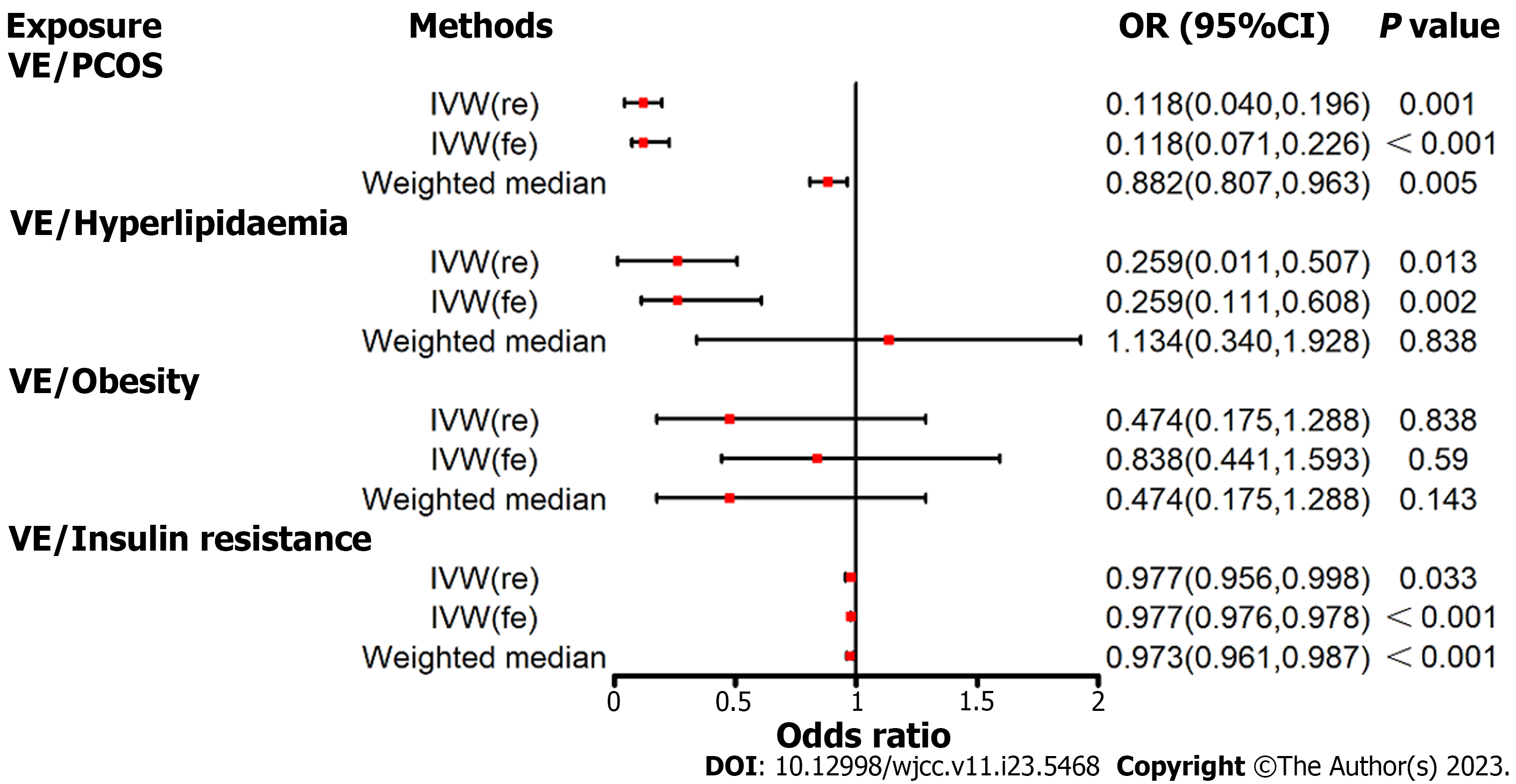
In the fixed-effects IVW estimations, genetically projected higher values of vitamin E were associated with a reduced risk of PCOS (Figure 2 and Supplementary Table 21). For 1-SD increase in genetically projected vitamin E concentrations, the combined odds ratio (OR) was 0.118 (95% confidence interval [CI]: 0.071–0.226, P < 0.001). The association remained consistent in complementary analyses when using the random-effects IVW and weighted median techniques. Higher vitamin E levels were correlated with a decreased risk of hyperlipidemia (OR = 0.259, 95%CI: 0.111–0.608, P = 0.002) and insulin resistance (OR = 0.977, 95%CI: 0.976–0.978, P < 0.001). However, no impact of vitamin E on obesity was observed via the fixed-effects IVW approach.
Genetically anticipated higher vitamin D concentrations were suggestive of a decreased risk of hyperlipidemia, as indicated via the random-effects IVW approach (OR = 0.749, 95%CI: 0.592–0.948, P = 0.016; Figure 3 and Supplemen
No evidence of directional pleiotropy was observed, but heterogeneity was present for vitamin D analysis on the key pathways of PCOS (Table 2). Furthermore, outlier SNPs were identified using the MR-PRESSO test, and the causal effect estimates of vitamin E on the risk of PCOS and key pathways implicated in its development were not statistically significant (Table 3).
| Exposure/Outcome | Heterogeneity | Pleiotropy | ||
| Cochran’s Q | P value | Egger-intercept | P value | |
| Vitamin D/PCOS | 180.692 | 0.368 | -0.006 | 0.487 |
| Vitamin D/Hyperlipidaemia | 449.648 | < 0.001 | -0.004 | 0.425 |
| Vitamin D/Obesity | 402.633 | < 0.001 | 0.002 | 0.494 |
| Vitamin D/Insulin resistance | 169.312 | 0.001 | -0.002 | 0.059 |
| Vitamin K/PCOS | 2.926 | 0.570 | 0.029 | 0.752 |
| Vitamin K/Hyperlipidaemia | 5.151 | 0.272 | 0.044 | 0.290 |
| Vitamin K/Obesity | 7.086 | 0.131 | 0.049 | 0.150 |
| Vitamin K/Insulin resistance | 7.932 | 0.094 | -0.008 | 0.469 |
| Vitamin B12/PCOS | 1.284 | 0.973 | 0.004 | 0.930 |
| Vitamin B12/Hyperlipidaemia | 3.346 | 0.764 | -0.022 | 0.297 |
| Vitamin B12/Obesity | 5.046 | 0.540 | 0.003 | 0.858 |
| Vitamin B12/Insulin resistance | 3.578 | 0.466 | -0.003 | 0.451 |
| Exposure trait | Outcome trait | N | Beta | P value |
| Vitamin D | PCOS | 172 | -0.092 | 0.645 |
| Vitamin D | Hyperlipidaemia | 169 | -0.141 | 0.126 |
| Vitamin D | Obesity | 174 | -0.031 | 0.618 |
| Vitamin D | Insulin resistance | 117 | -0.005 | 0.789 |
| Vitamin B12 | PCOS | 6 | -0.284 | 0.005 |
| Vitamin B12 | Hyperlipidaemia | 6 | -0.103 | 0.063 |
| Vitamin B12 | Obesity | 6 | -0.088 | 0.048 |
| Vitamin B12 | Insulin resistance | 6 | -0.015 | 0.319 |
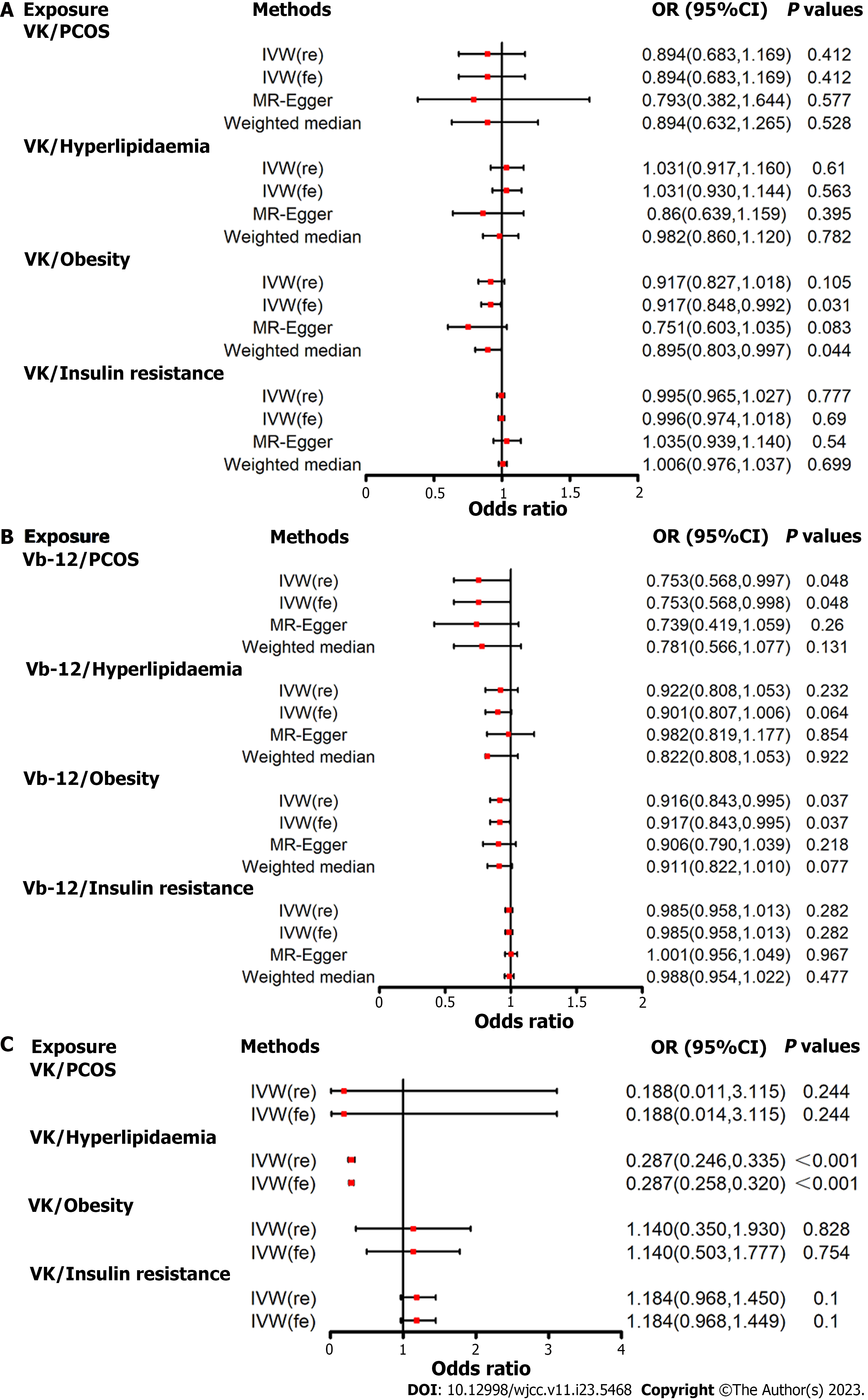
Figure 4A presents the MR estimation for the association of vitamin E supplementation with PCOS and key pathways implicated in its development. According to the fixed-effects IVW method, increased vitamin E levels were associated with a reduced risk of obesity (OR = 0.917, 95%CI: 0.848–0.992, P = 0.031; Figure 4A and Supplementary Table 21). However, no causal effect of vitamin E on PCOS, hyperlipidemia, and insulin resistance was observed. No evidence was observed of horizontal pleiotropy (P value for intercept > 0.05; Table 2) or heterogeneity as measured using Cochran's Q test (P value for Cochran's Q > 0.05; Table 3).
The fixed-effects IVW estimations suggested a link between genetically anticipated higher vitamin B12 Levels and a lower risk of PCOS (OR = 0.753, 95%CI: 0.568–0.998, P = 0.048) and obesity (OR = 0.917, 95%CI: 0.843–0.995, P = 0.037; Figure 4B and Supplementary Table 21). However, no correlation was observed between vitamin B12 levels and hyperlipidemia or insulin resistance.
No evidence of horizontal pleiotropy (P value for intercept > 0.05; Table 2) or heterogeneity as indicated by the Cochran's Q test (P value for Cochran’s Q > 0.05; Table 3) was found. After correcting for the outline SNPs, the causal effect estimates of vitamin B12 on the risk of PCOS (P = 0.005) and obesity (P = 0.048) remained statistically significant (Table 3).
The IVW estimate showed a significant association between genetically predicted vitamin A levels and hyperlipidemia risk (OR = 0.287, 95%CI: 0.258–0.320, P < 0.001; Figure 4C and Supplementary Table 21). This association was consistent with complementary analyses using the random-effects IVW method. However, no statistically significant causal effect estimates of vitamin A on the risk of PCOS, obesity, and insulin resistance were observed.
Supplementation with individual nutrients may improve health outcomes in women with PCOS by altering crucial PCOS-related pathways, such as insulin signaling, insulin resistance, and lipid metabolism. This MR analysis, based on large-scale genetic consortia, provides suggestive evidence supporting a causal effect of higher vitamins E and B12 levels on a decreased risk of PCOS. Our findings indicated that genetically predicted levels of vitamins K and B12 were related to a lower risk of obesity. Additionally, genetically predicted higher levels of vitamins E, D, and A were suggestively associated with a decreased risk of hyperlipidemia, while higher vitamin E levels were suggestively linked to a lower risk of insulin resistance.
Previous studies have employed cross-sectional, case-control, and cohort designs to investigate the association between vitamin supplementation and the risk of PCOS. However, the findings remain disputed. For instance, Panidis et al[28] found that patients with PCOS had lower levels of vitamin D compared with controls. Hahn et al[29] demonstrated a link between low serum 25-hydroxyvitamin D values and insulin resistance and obesity in women with PCOS. However, a meta-analysis of 30 trials did not provide evidence that vitamin D supplementation reduces or alleviates metabolic and hormonal dysregulations in PCOS[11]. This discrepancy may be attributed to the limitations of observational investigations, which are prone to residual confounding and imprecise measurements of confounders. In contrast, MR analysis offers highly accurate causal conclusions by leveraging the random assignment of genetic variations from parents to children.
A recent systemic review of 12 articles indicated that vitamin E supplementation improves lipid profile, reduces insulin levels, and decreases HOMA-IR values[30,31]. This is consistent with our findings suggesting that increased vitamin E concentrations are associated with a decreased risk of PCOS, hyperlipidemia, and insulin resistance. The anti-oxidative property of vitamin E, along with its effects on oxidative stress metrics, may explain its positive effects on lipid profile enhancement and insulin resistance[30]. Vitamin E acts as a substantial fat antioxidant, neutralizing peroxyl radicals and preventing the oxidation of polyunsaturated fatty acids[32,33]. Coenzyme Q10 is often supplemented together with vitamin E due to its synergistic roles in sustaining mitochondrial activity and integrity[10]. Foreign studies have shown favorable effects of coenzyme Q10 and vitamin E supplementation on blood insulin, HOMA-IR, and total testosterone levels in women with PCOS[34,35].
The role of vitamin B12 on PCOS remains unclear. B-group vitamins are responsible for breaking down Hcy in the blood, which is associated with insulin resistance[36]. However, our study provides no evidence of a causal effect of vitamin B12 on insulin resistance. A randomized controlled trial with B-group vitamin supplementation indicated a reduction in Hcy concentrations but no changes in insulin resistance[37].
Lipid metabolism is a key pathway in PCOS. Our MR analysis revealed that genetically predicted higher levels of vitamins E, D, and A are suggestively associated with a decreased risk of hyperlipidemia. This finding suggests the potential benefits of supplementing these vitamins. However, further functional studies in vivo are necessary to explore the underlying mechanisms. Additionally, adipose tissue plays a role as a metabolic and endocrine organ, and its overabundance can lead to alterations in body homeostasis and vitamin deficiency[2,38]. Therefore, vitamin supplementation may be beneficial in improving health outcomes in women with PCOS and obesity.
This study is the first MR investigation exploring the association between vitamin supplementation and PCOS and key pathways implicated in its development. The MR design strengthens causal inference by reducing residual confounding and other biases[39]. The use of data obtained from independent large GWAS ensures the reliability of the results. Furthermore, to address the potential influence of pleiotropic SNPs on our data, we implemented various techniques such as weighted median and MR-RAPS to minimize violations of the MR assumptions. Additionally, we used MR-PRESSO to identify and assess the probable presence of pleiotropy among the SNPs. Lastly, the genetic variants used as IVs were located on different chromosomes, minimizing the potential gene-gene interactions in our findings.
Nevertheless, our study has some limitations. First, the vitamin levels analyzed were genetically predicted concentrations, approximating average effects over the life course. The concentration of vitamins is influenced by the diet. Second, the analysis was restricted to participants of European ancestry to minimize bias due to population stratification; this limits the generalizability of the findings to non-European populations. Third, weak instrument bias may be present, given the low variability of vitamin levels explained by the SNPs. Fourth, although our study incorporated data from extensive genetic epidemiology networks, it may not be adequately powered to detect considerably small effects. Fifth, we were unable to obtain data stratified by the PCOS phenotype, warranting further investigation into the effect of vitamin supplements on different PCOS phenotypes. Lastly, we were unable to assess linear associations between vitamin levels and PCOS. Further prospective and functional studies are warranted to elucidate the role of vitamin supplements in PCOS.
Our MR analysis suggests that higher levels of vitamins A, D, E, K, and B12 are causally related to a reduced risk of PCOS or key pathways implicated in its development. Further prospective population-based studies and in vivo and in vitro trials are required to clarify the precise role of vitamin supplements in the onset of PCOS and key pathways implicated in its development.
Outcomes from conventional observational investigations are often based on the limited sample size and influenced by confounding factors.
To conduct a two-sample mendelian randomization (MR) analysis to assess the impact of plasma levels of vitamins A, B12, D, E, and K on polycystic ovary syndrome (PCOS) and key pathways implicated in its development, namely, insulin resistance, hyperlipidemia, and obesity.
To explore the causal relationship between increased vitamins A, D, E, K, and B12 values and a reduced risk of PCOS or primary pathways implicated in its development.
The inverse variance weighted (IVW) method is considered highly reliable when there is no evidence of directional pleiotropy among the selected instrumental variables. Complementary analyses were conducted using the weighted median and MR-Egger methods as supplements to the IVW method. Furthermore, the MR-robust adjusted profile score (MR-RAPS) and MR-PRESSO approaches were used to identify outlier single nucleotide polymorphisms (SNPs) and provide causal estimations after removing probable outliers, assuming that the employed SNPs are valid.
This MR analysis, based on large-scale genetic consortia, provided suggestive evidence supporting a causal effect of higher vitamins E and B12 levels on a decreased risk of PCOS. Our findings indicated that genetically predicted levels of vitamins K and B12 were related to a lower risk of obesity. Additionally, genetically predicted higher levels of vitamins E, D, and A were suggestively associated with a decreased risk of hyperlipidemia, while higher vitamin E levels were suggestively linked to a lower risk of insulin resistance.
Higher levels of vitamins A, D, E, K, and B12 are causally related to a reduced risk of PCOS or key pathways implicated in its development.
Further prospective population-based studies and in vivo and in vitro trials are required to clarify the precise role of vitamin supplements in the onset of PCOS and key pathways implicated in its development.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Reproductive biology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rahmoune H, Algeria S-Editor: Liu JH L-Editor: Wang TQ P-Editor: Cai YX
| 1. | Sadeghi HM, Adeli I, Calina D, Docea AO, Mousavi T, Daniali M, Nikfar S, Tsatsakis A, Abdollahi M. Polycystic Ovary Syndrome: A Comprehensive Review of Pathogenesis, Management, and Drug Repurposing. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 195] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 2. | Shrivastava S, Conigliaro RL. Polycystic Ovarian Syndrome. Med Clin North Am. 2023;107:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 3. | Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-2855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1043] [Cited by in RCA: 863] [Article Influence: 95.9] [Reference Citation Analysis (0)] |
| 4. | Anagnostis P, Tarlatzis BC, Kauffman RP. Polycystic ovarian syndrome (PCOS): Long-term metabolic consequences. Metabolism. 2018;86:33-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 221] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 5. | Ibáñez L, de Zegher F. Adolescent PCOS: a postpubertal central obesity syndrome. Trends Mol Med. 2023;29:354-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 46] [Reference Citation Analysis (0)] |
| 6. | Joham AE, Norman RJ, Stener-Victorin E, Legro RS, Franks S, Moran LJ, Boyle J, Teede HJ. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. 2022;10:668-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 323] [Article Influence: 107.7] [Reference Citation Analysis (0)] |
| 7. | Zeng X, Xie YJ, Liu YT, Long SL, Mo ZC. Polycystic ovarian syndrome: Correlation between hyperandrogenism, insulin resistance and obesity. Clin Chim Acta. 2020;502:214-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 248] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 8. | Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T, Norman RJ; International PCOS Network. Erratum. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2019;34:388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Menichini D, Ughetti C, Monari F, Di Vinci PL, Neri I, Facchinetti F. Nutraceuticals and polycystic ovary syndrome: a systematic review of the literature. Gynecol Endocrinol. 2022;38:623-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Heidari H, Hajhashemy Z, Saneei P. A meta-analysis of effects of vitamin E supplementation alone and in combination with omega-3 or magnesium on polycystic ovary syndrome. Sci Rep. 2022;12:19927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 11. | He C, Lin Z, Robb SW, Ezeamama AE. Serum Vitamin D Levels and Polycystic Ovary syndrome: A Systematic Review and Meta-Analysis. Nutrients. 2015;7:4555-4577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 142] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 12. | Alesi S, Ee C, Moran LJ, Rao V, Mousa A. Nutritional Supplements and Complementary Therapies in Polycystic Ovary Syndrome. Adv Nutr. 2022;13:1243-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 13. | Emdin CA, Khera AV, Kathiresan S. Mendelian Randomization. JAMA. 2017;318:1925-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 2189] [Article Influence: 273.6] [Reference Citation Analysis (0)] |
| 14. | Revez JA, Lin T, Qiao Z, Xue A, Holtz Y, Zhu Z, Zeng J, Wang H, Sidorenko J, Kemper KE, Vinkhuyzen AAE, Frater J, Eyles D, Burne THJ, Mitchell B, Martin NG, Zhu G, Visscher PM, Yang J, Wray NR, McGrath JJ. Genome-wide association study identifies 143 loci associated with 25 hydroxyvitamin D concentration. Nat Commun. 2020;11:1647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 271] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 15. | Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, Liu B, Matthews P, Ong G, Pell J, Silman A, Young A, Sprosen T, Peakman T, Collins R. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6354] [Cited by in RCA: 7487] [Article Influence: 748.7] [Reference Citation Analysis (0)] |
| 16. | Grarup N, Sulem P, Sandholt CH, Thorleifsson G, Ahluwalia TS, Steinthorsdottir V, Bjarnason H, Gudbjartsson DF, Magnusson OT, Sparsø T, Albrechtsen A, Kong A, Masson G, Tian G, Cao H, Nie C, Kristiansen K, Husemoen LL, Thuesen B, Li Y, Nielsen R, Linneberg A, Olafsson I, Eyjolfsson GI, Jørgensen T, Wang J, Hansen T, Thorsteinsdottir U, Stefánsson K, Pedersen O. Genetic architecture of vitamin B12 and folate levels uncovered applying deeply sequenced large datasets. PLoS Genet. 2013;9:e1003530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 17. | Mondul AM, Yu K, Wheeler W, Zhang H, Weinstein SJ, Major JM, Cornelis MC, Männistö S, Hazra A, Hsing AW, Jacobs KB, Eliassen H, Tanaka T, Reding DJ, Hendrickson S, Ferrucci L, Virtamo J, Hunter DJ, Chanock SJ, Kraft P, Albanes D. Genome-wide association study of circulating retinol levels. Hum Mol Genet. 2011;20:4724-4731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 18. | Dashti HS, Shea MK, Smith CE, Tanaka T, Hruby A, Richardson K, Wang TJ, Nalls MA, Guo X, Liu Y, Yao J, Li D, Johnson WC, Benjamin EJ, Kritchevsky SB, Siscovick DS, Ordovás JM, Booth SL. Meta-analysis of genome-wide association studies for circulating phylloquinone concentrations. Am J Clin Nutr. 2014;100:1462-1469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Locke AE, Steinberg KM, Chiang CWK, Service SK, Havulinna AS, Stell L, Pirinen M, Abel HJ, Chiang CC, Fulton RS, Jackson AU, Kang CJ, Kanchi KL, Koboldt DC, Larson DE, Nelson J, Nicholas TJ, Pietilä A, Ramensky V, Ray D, Scott LJ, Stringham HM, Vangipurapu J, Welch R, Yajnik P, Yin X, Eriksson JG, Ala-Korpela M, Järvelin MR, Männikkö M, Laivuori H; FinnGen Project, Dutcher SK, Stitziel NO, Wilson RK, Hall IM, Sabatti C, Palotie A, Salomaa V, Laakso M, Ripatti S, Boehnke M, Freimer NB. Exome sequencing of Finnish isolates enhances rare-variant association power. Nature. 2019;572:323-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 133] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 20. | Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, Wheeler E, Glazer NL, Bouatia-Naji N, Gloyn AL, Lindgren CM, Mägi R, Morris AP, Randall J, Johnson T, Elliott P, Rybin D, Thorleifsson G, Steinthorsdottir V, Henneman P, Grallert H, Dehghan A, Hottenga JJ, Franklin CS, Navarro P, Song K, Goel A, Perry JR, Egan JM, Lajunen T, Grarup N, Sparsø T, Doney A, Voight BF, Stringham HM, Li M, Kanoni S, Shrader P, Cavalcanti-Proença C, Kumari M, Qi L, Timpson NJ, Gieger C, Zabena C, Rocheleau G, Ingelsson E, An P, O'Connell J, Luan J, Elliott A, McCarroll SA, Payne F, Roccasecca RM, Pattou F, Sethupathy P, Ardlie K, Ariyurek Y, Balkau B, Barter P, Beilby JP, Ben-Shlomo Y, Benediktsson R, Bennett AJ, Bergmann S, Bochud M, Boerwinkle E, Bonnefond A, Bonnycastle LL, Borch-Johnsen K, Böttcher Y, Brunner E, Bumpstead SJ, Charpentier G, Chen YD, Chines P, Clarke R, Coin LJ, Cooper MN, Cornelis M, Crawford G, Crisponi L, Day IN, de Geus EJ, Delplanque J, Dina C, Erdos MR, Fedson AC, Fischer-Rosinsky A, Forouhi NG, Fox CS, Frants R, Franzosi MG, Galan P, Goodarzi MO, Graessler J, Groves CJ, Grundy S, Gwilliam R, Gyllensten U, Hadjadj S, Hallmans G, Hammond N, Han X, Hartikainen AL, Hassanali N, Hayward C, Heath SC, Hercberg S, Herder C, Hicks AA, Hillman DR, Hingorani AD, Hofman A, Hui J, Hung J, Isomaa B, Johnson PR, Jørgensen T, Jula A, Kaakinen M, Kaprio J, Kesaniemi YA, Kivimaki M, Knight B, Koskinen S, Kovacs P, Kyvik KO, Lathrop GM, Lawlor DA, Le Bacquer O, Lecoeur C, Li Y, Lyssenko V, Mahley R, Mangino M, Manning AK, Martínez-Larrad MT, McAteer JB, McCulloch LJ, McPherson R, Meisinger C, Melzer D, Meyre D, Mitchell BD, Morken MA, Mukherjee S, Naitza S, Narisu N, Neville MJ, Oostra BA, Orrù M, Pakyz R, Palmer CN, Paolisso G, Pattaro C, Pearson D, Peden JF, Pedersen NL, Perola M, Pfeiffer AF, Pichler I, Polasek O, Posthuma D, Potter SC, Pouta A, Province MA, Psaty BM, Rathmann W, Rayner NW, Rice K, Ripatti S, Rivadeneira F, Roden M, Rolandsson O, Sandbaek A, Sandhu M, Sanna S, Sayer AA, Scheet P, Scott LJ, Seedorf U, Sharp SJ, Shields B, Sigurethsson G, Sijbrands EJ, Silveira A, Simpson L, Singleton A, Smith NL, Sovio U, Swift A, Syddall H, Syvänen AC, Tanaka T, Thorand B, Tichet J, Tönjes A, Tuomi T, Uitterlinden AG, van Dijk KW, van Hoek M, Varma D, Visvikis-Siest S, Vitart V, Vogelzangs N, Waeber G, Wagner PJ, Walley A, Walters GB, Ward KL, Watkins H, Weedon MN, Wild SH, Willemsen G, Witteman JC, Yarnell JW, Zeggini E, Zelenika D, Zethelius B, Zhai G, Zhao JH, Zillikens MC; DIAGRAM Consortium; GIANT Consortium; Global BPgen Consortium, Borecki IB, Loos RJ, Meneton P, Magnusson PK, Nathan DM, Williams GH, Hattersley AT, Silander K, Salomaa V, Smith GD, Bornstein SR, Schwarz P, Spranger J, Karpe F, Shuldiner AR, Cooper C, Dedoussis GV, Serrano-Ríos M, Morris AD, Lind L, Palmer LJ, Hu FB, Franks PW, Ebrahim S, Marmot M, Kao WH, Pankow JS, Sampson MJ, Kuusisto J, Laakso M, Hansen T, Pedersen O, Pramstaller PP, Wichmann HE, Illig T, Rudan I, Wright AF, Stumvoll M, Campbell H, Wilson JF; Anders Hamsten on behalf of Procardis Consortium; MAGIC investigators, Bergman RN, Buchanan TA, Collins FS, Mohlke KL, Tuomilehto J, Valle TT, Altshuler D, Rotter JI, Siscovick DS, Penninx BW, Boomsma DI, Deloukas P, Spector TD, Frayling TM, Ferrucci L, Kong A, Thorsteinsdottir U, Stefansson K, van Duijn CM, Aulchenko YS, Cao A, Scuteri A, Schlessinger D, Uda M, Ruokonen A, Jarvelin MR, Waterworth DM, Vollenweider P, Peltonen L, Mooser V, Abecasis GR, Wareham NJ, Sladek R, Froguel P, Watanabe RM, Meigs JB, Groop L, Boehnke M, McCarthy MI, Florez JC, Barroso I. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42:105-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1722] [Cited by in RCA: 1721] [Article Influence: 114.7] [Reference Citation Analysis (0)] |
| 21. | Park S, Lee S, Kim Y, Lee Y, Kang MW, Kim K, Kim YC, Han SS, Lee H, Lee JP, Joo KW, Lim CS, Kim YS, Kim DK. Atrial fibrillation and kidney function: a bidirectional Mendelian randomization study. medRxiv. 2020;. [DOI] [Full Text] |
| 22. | Holmes MV, Ala-Korpela M, Smith GD. Mendelian randomization in cardiometabolic disease: challenges in evaluating causality. Nat Rev Cardiol. 2017;14:577-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 488] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 23. | Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol. 2016;40:304-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4015] [Cited by in RCA: 5756] [Article Influence: 639.6] [Reference Citation Analysis (0)] |
| 24. | Burgess S, Thompson SG. Erratum to: Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32:391-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 25. | Fu Y, Xu F, Jiang L, Miao Z, Liang X, Yang J, Larsson SC, Zheng JS. Circulating vitamin C concentration and risk of cancers: a Mendelian randomization study. BMC Med. 2021;19:171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 26. | Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693-698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2495] [Cited by in RCA: 5399] [Article Influence: 771.3] [Reference Citation Analysis (0)] |
| 27. | Chen L, Sun X, Wang Z, Lu Y, Chen M, He Y, Xu H, Zheng L. The impact of plasma vitamin C levels on the risk of cardiovascular diseases and Alzheimer's disease: A Mendelian randomization study. Clin Nutr. 2021;40:5327-5334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 28. | Panidis D, Balaris C, Farmakiotis D, Rousso D, Kourtis A, Balaris V, Katsikis I, Zournatzi V, Diamanti-Kandarakis E. Serum parathyroid hormone concentrations are increased in women with polycystic ovary syndrome. Clin Chem. 2005;51:1691-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Hahn S, Haselhorst U, Tan S, Quadbeck B, Schmidt M, Roesler S, Kimmig R, Mann K, Janssen OE. Low serum 25-hydroxyvitamin D concentrations are associated with insulin resistance and obesity in women with polycystic ovary syndrome. Exp Clin Endocrinol Diabetes. 2006;114:577-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 196] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 30. | Tefagh G, Payab M, Qorbani M, Sharifi F, Sharifi Y, Ebrahimnegad Shirvani MS, Pourghazi F, Atlasi R, Shadman Z, Rezaei N, Mohammadi-Vajari E, Larijani B, Ebrahimpur M. Effect of vitamin E supplementation on cardiometabolic risk factors, inflammatory and oxidative markers and hormonal functions in PCOS (polycystic ovary syndrome): a systematic review and meta-analysis. Sci Rep. 2022;12:5770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 31. | Yalle-Vásquez S, Osco-Rosales K, Nieto-Gutierrez W, Benites-Zapata V, Pérez-López FR, Alarcon-Ruiz CA. Vitamin E supplementation improves testosterone, glucose- and lipid-related metabolism in women with polycystic ovary syndrome: a meta-analysis of randomized clinical trials. Gynecol Endocrinol. 2022;38:548-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 32. | Lee GY, Han SN. The Role of Vitamin E in Immunity. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 294] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 33. | Palamanda JR, Kehrer JP. Involvement of vitamin E and protein thiols in the inhibition of microsomal lipid peroxidation by glutathione. Lipids. 1993;28:427-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 45] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Izadi A, Ebrahimi S, Shirazi S, Taghizadeh S, Parizad M, Farzadi L, Gargari BP. Hormonal and Metabolic Effects of Coenzyme Q10 and/or Vitamin E in Patients With Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2019;104:319-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Zhang J, Xing C, Zhao H, He B. The effectiveness of coenzyme Q10, vitamin E, inositols, and vitamin D in improving the endocrine and metabolic profiles in women with polycystic ovary syndrome: a network Meta-analysis. Gynecol Endocrinol. 2021;37:1063-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Kennedy DO. B Vitamins and the Brain: Mechanisms, Dose and Efficacy--A Review. Nutrients. 2016;8:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 514] [Cited by in RCA: 521] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 37. | Kilicdag EB, Bagis T, Tarim E, Aslan E, Erkanli S, Simsek E, Haydardedeoglu B, Kuscu E. Administration of B-group vitamins reduces circulating homocysteine in polycystic ovarian syndrome patients treated with metformin: a randomized trial. Hum Reprod. 2005;20:1521-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | Thomas-Valdés S, Tostes MDGV, Anunciação PC, da Silva BP, Sant'Ana HMP. Association between vitamin deficiency and metabolic disorders related to obesity. Crit Rev Food Sci Nutr. 2017;57:3332-3343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 39. | Yuan S, Mason AM, Carter P, Burgess S, Larsson SC. Homocysteine, B vitamins, and cardiovascular disease: a Mendelian randomization study. BMC Med. 2021;19:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |