Published online Aug 6, 2023. doi: 10.12998/wjcc.v11.i22.5244
Peer-review started: March 29, 2023
First decision: June 1, 2023
Revised: June 6, 2023
Accepted: July 3, 2023
Article in press: July 3, 2023
Published online: August 6, 2023
Processing time: 126 Days and 15.5 Hours
The effect of the sympathetic nervous system on peripheral arteries causes vasoconstriction when smooth muscle cells in the walls of blood vessels contract, which leads to narrowing of arteries and reduction of the blood flow.
To compare sympathetic vasomotor activation of the brachial arteries in healthy subjects and patients with painful diabetic neuropathy; and therefore, to assess whether there is significant vasomotor dysfunction of medium sized arteries in diabetic neuropathy.
The study included 41 diabetic neuropathy patients and 41 healthy controls. Baseline diameter and flow rate of the brachial arteries were measured. Then, using a bipolar stimulus electrode, a 10 mA, 1 Hz electrical stimulus was admini
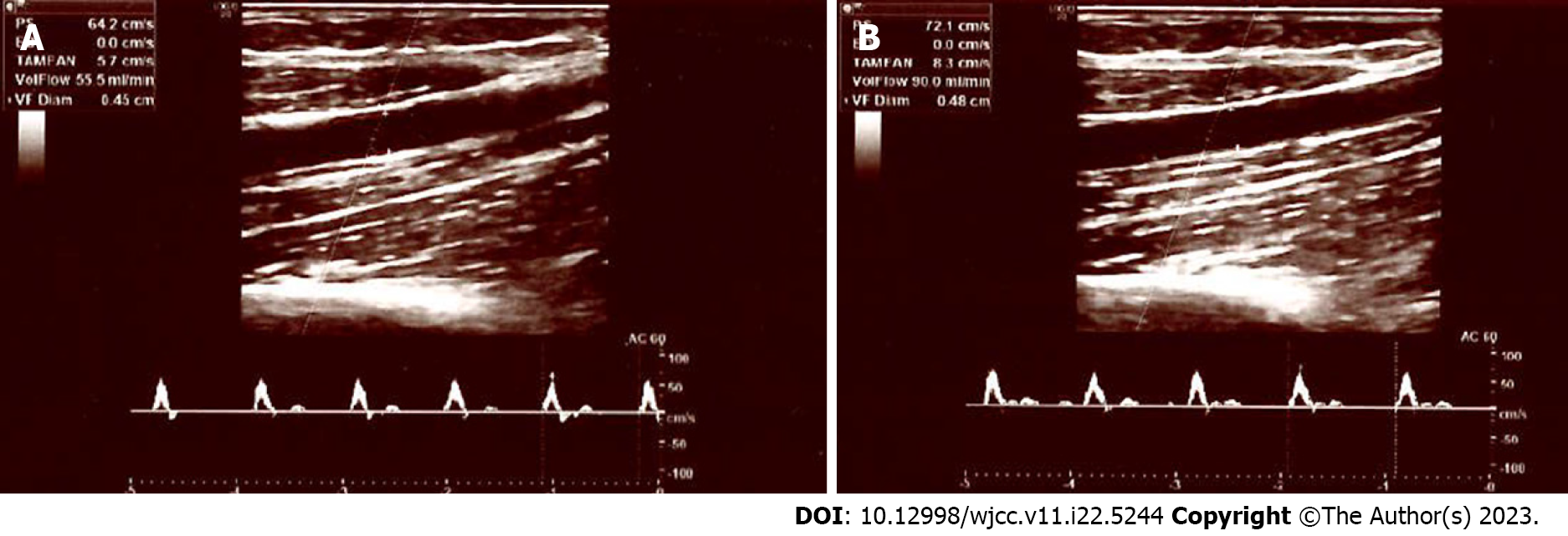
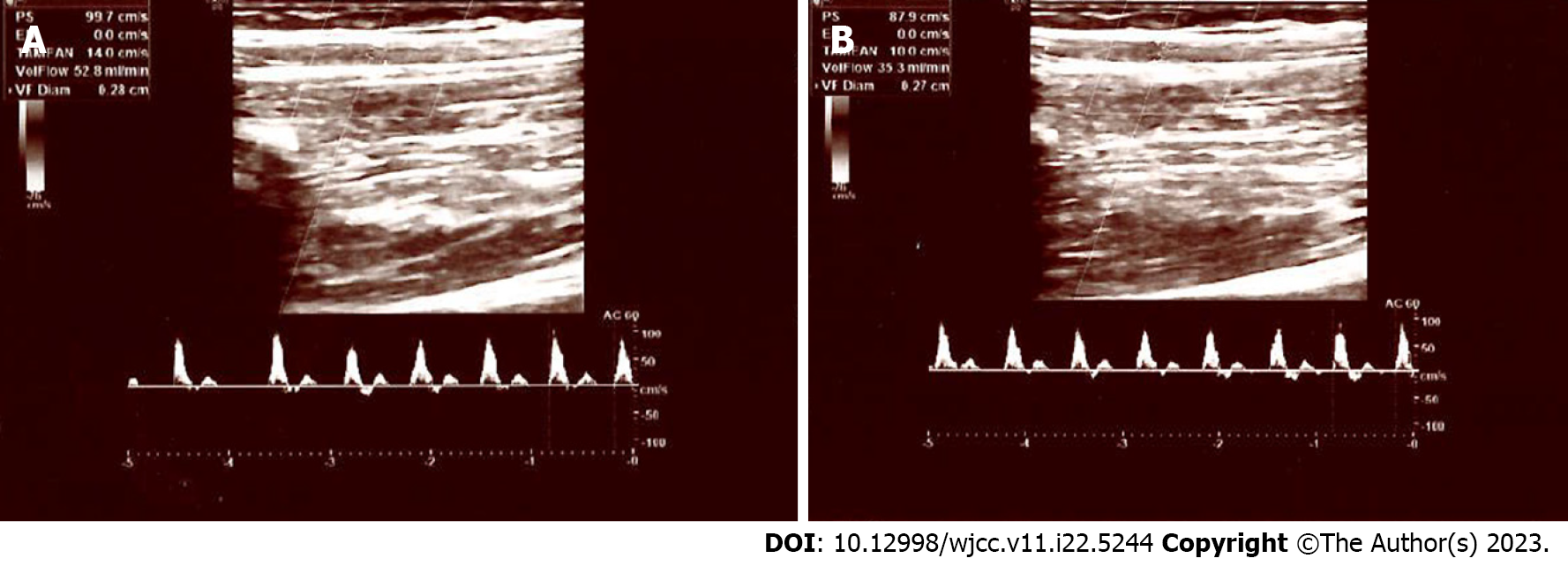
In the control group, the median flow rate was 70.0 mL/min prior to stimulation and 35.0 mL/min after stimulation, with a statistically significant decrease (P < 0.001), which is consistent with sympathetic nervous system functioning (vasoconstriction). In the diabetic neuropathy group, median flow rate before the stimulation was 35.0 mL/min. After stimulation, the median flow rate was 77.0 mL/min; thus, no significant decrease in the flow rate was detected. In the control group, the median brachial artery diameter, which was 3.6 mm prior to stimulation, decreased to 3.4 mm after stimulation, and this decrease was also statistically significant (P = 0.046). In the diabetic neuropathy group, the median brachial artery diameter increased from 3.4 mm to 3.6 mm following nerve stimulation. Once again, no narrowing was observed.
Our research suggests that diabetic neuropathy results in significant vasomotor dysfunction of medium sized peripheral arteries. Physiological vasoconstriction in response to sympathetic activation is impaired in diabetic neuropathy.
Core Tip: Our research is consistent with the observations that diabetic neuropathy results in sympathetic vasomotor dysfunction. Furthermore, the new non-invasive, inexpensive, and user-friendly technique that we offer is sensitive to this phenomenon in medium sized arteries. It also has the potential to be used as a tool for early detection of sympathetic dysfunction due to other causes. Our research suggests that diabetic neuropathy results in significant vasomotor dysfunction of medium sized peripheral arteries. Moreover, this is the first study to investigate diabetic autonomic neuropathy in medium sized arteries. Physiological vasoconstriction in response to sympathetic activation is impaired in painful diabetic neuropathy.
- Citation: Ege F, Kazci Ö, Aydin S. Diabetic neuropathy results in vasomotor dysfunction of medium sized peripheral arteries. World J Clin Cases 2023; 11(22): 5244-5251
- URL: https://www.wjgnet.com/2307-8960/full/v11/i22/5244.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i22.5244
Diabetes mellitus is a chronic metabolic disorder that affects millions of people worldwide[1]. One of the most common complications associated with diabetes mellitus is distal symmetrical peripheral neuropathy, which affects the peripheral nerves and can result in a variety of symptoms, including pain, paraesthesia, numbness, tingling, discomfort, and sensations of burning or cold etc. Diabetic neuropathy with painful symptoms, also known as painful diabetic neuropathy (PDN), is a particularly debilitating form of the condition.
There are several factors associated with PDN, which have been extensively studied in the scientific literature. One of the main factors associated with PDN is hyperglycaemia, or dysregulation of blood sugar levels. Chronic hyperglycaemia may harm the nerves through a variety of mechanisms, including oxidative stress, inflammation, and advanced glycation end products[2]. Additionally, hyperglycaemia can impair the microcirculation of the nerves, leading to ischemia and further nerve damage[3]. Other factors associated with PDN include dyslipidaemia, which results in nerve damage through similar mechanisms to hyperglycaemia[4]; chronic inflammation, which can contribute to nerve damage and sensitization of pain pathways[5]; and genetic factors, which may predispose individuals to develop PDN[6].
In patients with diabetic neuropathy, autonomic nervous system dysfunctions have also been observed. Signs and symptoms related to cardiovascular, sudomotor, gastrointestinal and thermoregulatory systems are frequently reported[7]. Cardiovascular autonomic neuropathy and sympathetic failure such as orthostatic hypotension secondary to diabetes mellitus can currently be diagnosed with multiple autonomous tests[8]. It was reported that sympathetic skin response (SSR) amplitudes were significantly lower in diabetics than in controls[9]. Additionally, the SSR test is suggested to be a sensitive indicator of autonomic dysfunction in diabetes[10]. All these data indicate that sympathetic nervous system (SNS) dysfunction is frequently complicated by PDN.
The SNS plays crucial role in regulating blood flow to peripheral vascular structures[11]. The effect of the system on peripheral arteries is that vasoconstriction occurs when smooth muscle cells in the walls of blood vessels contract, which leads to narrowing of arteries and a reduction of the blood flow[12]. On the other hand, peripheral vasodilator response of extremities is not mediated by the parasympathetic nervous system but by local nitrergic mechanisms[13]. The SNS induces vasoconstriction through the release of norepinephrine from sympathetic nerve terminals, which binds to alpha-adrenergic receptors on smooth muscle cells. Activation of alpha-adrenergic receptors triggers the opening of calcium channels in smooth muscles, vessel wall contraction, and arterial vasoconstriction[14].
There are several tests that can be used to diagnose autonomic dysfunction in diabetic neuropathy. The Heart rate variability test, Tilt table test, Valsalva Ratio, Quantitative sudomotor axon reflex testing (QSART) and Sympathetic Skin Response (SSR) are some of these tests[15].
Doppler ultrasound combined with low-dose electrical stimulation of a peripheral nerve with the “electroneuromyography, evoked potential” (ENMG/EP) system to evaluate autonomous system functioning can also be used as an alternative test to these procedures. This test specifically measures the sympathetic vasomotor response to peripheric neural activation and was previously defined by us in active and healthy subjects elsewhere. In our previous study, it was demonstrated that after electrical stimulation of the median nerve, blood flow and diameter of the brachial artery decreased in healthy subjects[16]. We also previously demonstrated that the sympathetic fibres in the median nerve contribute on the brachial artery’s vasomotor functioning[17]. Hence, the purpose of this study was to compare sympathetic vasomotor activation of the brachial artery in response to median nerve activation in healthy subjects and PDN patients; and therefore, to assess whether there is significant vasomotor dysfunction in PDN patients. In this study, our main purpose was to determine the impact of diabetic neuropathies on medium sized arteries.
This experimental study was conducted at VM Medicalpark Ankara Hospital in 2022. The studies adhered to the most recent version of the Declaration of Helsinki, and the No. 2 Clinical Research Ethics Committee of Ankara City Hospital allowed the procedures. The ethics committee at the Ankara City Hospital is the most approved ethics body within the Turkish Ministry of Health. Due to the nature and significance of our research, we deemed it necessary to obtain ethical approval from Ankara City Hospital (Approval number: E3-22-1307). Each participant and/or guardian gave informed consent (s).
Our study included 41 diabetic individuals with painful neuropathy and 41 healthy controls. Diabetic patients were randomly chosen from follow-up patients in the Endocrinology Clinic who had neuropathic symptoms. These subjects were diagnosed with Diabetes Mellitus due to abnormal fasting and postprandial glucose levels and high HbA1c values. Diabetic neuropathy was diagnosed by neuropathic symptoms, neurological examination, and electrophysiological methods performed by an experienced neurologist. In both groups, participants with heart disease, essential hypertension, and carpal tunnel syndrome; and in the control group, diabetes mellitus and polyneuropathy from any cause were excluded. The ratio of females to males was maintained in both the patient and control groups. For each patient in the patient group, age-matched controls were assigned.
Similar methods to "sensory nerve conduction studies" were conducted. Such non-invasive methods are routinely used for the diagnosis of peripheral sensorial neuropathies in electrophysiology laboratories. There was a four-hour period before the experiment in which smoking, consumption of coffee, tea and alcohol, and exercise were discouraged. At the beginning of the experiment, individuals sat quietly for 10 min with their right forearms supinated. Their blood pressure, heart rate, and body temperature were then measured, and individuals with abnormal results (hypertension, fever, tachycardia etc.) were excluded. Thus, normal hemodynamic parameters required for the experiment were maintained. After a 10-min break, individuals were asked to remain as still as possible and they were given an oral briefing on what to expect at each stage of the test. In this study, we used a 9-Hz linear probe of a General Electric LOQIC P9 Doppler device to measure the baseline diameter and flow rate of the right brachial artery 2 cm above the antecubital fossa. These measurements were taken by a radiologist who has worked in the field of Doppler for eight years. The Doppler sensor was stationary during the experiment so that continuous data could be collected. Then, using the bipolar stimulus electrode of the Nihon Kohden MEB-9400A EMG/EP system, we administered a 10 mA (milliampere), 1 Hz (Hertz) electrical stimulus orthodromically (that is, through physiological transmission of the sensorial nerve fibres) to the median nerve at the wrist level for 5 s. The artery diameter and blood flow rate were re-measured immediately after the sixth stimulation.
The variables were investigated using analytical methods (Kolmogorov-Smirnov/Shapiro-Wilk’s test) to determine whether they were normally distributed. Descriptive analyses are presented using frequencies and percentages for the categorical variables, mean ± SD for normally distributed variables and median (25P-75P) for the non-normally distributed variables. The Student-t Test or Mann Whitney U test was used for the comparison of continuous variables in independent groups, and the Paired-Sample T-test or Wilcoxon test was used in dependent groups.
A P value of less than 0.05 was considered statistically significant. Statistical analyses were performed using the SPSS (20.0. Armonk, NY, United States).
In addition to sociodemographic data such as age and gender, the diameters and flow rates of the brachial arteries were recorded as final values before and after electrical stimulation in patients with diabetic neuropathy and in the control group.
The study included 82 participants, 41 of whom had PDN and 41 of whom were controls. The mean age of the 82 participants was 59.7 ± 11.2 years, and 54 (65.9%) of the participants were female (min. 38 - max. 84).
Table 1 displays the flow measurements of the brachial artery before and after stimulation of the median nerve in the PDN and control groups. In the control group, the median flow rate was 70.0 mL/min prior to stimulation (Min: 23 mL/min and Max: 204 mL/min) and 35.0 mL/min after stimulation (Min: 7 mL/min and Max: 103 mL/min), with a statistically significant decrease detected following stimulation (P < 0.001) (Table 1). Before nerve stimulation, median flow rate in the PDN group was 35.0 mL/min (Min: 8 mL/min and Max: 103 mL/min). After stimulation, the median flow rate was 77.0 mL/min (Min: 23 mL/min and Max: 201 mL/min); thus, no significant decrease in the flow rate was detected (besides, significant higher values were observed).
| Diabetic neuropathy (n = 41) | Control (n = 41) | P value1 | |
| Flow before stimulation (mL/min) | < 0.001 | ||
| mean ± SD | 40.2 ± 19.8 | 75.5 ± 31.9 | |
| Median (25P-75P) | 35.0 (30.0-53.0) | 70.0 (65.0-86.0) | |
| Min.-Max | 8.0-103.0 | 23.0-204.0 | |
| Flow after stimulation (mL/min) | < 0.001 | ||
| mean ± SD | 88.5 ± 40.6 | 38.1 ± 17.3 | |
| Median (25P-75P) | 77.0 (65.0-112.0) | 35.0 (26.0-45.0) | |
| Min.-Max. | 23.0-201.0 | 7.0-103.0 | |
| P value2 | < 0.001 | < 0.001 |
In the control group, the median brachial artery diameter, which was 3.6 mm prior to stimulation (Min: 2.8 mm and Max: 4.4 mm), decreased to 3.4 mm after stimulation (Min: 2.3 mm and Max: 4.7 mm), and this decrease was statistically significant (P = 0.046) (Table 2). In the PDN group, the median brachial artery diameter increased from 3.4 mm (Min: 2.1 mm and Max: 5.1 mm) to 3.6 mm following nerve stimulation (Min: 2.9 mm and Max: 4.6 mm). Once again, no narrowing was observed.
| Diabetic neuropathy (n = 41) | Control (n = 41) | P value1 | |
| Diameter before stimulation (mm) | 0.107 | ||
| mean ± SD | 3.4 ± 0.6 | 3.6 ± 0.4 | |
| Median (25P-75P) | 3.4 (3.1-3.8) | 3.6 (3.2-4.0) | |
| Min.-Max. | 2.1-5.1 | 2.9-4.6 | |
| Diameter after stimulation (mm) | 0.055 | ||
| mean ± SD | 3.6 ± 0.4 | 3.4 ± 0.5 | |
| Median (25P-75P) | 3.6 (3.2-4.0) | 3.4 (3.1-3.8) | |
| Min.-Max. | 2.9-4.6 | 2.3-4.7 | |
| P value2 | 0.072 | 0.046 |
Table 3 summarizes the changes in brachial artery flow and diameter measurements induced by stimulation of the median nerve. In the PDN group, the brachial artery output increased by a median of 39.0 mL/min, whereas it decreased by a median of 34.0 mL/min in the control group (P < 0.001). In the PDN group, the average diameter increased by 0.2 mm, whereas the diameter decreased by 0.2 mm in the control group (P = 0.007). These variations were deemed statistically significant (Table 3).
| Diabetic neuropathy (n = 41) | Control (n = 41) | P value | |
| Change in flow (mL/min) | < 0.0011 | ||
| mean ± SD | 48.3 ± 33.1 | -37.4 ± 21.2 | |
| Median (25P-75P) | 39.0 (26.0-60.0) | -34.0 [-46.0-(-25.0)] | |
| Min.-Max. | 1.0-168.0 | -102.0-(-0.2) | |
| Change in diameter (mm) | 0.0072 | ||
| mean ± SD | 0.2 ± 0.7 | -0.2 ± 0.6 | |
| Median (25P-75P) | 0.2 (-0.2-0.4) | -0.2 (-0.6-0.3) | |
| Min.-Max. | -1.4-1.7 | -1.5-0.9 |
Tables 4 and 5 show the findings of the investigation undertaken to identify the effect of gender on the change in flow rate and diameter according to chronic illness status. In the presence of diabetic neuropathy, the change in flow between men and women was statistically substantially different (P = 0.004), but there was no statistically significant difference between the changes in flow rate between men and women in the control group (P = 0.137). Increases in circumference were observed to be statistically equivalent between men and women in both the diabetic and control groups (P > 0.05).
| Flow rate (mL/min) | P value1 | |||
| Median (25P-75P) | Min.-Max. | |||
| Diabetic neuropathy | Female (n = 27) | 43.0 (35.0-77.0) | 1.0-168.0 | 0.004 |
| Male (n = 14) | 28.5 (22.0-40.0) | 10.0-59.0 | ||
| Control | Female (n = 27) | -31.0 (-44.7-(-22.0) | -66.9-(-0.2) | 0.137 |
| Male (n = 14) | -40.0 (-50.0-(-32.0) | -102.0-(-9.0) | ||
| Artery diameter (mm) | P value1 | |||
| mean ± SD | Min.-Max. | |||
| Diabetic neuropathy | Female (n = 27) | 0.3 ± 0.6 | -1.3-1.7 | 0.223 |
| Male (n = 14) | 0.0 ± 0.7 | -1.4-1.2 | ||
| Control | Female (n = 27) | -0.3 ± 0.7 | -1.5-0.9 | 0.250 |
| Male (n = 14) | 0.0 ± 0.5 | -1.3-0.6 | ||
Our results indicate that vasomotor responses of medium sized arteries are impaired in diabetic neuropathy. In PDN, the distal sensory and autonomic fibres are initially damaged[18]. Therefore, autonomic involvement of differing degrees is frequently complicated by painful diabetic distal neuropathy. Diabetic autonomic neuropathy (DAN) is a complication of diabetes that affects the autonomic nervous system, which controls the involuntary functions of the body such as heart rate, blood pressure and digestion[19]. There are several tests used in the detection of DAN, including much more standardized cardiovascular reflex tests[20], less standardized quantitative sensory tests and sudomotor tests[19]. However, autonomic dysfunction measured by the vasomotor response of medium sized arteries has not been reported in the literature.
Cardiovascular reflex tests are the most frequently used tests for the diagnosis of DAN. These tests measure the heart rate and blood pressure responses to various stimuli such as deep breathing, standing up, and the Valsalva manoeuvre. Abnormal responses to these tests indicate dysfunction of the autonomic nervous system. The most widely used cardiovascular reflex tests are the heart rate response to deep breathing (HRDB), the heart rate response to standing (HRS), and the blood pressure response to standing (BPS). A reduction in HRDB, HRS or BPS is indicative of autonomic dysfunction[15,19,21].
Quantitative sensory tests measure the ability of a person to perceive changes in temperature, pressure, and vibration. These tests are useful for detecting early changes in peripheral autonomic nerve functioning. Abnormalities in these tests suggest damage to small sensory nerves, which is a common manifestation of DAN[20].
Sudomotor tests measure the function of sweat glands, which are controlled by the SNS. These tests assess the ability of the sweat glands to produce sweat in response to a stimulus such as heat or electrical stimulation. Abnormal results in these tests suggest dysfunction of the autonomic nervous system[15,21].
One of the most widely used tests for sudomotor function is the QSART, which measures the ability of the sweat glands to produce sweat in response to an electrical stimulus. Other sudomotor tests include the thermoregulatory sweat test, which measures the ability of the body to regulate temperature, and the silicone impression method, which measures the density of sweat gland openings on the skin[21].
In diabetic neuropathy, sympathetic vasomotor response in peripheral arteries is also impaired[22,23]. However, laser Doppler flowmetry utilized in these tests is not widely used in clinical practise at present. Additionally, these tests specifically measure the vasomotor alterations in smaller arteries and arterioles, and the vasomotor response of medium sized arteries is unknown. However, in this study, we examined the sympathetic vasomotor response of the brachial artery, which is one of the medium sized peripheral arteries in the human body. In our control subjects, both the diameter and flow rate of the brachial artery were significantly decreased following electrical stimulation of the median nerve, i.e., when peripheral sympathetic fibres are activated. The opposite is true for PDN patients; following stimulation, no significant vasoconstriction response was observed. These results suggest that painful neuropathy is frequently complicated by SNS dysfunction and impaired vasomotor tone of medium arteries. Figures 1 and 2 depict the Doppler parameters of healthy participants and diabetic neuropathy patients.
Our research is consistent with the observation that diabetic neuropathy results in sympathetic vasomotor dysfunction. Furthermore, the new non-invasive, inexpensive, and user-friendly technique that we offer is sensitive to this phenomenon in medium sized arteries. It also has the potential to be used as a tool for early detection of sympathetic dysfunction due to other causes (amyloid neuropathy, Guillain Barre Syndrome, transverse myelitis etc.). The limitation of this study is that types and duration of diabetes mellitus were not taken into consideration and patient sub-groups were not formed. Further studies are required to make decisions on how vascular tone differs in subtypes.
In conclusion, our research suggests that diabetic neuropathy results in significant vasomotor dysfunction of medium sized peripheral arteries. Moreover, this is the first study to investigate DAN in medium sized arteries. Physiological vasoconstriction in response to sympathetic activation is impaired in patients with painful diabetic neuropathy.
When the smooth muscle cells lining blood vessels contract in response to stimulation from the sympathetic nervous system, the result is vasoconstriction and a decrease in blood flow to the extremities.
To identify a different method for the detection of diabetic autonomic neuropathy that does not impair patient comfort.
The goal of this research was to determine whether there is major vasomotor dysfunction of medium-sized arteries in diabetic neuropathy by comparing the sympathetic vasomotor activation of the brachial arteries in healthy people and patients with painful diabetic neuropathy.
A 10 mA, 1 Hz electrical stimulus was applied to the median nerve in the wrist for 5 s using a bipolar stimulus electrode. Following stimulation, the diameter of the brachial artery and the blood flow rate were re-measured.
In the control group, the median flow rate was 70.0 mL/min prior to stimulation and 35.0 mL/min after stimulation, with a statistically significant decrease (P < 0.001) which is consistent with sympathetic nervous system functioning (vasoconstriction). In the diabetic neuropathy group, median flow rate before stimulation was 35.0 mL/min. After stimulation, the median flow rate was 77.0 mL/min; thus, no significant decrease in the flow rate was detected. In the control group, the median brachial artery diameter, which was 3.6 mm prior to stimulation, decreased to 3.4 mm after stimulation, and this decrease was also statistically significant (P = 0.046). In the diabetic neuropathy group, the median brachial artery diameter increased from 3.4 mm to 3.6 mm following nerve stimulation. Once again, no narrowing was observed.
Our study shows that diabetic neuropathy causes medium-sized peripheral arteries to have blood flow problems. In diabetic neuropathy, the body cannot constrict blood vessels normally when the sympathetic nervous system is activated.
We will try this new method in the clinic in the future.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Beg MMA, Kyrgyzstan; Glumac S, Croatia S-Editor: Liu JH L-Editor: Webster JR P-Editor: Liu JH
| 1. | Koye DN, Magliano DJ, Nelson RG, Pavkov ME. The Global Epidemiology of Diabetes and Kidney Disease. Adv Chronic Kidney Dis. 2018;25:121-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 408] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 2. | Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, Sosenko JM, Ziegler D. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care. 2017;40:136-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1426] [Cited by in RCA: 1439] [Article Influence: 179.9] [Reference Citation Analysis (1)] |
| 3. | Obrosova IG. Diabetes and the peripheral nerve. Biochim Biophys Acta. 2009;1792:931-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 136] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Vincent AM, Callaghan BC, Smith AL, Feldman EL. Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat Rev Neurol. 2011;7:573-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 381] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 5. | Tavakoli M, Malik RA. Corneal confocal microscopy: a novel non-invasive technique to quantify small fibre pathology in peripheral neuropathies. J Vis Exp. 2011;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 6. | Veluchamy A, Hébert HL, Meng W, Palmer CNA, Smith BH. Systematic review and meta-analysis of genetic risk factors for neuropathic pain. Pain. 2018;159:825-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 7. | Freeman R. Diabetic autonomic neuropathy. Handb Clin Neurol. 2014;126:63-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 8. | Agashe S, Petak S. Cardiac Autonomic Neuropathy in Diabetes Mellitus. Methodist Debakey Cardiovasc J. 2018;14:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 9. | Zgur T, Vodusek DB, Krzan M, Vrtovec M, Denislic M, Sibanc B. Autonomic system dysfunction in moderate diabetic polyneuropathy assessed by sympathetic skin response and Valsalva index. Electromyogr Clin Neurophysiol. 1993;33:433-439. [PubMed] |
| 10. | Jha S, Nag D. Sympathetic skin response and autonomic dysfunction in diabetes. Indian J Physiol Pharmacol. 1995;39:149-153. [PubMed] |
| 11. | Aalkjær C, Nilsson H, De Mey JGR. Sympathetic and Sensory-Motor Nerves in Peripheral Small Arteries. Physiol Rev. 2021;101:495-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | LeBouef T, Yaker Z, Whited L. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2022. [DOI] [Full Text] |
| 13. | Karemaker JM. An introduction into autonomic nervous function. Physiol Meas. 2017;38:R89-R118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 142] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 14. | Joyner MJ, Charkoudian N. The sympathetic nervous system and blood pressure in humans: implications for hypertension. J Hum Hypertens. 2010;24:171-181. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Cheshire WP, Freeman R, Gibbons CH, Cortelli P, Wenning GK, Hilz MJ, Spies JM, Lipp A, Sandroni P, Wada N, Mano A, Ah Kim H, Kimpinski K, Iodice V, Idiáquez J, Thaisetthawatkul P, Coon EA, Low PA, Singer W. Electrodiagnostic assessment of the autonomic nervous system: A consensus statement endorsed by the American Autonomic Society, American Academy of Neurology, and the International Federation of Clinical Neurophysiology. Clin Neurophysiol. 2021;132:666-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 16. | Kazci O, Ege F. Evaluation of Sympathetic Vasomotor Activity of the Brachial Arteries Using Doppler Ultrasound. Med Sci Monit. 2023;29:e939352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 17. | Ege F, Kazcı O. Brachial arteries sympathetic innervation: A contribution to anatomical knowledge. World J Neurol. 2023;9:1-7. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Bodman MA, Varacallo M. Peripheral Diabetic Neuropathy. 2022 Sep 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. [PubMed] |
| 19. | Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26:1553-1579. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1258] [Cited by in RCA: 1215] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 20. | Lin K, Wei L, Huang Z, Zeng Q. Combination of Ewing test, heart rate variability, and heart rate turbulence analysis for early diagnosis of diabetic cardiac autonomic neuropathy. Medicine (Baltimore). 2017;96:e8296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, Bernardi L, Valensi P; Toronto Diabetic Neuropathy Expert Group. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285-2293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1948] [Cited by in RCA: 1747] [Article Influence: 116.5] [Reference Citation Analysis (0)] |
| 22. | Kohriyama T, Mimori Y, Katayama S, Yamamura Y, Nakamura S. [Vasomotor impairment in patients with diabetic neuropathy--evaluation by laser Doppler flowmetry]. Rinsho Shinkeigaku. 1995;35:8-13. [PubMed] |
| 23. | Sun PC, Kuo CD, Chi LY, Lin HD, Wei SH, Chen CS. Microcirculatory vasomotor changes are associated with severity of peripheral neuropathy in patients with type 2 diabetes. Diab Vasc Dis Res. 2013;10:270-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |