Published online Aug 6, 2023. doi: 10.12998/wjcc.v11.i22.5224
Peer-review started: May 29, 2023
First decision: June 15, 2023
Revised: June 26, 2023
Accepted: July 10, 2023
Article in press: July 10, 2023
Published online: August 6, 2023
Processing time: 66 Days and 2.8 Hours
Most physicians consider molars with chronic apical periodontitis (CAP) lesions as contraindications for immediate implant placement. At the patient’s request, we perform immediate implant placement of the mandibular molars with CAP in clinical practice.
To retrospectively analyze and compare the 5-year outcomes of immediate implant placement of the mandibular molars with CAP and those without obvious inflammation.
The clinical data of patients with immediate implant placement of the mandibular molars in the Department of Oral and Maxillofacial Surgery, the Affiliated Hospital of Qingdao University, from June 2015 to June 2017 were collected. The patients were divided into CAP (n = 52) and no-CAP (n = 45) groups. Changes in bone mineral density and bone mass around implants were analyzed 5 years after implant restoration.
At 5 years after implantation, the peri-implant bone mineral density was 528.2 ± 78.8 Hounsfield unit (HU) in the CAP group and 562.6 ± 82.9 HU in the no-CAP group (P = 0.126). Marginal bone resorption around implants did not differ significantly between the two groups, including buccal (P = 0.268) or lingual (P = 0.526) resorption in the vertical direction or buccal (P = 0.428) or lingual (P = 0.560) resorption in the horizontal direction. Changes in the peri-implant jump space did not differ significantly between the two groups, including the buccal (P = 0.247) or lingual (P = 0.604) space in the vertical direction or buccal (P = 0.527) or lingual (P = 0.707) space in the horizontal direction. The gray value of cone-beam computed tomography measured using Image J software can reflect the bone mineral density. In the CAP area, the gray values of the bone tissue immediately and 5 years after implant placement differed significantly from those of the surrounding bone tissue (P < 0.01).
The results of this study suggest that immediate implant placement of the mandibular molars with CAP can achieve satisfactory 5-year clinical results, without significant differences in the complications, survival rate, or bone tissue condition from the no-CAP mandibular molars.
Core Tip: This study was aimed at retrospectively analyzing and comparing the 5-year outcomes of immediate implant placement of the mandibular molars with chronic apical periodontitis and those without obvious inflammation based on changes in bone mineral density and bone mass around implants 5 years after implant restoration.
- Citation: Yang H, Luo D, Yuan MJ, Yang JJ, Wang DS. Five-year outcomes of immediate implant placement for mandibular molars with and without chronic apical periodontitis: A retrospective study. World J Clin Cases 2023; 11(22): 5224-5235
- URL: https://www.wjgnet.com/2307-8960/full/v11/i22/5224.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i22.5224
Chronic apical periodontitis (CAP) is a globally prevalent infectious disease, characterized by the inflammatory response of periapical tissues and local alveolar bone destruction caused by intramedullary microbial infection[1,2]. Compared to other bones in the body, the alveolar bone can communicate directly with the outside environment through the dental pulp; if the pulp is necrotic and infected, no epithelial barrier exists to resist infection or inflammatory factors[3]. In the development of CAP, microbial infection and immune defense response jointly lead to local alveolar bone destruction[4-6]. Root canal therapy is the main treatment for CAP. However, after root canal treatment, CAP may persist as asymptomatic inflammation[7]. Persistent CAP after root canal treatment may be caused by failure to strictly follow the principle of asepsis, a poor approach design, residual accessory canals, improper use of instruments, incomplete de
Due to the complexity of the main and accessory root canal systems and residual infection at the root canal branches and anastomosis, the mandibular molars may develop persistent CAP even after following the most stringent root canal treatment procedures, resulting in persistent destruction of the periapical bone[1,2,9]. When the periapical shadow area of the affected tooth is enlarged in the periapical radiograph, the root canal retreatment is difficult to perform, and the affected tooth cannot be retained, the method of restoring the affected tooth after extraction should be considered[10].
Since Branemark successfully implanted the first dental implant in 1965, the dental implant technology has been an effective method to restore the masticatory and esthetic functions of the dentition for patients with missing teeth[11,12]. The initial treatment protocol involves implant placement in the healed extraction socket, referring to the alveolar bone after healing for at least 5-6 mo after extraction of the affected tooth, a delayed dental implant. In 1989, Lazzara et al[13] introduced implants placed immediately after tooth extraction. In recent years, many studies have confirmed the reliability of implants placed during tooth extraction[14]. With advancements in the implant technology, immediate implant placement has become the first choice to restore missing teeth in clinical practice[15-17]. Hansson and Ekestubbe[18] and Ericsson et al[19] found that immediate dental implant surgery is minimally invasive, which reduces the risk of osteonecrosis and promotes the process of bone remodeling, shortening the healing period of the alveolar bone and promoting the transformation of woven bone into lamellar bone. Compared to delayed dental implant placement, immediate implant placement has advantages of shortening the treatment course, reducing the procedural pain of patients, and reducing the alveolar bone absorption in the surgical area[20]. Currently, immediate implant placement is mainly used in the anterior esthetic area in clinical practice[21].
Most molars cannot be retained after severe CAP. The complex shape of the extraction socket after extraction of the affected teeth, severe destruction of the periodontal soft tissue and alveolar bone, and presence of abundant inflammatory granulation tissue adversely affect the success rate of immediate implant placement[16]. In a prospective study, Alsaadi et al[22] found that immediate implant placement in teeth with CAP lesions resulted in a greater rate of implant failure compared to delayed implant placement. In addition, the retrograde peri-implantitis that occurred in the study was thought to be caused by immediate implant placement at the CAP tooth position[23]. Most physicians consider the molars with severe CAP as a contraindication for immediate implant placement. However, in recent years, data from animal studies, case reports, and prospective studies have shown that the success rate of implant placement in the teeth with CAP is similar to that in the teeth without CAP[24,25].
The aim of this study was to retrospectively analyze and compare the 5-year outcomes of immediate implant placement of the mandibular molars with CAP and those without obvious inflammation based on quantitative peri-implant bone mass changes evaluated using Simplant and Image J software.
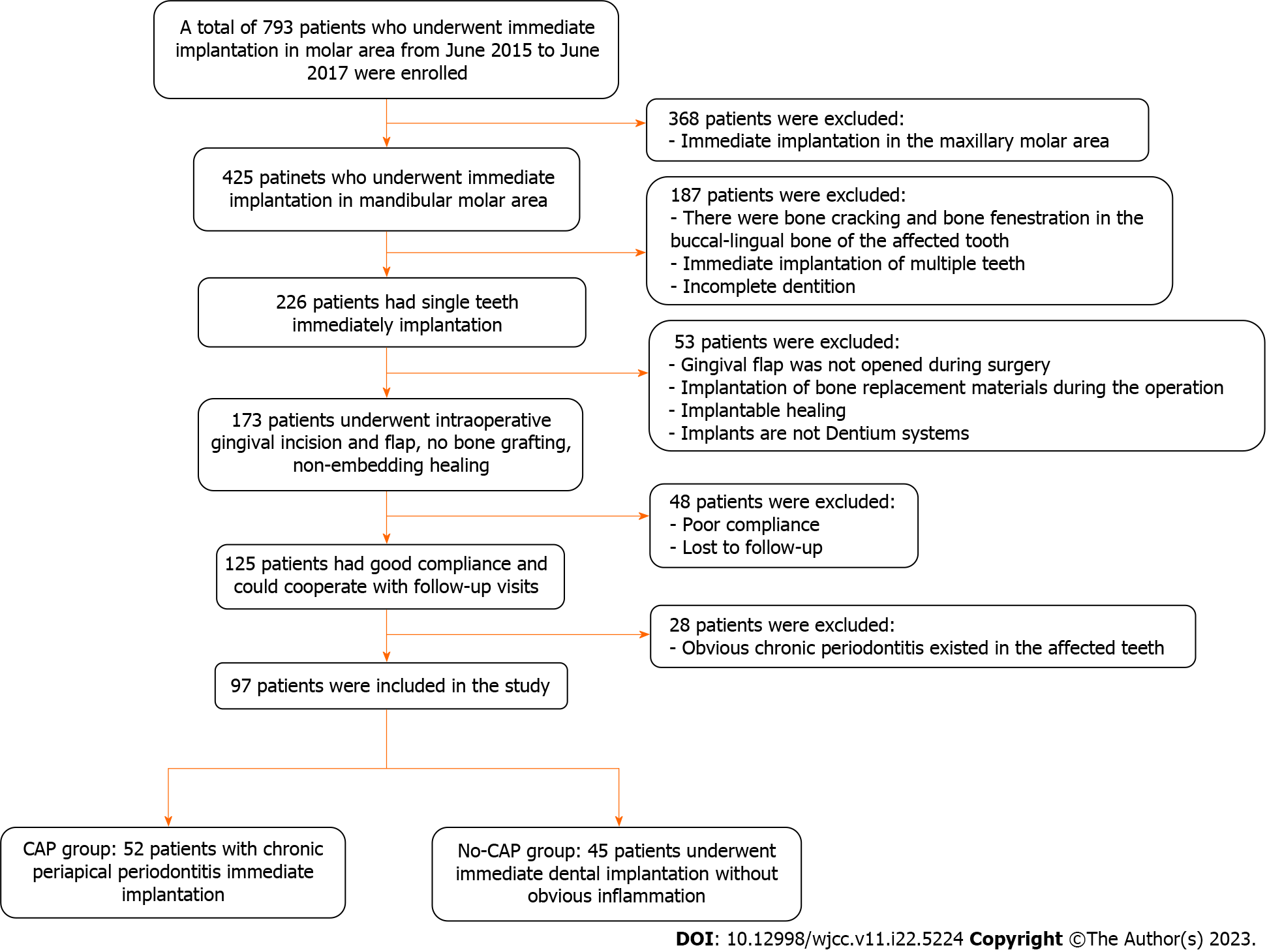
Clinical cases of the molars that could not be retained or received immediate implant placement were collected from the Oral and Maxillofacial Surgery Department of the Affiliated Hospital of Qingdao University from June 2015 to June 2017. Figure 1 shows the patients’ screening process.
This study was conducted in accordance with Declaration of Helsinki guidelines and regulations, and all study methods were approved by the Ethics Committee of the Affiliated Hospital of Qingdao University (QYFYKYLL
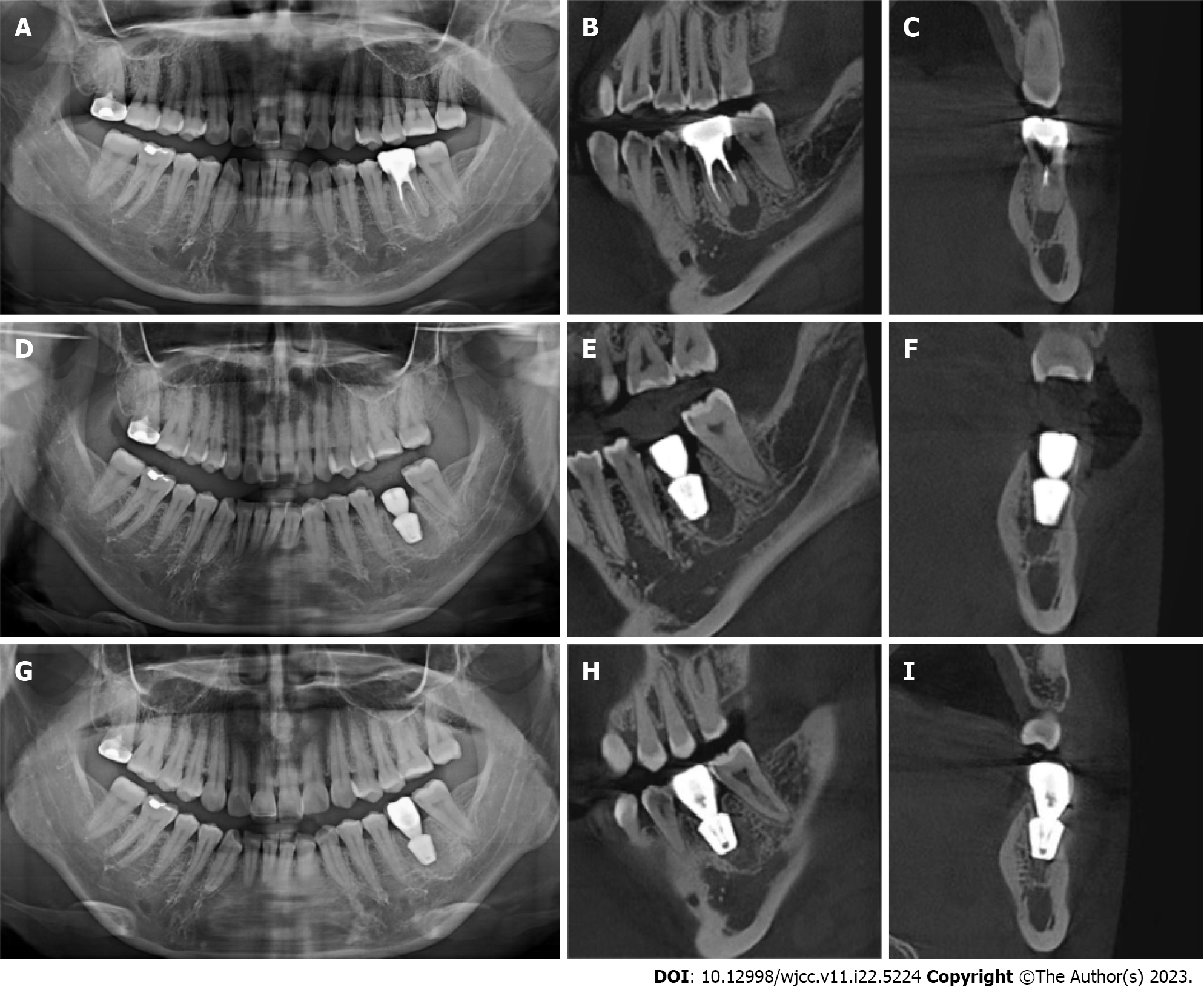
Inclusion criteria were: Age ≥ 18 years; no pregnancy or lactation; no systemic disease or the use of related drugs; good oral hygiene with no acute inflammation in the dentition; no retentive value of the affected teeth in the posterior mandibular region as confirmed by oral physicians and prosthodontists (Figure 2A-C); patient consent for immediate implant placement; and willingness to attend regular follow-ups. The same chief physician with 25 years of work experience completed the implant placement surgery and crown restoration, under assistance by doctors with more than 5 years of clinical experience.
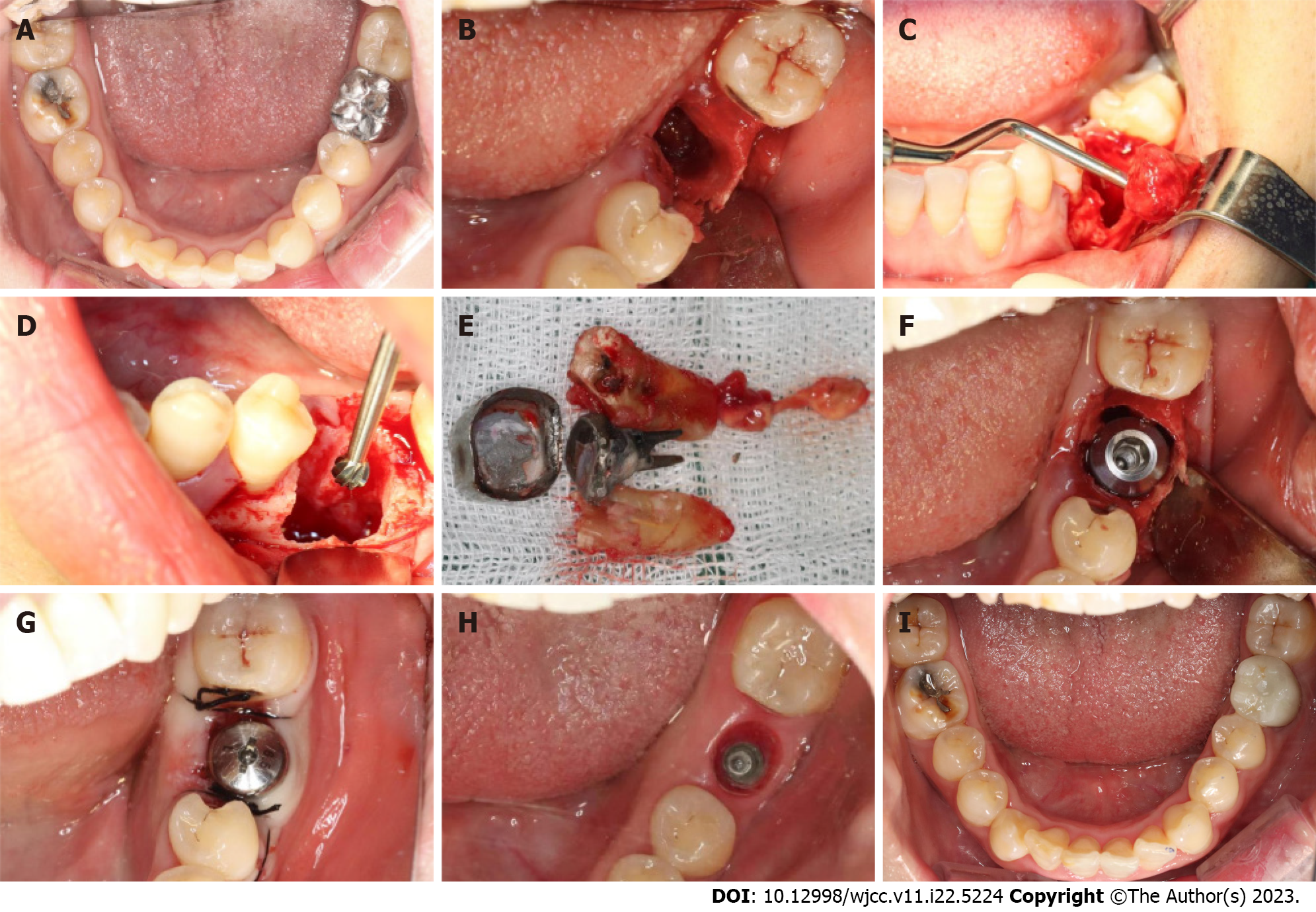
All study participants underwent routine disinfection and draping in the intraoral and maxillofacial regions. We used 1% iodophor for disinfection and asked the patient to gargle for 20 s before sterilizing the maxillofacial area, up to the palpebral fissure, down to the level of the hyoid bone, and bilaterally to the front of the tragus. The angular mucoperiosteal flap on the buccal side of the affected tooth was completely turned over. A high-speed turbine extraction handpiece was used to extract the affected teeth by root separation to protect the integrity of the apical septum and bone wall of the alveolar fossa to the greatest extent (Figure 3A and B). The inflammatory tissues on the edge and inside of the mucoperiosteal flap were pruned. The inflammatory tissue attached to the inner wall of the alveolar fossa was scraped with an appropriate type of scraping spoon and then polished with large, medium, and small ball drills until no fibrous tissue remained on the bone wall of the alveolar fossa (Figure 3C and D). The implant cavities were prepared step-wise, and the corresponding implants were selected and rotated into the implant socket using a ratchet wrench to ensure that the implants had been placed in the correct three-dimensional position, with the neck and shoulder of the implants placed 1.0-1.5 mm below the edge of the alveolar bone. The torque force after implant placement was tested using a force measuring wrench to ensure that the torque force exceeded 35 N. cm (Figure 3E and F). The implant stability quotient was measured with the resonance frequency analyzer (Osstell, Sweden) to confirm the initial stability of the implant and install the healing abutment with appropriate diameter and penetration height. The buccal-lingual mucoperiosteal flap was tightly sutured around the healing abutment (Figure 3G). Cone-beam computed tomography (CBCT) was performed after immediate implant placement to ensure that the implants were in the correct tooth and spatial positions (Figure 2D-F).
At 3 mo postoperatively, an impression was taken, and an all-porcelain crown was fabricated (Figure 3H and I).
All patients underwent CBCT before and after the implant placement and at the follow-up visits (Figure 2). CBCT was performed by the same dental radiologist with 10 years of experience. The clinical examination and CBCT image measurement and analysis were performed by three dentists with 10, 10, and 5 years of working experience, respectively. Each dentist measured and analyzed the CBCT scans of the two groups of patients, and the results of each patient’s evaluation index were averaged and recorded. The intraclass correlation coefficient (ICC) was used to test the difference in the observation results of the three observers. According to the specificity of the evaluation indicators in this study, the peri-implant bone mineral density with the most complicated detection steps was selected for ICC calculation, which could reflect the differences in the observation results of the three observers in this study.
The bone tissue around the implant was analyzed 5 years after crown restoration. CBCT equipment and parameters were as follows: Brand model, Kava i-CAT 17-19; tube voltage, 120 kV; current, 5 mA; exposure time, 26.9 s; diameter, 16 cm; height, 11 cm; and resolution, 0.25 mm.
The patient sat in an upright position. The dental chair was adjusted to the appropriate height according to the patient’s height. The patient’s head was rested on the head support. The dentist wore protective clothing for infection control, adjusted the front center line to the center of the patient’s face, directed the patient’s eyes to look forward, adjusted the orbital ear plane to be parallel to the ground, and held the patient’s jaw bracket. “Preview” determined that the scanning range included the patient’s complete upper and lower dentition and alveolar bone, and the CBCT scan was captured.
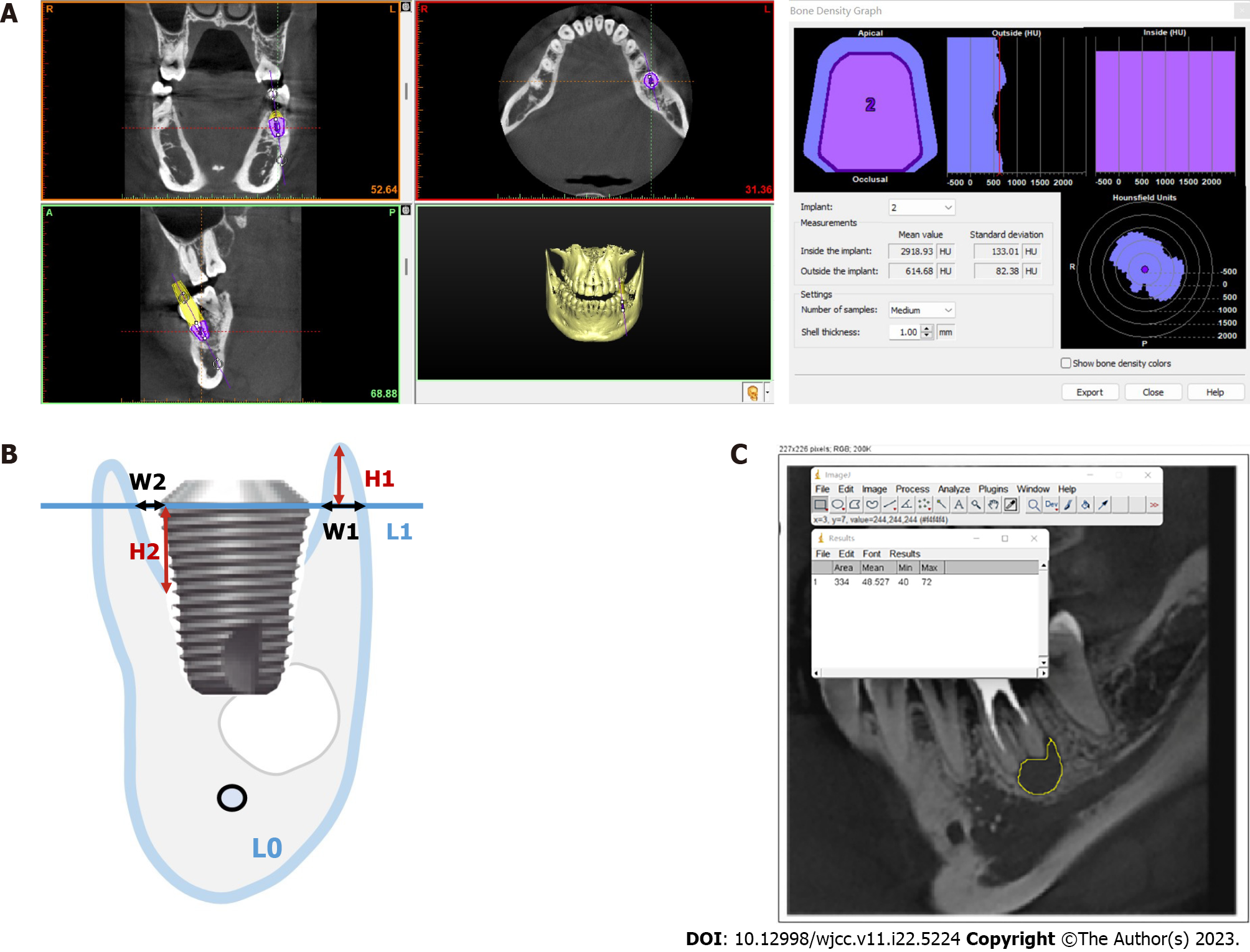
Peripheral bone density: The CBCT scans of the CAP and no-CAP groups 5 years after dental crown restoration were exported in the Digital Imaging and Communications in Medicine format and imported into Simplant software (Materialise Dental, Belgium). The alveolar bone density was measured around the virtual implant (Figure 4A).
Vertical and horizontal marginal bone losses: The implant long axis L0 and the implant shoulder plane L1 perpendicular to L0 were determined. In the vertical direction, the vertical distance from the crest of the buccal and lingual alveolar crests to L1 (H1) was measured. In the horizontal direction, the distance (W1) between L1 and the lateral side of the buccal and lingual bone walls and the intersection of the implant edge was measured (Figure 4B). Each group of data was measured thrice, with an average accuracy of 0.01 mm. The buccal and lingual vertical and horizontal marginal bone loss from immediately after implant placement to 5 years after crown restoration in the CAP and no-CAP groups were calculated.
Changes in the jump gap: The long axis L0 of the implant body and the shoulder plane L1 perpendicular to L0 were determined. The vertical distance from the highest point of implant-bone contact to L1 (H2) was measured immediately after surgery and 5 years after crown restoration, and the distance between L1 and the intersections of buccal and lingual bone walls and implant edges (W2) was measured (Figure 4B). The data in each group were measured thrice, with an average accuracy of 0.01 mm. Changes in buccal and lingual jump gaps in the CAP and no-CAP groups 5 years after crown restoration were calculated.
Changes in bone density in the CAP-damaged areas: The CBCT scans of the largest area of bone destruction in the horizontal plane of the CAP group were selected and exported in the jpg format. With the Image J software, the region of interest was selected in the image, avoiding areas such as the surrounding alveolar bone and root tip as much as possible. After determining the region of interest, the “Measure” option in the toolbar was selected, followed by the “Analyze” option to obtain the gray value of the inflammatory bone destruction area and surrounding bone tissue (Figure 4C). Differences in the gray value between the two were calculated and recorded. The gray values of the bone destruction area and surrounding bone tissue in the same area 5 years after crown restoration were obtained with the same method, and the difference in the gray values between the two was calculated and recorded. The obtained gray value cannot directly be used as the bone mineral density value, but the difference in the gray values between the area of inflammatory bone destruction and the surrounding bone tissue immediately and 5 years after surgery can represent the change in bone mineral density in this area.
ICC calculations and statistical tests were performed using SPSS 20.0 (IBM, Chicago, IL). Patient age belonged to non-normally distributed data, and the Wilcoxon signed-rank test was used for the analysis. The data of peri-implant bone tissue changes (bone mineral density, marginal bone loss, jump gap, and gray value) belonged to normally distributed data and were analyzed with the independent-samples t-test. For patient sex, the chi-square test was used. A P value < 0.05 was considered to indicate statistical significance.
We enrolled 97 teeth of 97 patients, with 52 patients in the CAP group, including 28 women and 24 men, aged 35.6 ± 5.28 (68-18) years, and 45 patients in the no-CAP group, including 19 women and 26 men, aged 36.8 ± 4.79 (66-20) years. Patient age (P = 0.385) or sex (P = 0.314) distribution did not differ significantly between the two groups (Tables 1 and 2). The peri-implant bone mineral density values measured by three observers in this study were tested for inter-observer differences, and the calculated ICC value was 0.816, falling between 0.75 and 0.9, indicating a good consistency of the observation results[26].
| Group | Max | Min | Mean | SD | P value |
| CAP group (n = 52) | 68 | 18 | 35.6 | 5.28 | 0.385 |
| No-CAP group (n = 45) | 66 | 20 | 36.8 | 4.79 |
| Group | Sex | Total | P value | |
| Female | Male | |||
| CAP group (n = 52) | 28 | 24 | 52 | 0.314 |
| No-CAP group (n = 45) | 19 | 26 | 45 | |
| Total (n = 97) | 47 | 50 | 97 | |
The clinical records of the two groups within 5 years were analyzed. In the CAP group, one patient developed central screw loosening, and one patient experienced restoration chipping. In the no-CAP group, one patient’s prosthesis fell off, and one patient’s prosthesis chipped off. After the corresponding treatment, the implant condition was good. The implants in the two groups were in position and functioned well, and the survival rate was 100%.
The CBCT data were imported into Simplant software to measure and compare changes in bone mineral density of peri-implant bone tissue after 5 years of implant denture restoration. The peri-implant bone densities were 528.2 ± 78.8 Hounsfield unit (HU) and 562.6 ± 82.9 HU after 5 years of implant restoration in the CAP and no-CAP groups, respectively. The independent-samples t-test showed no significant difference in the peri-implant bone density between the two groups (P = 0.126).
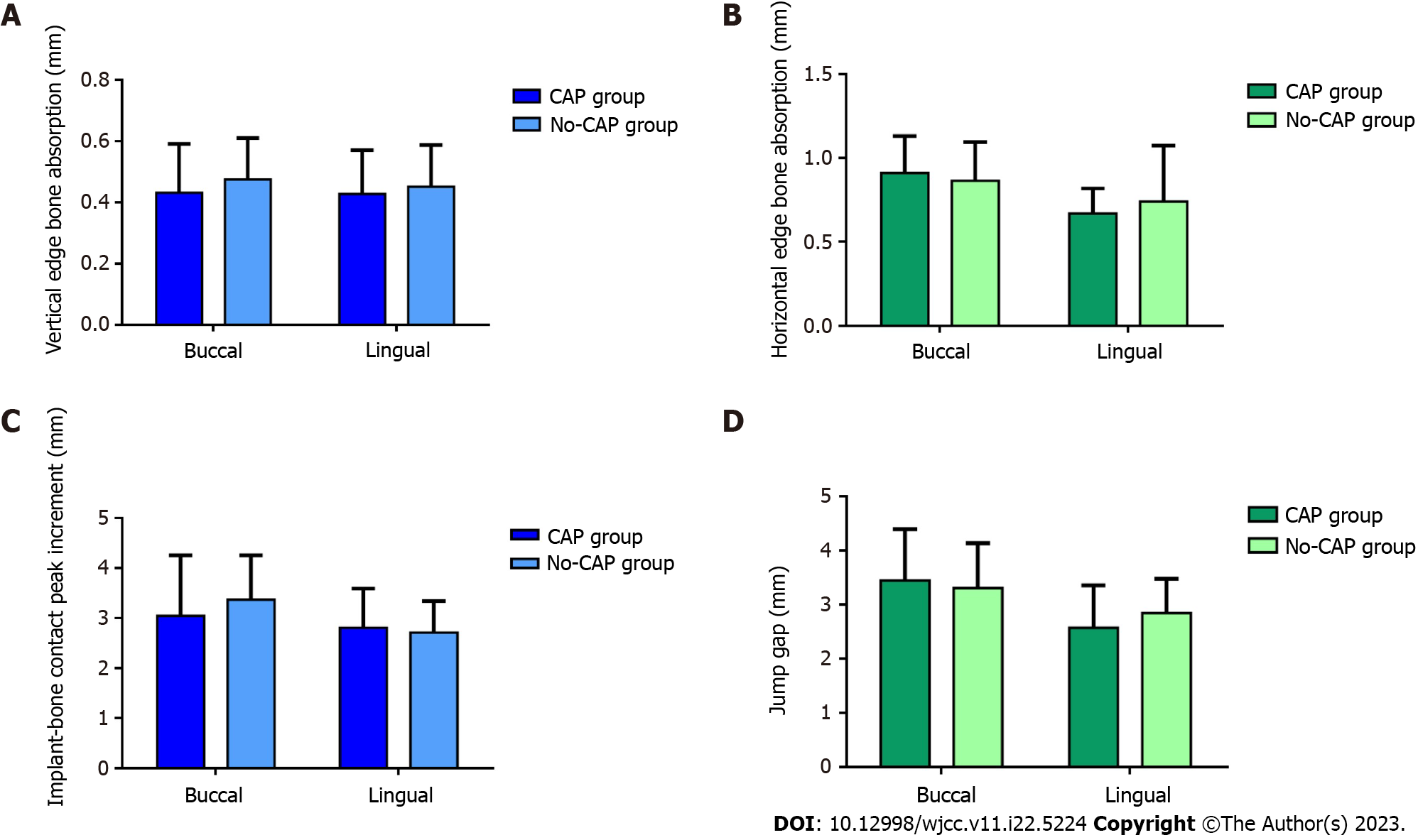
Figure 5A and B shows the amount of bone resorption at the implant edge. In the vertical direction, the buccal marginal bone loss was 0.43 ± 0.16 mm in the CAP group and 0.47 ± 0.14 mm in the no-CAP group, showing no significant difference between the two groups (P = 0.268). The marginal bone loss did not differ significantly between the CAP (0.43 ± 0.14 mm) and no-CAP (0.45 ± 0.14 mm) groups (P = 0.526). In the horizontal direction, the buccal marginal bone loss was 0.91 ± 0.22 mm in the CAP group and 0.86 ± 0.23 mm in no-CAP group, showing no significant difference between the two groups (P = 0.428). The marginal bone loss was 0.67 ± 0.15 mm in the CAP group and 0.64 ± 0.22 mm in the no-CAP group (P = 0.560), showing no significant difference.
In the vertical direction, the incremental value of the highest point of buccal implant-bone contact was 3.04 ± 1.21 mm in the CAP group and 3.36 ± 0.89 mm in the no-CAP group, showing no significant difference (P = 0.247). The implant bone increment at the highest point of lingual implant-bone contact was 2.80 ± 0.78 mm in the CAP group and 2.71 ± 0.63 mm in the no-CAP group, showing no significant difference (P = 0.604; Figure 5C). In the horizontal direction, the width change in the buccal jump gap was 3.44 ± 0.95 mm in the CAP group and 3.30 ± 0.83 mm in the no-CAP group, showing no significant difference (P = 0.527). The width change in the lingual jump gap was 2.57 ± 0.78 mm in the CAP group and 2.84 ± 0.63 mm in the no-CAP group, showing no significant difference (P = 0.707; Figure 5D).
The inflammatory bone destruction area of the alveolar bone in the CAP group disappeared 5 years after implant denture restoration. The gray value difference between the CAP lesion area and the surrounding bone tissue was 107.6 ± 21.7 immediately after surgery and 32.5 ± 15.3 5 years after implant restoration, with significant differences between the two groups (P < 0.01).
Currently, immediate implant placement is the preferred modality to restore the missing anterior teeth[27]. For the mandibular molars, immediate implant placement is often contraindicated because of the large area of CAP lesions around the root apices and severe alveolar bone destruction[28]. Therefore, delayed dental implant placement is chosen, which prolongs the restoration time of the missing teeth and aggravates pain[29]. In this retrospective study, the patients who had received immediate implant placement and crown restoration with successful outcomes after 5 years were selected. The aim of the present study was to investigate the clinical effect of immediate implant placement for the missing mandibular molars.
Osseointegration is the decisive factor for the success of implant restoration[30]. The larger the contact area between the implant surface and the trabecular bone in the surrounding alveolar bone, the better is implant osseointegration[31]. The study revealed no significant differences in bone mineral density between the chronic periapical teeth with immediate restoration and the teeth with conventional immediate restoration 5 years after immediate restoration. Microbial antigens derived from root canal infections can stimulate specific and nonspecific immune responses in periapical tissues[32]. Macrophages, mast cells, T cells, and neutrophils are involved in the formation of CAP tissue, including cytokines and chemokines[32,33]. Therefore, the inflammatory tissue in the alveolar fossa should be completely removed to reduce the influence of inflammatory factors around the implant on the bone tissue.
The marginal bone level plays a crucial role in maintaining the stability and function of implants, with great sig
The gap between the medial wall of the alveolar bone and the implant surface is called the jump gap[17,38]. The implant may be wrapped in blood clots after placement. At 1-2 wk postoperatively, osteoblasts form new woven bone on the implant surface and a bridge with the bone wall of the alveolar socket. As the woven bone continues to mineralize, it can transform into lamellar bone[38]. The present study involved no bone grafting in the jumping gap in the CAP or no-CAP group. The jumping gap healed naturally after the operation. Five years after implant placement and loading, CBCT showed that the original jumping gap had disappeared.
Bone mass loss is a clinical feature of CAP caused by microbial factors and immune defense responses[39]. As a chronic inflammatory disease, CAP causes an imbalance between bone resorption and remodeling, which leads to bone mass loss[28]. Bone resorption and formation are antagonistic and coupled processes of osteoblasts and osteoclasts, which together constitute the normal bone mass[40]. Several factors affect periapical bone remodeling, including microbes, human signaling pathways, and the immune system[41]. CAP may directly be caused by bacterial infection and trigger an immune response from the host, resulting in destruction of periapical tissues[40,42]. In the progression of pulpitis, the flora is simple in the early stage, but with the dominance of gram-negative anaerobic bacteria, such as porphyromonas, the complexity of root canal flora increases[42]. Of all bacterial species, 54.6% are strictly anaerobic, while anaerobic gram-negative bacteria dominate the root canals with periapical lesions[43]. In the periapical infected tissues, bacterial abundance and diversity are significantly reduced, and the microbial balance in the biofilm is disrupted[44]. Endotoxin in the cell wall of gram-negative bacteria can cause local tissue swelling and bone resorption and mobilize the host immune response. Further, its content is positively correlated with the degree of bone injury[45]. It can promote osteoclast differentiation and bone resorption induced by lipoic acid and participate in maintaining the survival of mature osteoclasts, thereby jointly causing inflammatory alveolar bone loss[45].
Therefore, in the immediate implant surgery in the present study, we should removed the inflammatory tissue in the alveolar fossa and the bacteria and inflammatory tissue in the periapical bone destruction area. The results showed no significant difference between the CAP and no-CAP groups in the gray value of the apical bone destruction area and the surrounding bone tissue 5 years after loading of immediate dental implant restoration. This indicated that the mandibular molars with various inflammatory periapical bone tissue lesions could be effectively removed by improving the implant socket approach during immediate implant placement to promote bone tissue reconstruction.
This study has some limitations. First, this was a retrospective study; therefore, the credibility of the findings is weaker. However, strict inclusion and exclusion criteria were set to minimize variables not relevant to the purpose of the study. Second, the study sample size was small; therefore, the data may not accurately represent the entire population. Third, all patients showed good compliance, which caused a selection bias. In the future, we will conduct a prospective study corresponding to this study to expand the sample size and follow-up duration and further explore the soft and hard tissue conditions of immediate dental implant placement in the mandibular molars with CAP.
The results of this study suggest that immediate implant placement of the mandibular molars with CAP can achieve satisfactory 5-year clinical results, without significant differences in the complications, survival rate, or bone tissue condition from the no-CAP mandibular molars.
At the patient’s request, we tried to perform immediate implant placement of the mandibular molars with CAP in clinical practice.
Most physicians consider the molars with CAP lesions as contraindications for immediate implant placement.
Immediate implant placement of the mandibular molars with CAP can achieve satisfactory 5-year clinical results.
In this study, we retrospectively analyzed and compared the 5-year outcomes of immediate implant placement of the mandibular molars with CAP and those without obvious inflammation based on quantitative peri-implant bone mass changes evaluated using Simplant and Image J software.
The peri-implant bone density was 528.2 ± 78.8 Hounsfield unit (HU) in the CAP group and 562.6 ± 82.9 HU in the no-CAP group 5 years after implant placement. The peri-implant bone density did not differ significantly between the two groups. The marginal bone resorption or jump gap did not differ significantly between the two groups. In the CAP area, the gray values of the bone tissue immediately and 5 years after implant placement differed significantly from those of the surrounding bone tissue (P < 0.01).
The results of this study suggest that immediate implant placement of the mandibular molars with CAP can achieve satisfactory 5-year clinical results, without significant differences in the complications, survival rate, or bone tissue condition from the no-CAP mandibular molars.
Immediate implant placement of the mandibular molars with chronic apical periodontitis (CAP) can achieve good clinical outcomes.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ardila CM, Colombia; Utharavalli USK, India S-Editor: Lin C L-Editor: A P-Editor: Yuan YY
| 1. | Azuma MM, Samuel RO, Gomes-Filho JE, Dezan-Junior E, Cintra LT. The role of IL-6 on apical periodontitis: a systematic review. Int Endod J. 2014;47:615-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 2. | Graunaite I, Lodiene G, Maciulskiene V. Pathogenesis of apical periodontitis: a literature review. J Oral Maxillofac Res. 2012;2:e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Silva TA, Garlet GP, Lara VS, Martins W Jr, Silva JS, Cunha FQ. Differential expression of chemokines and chemokine receptors in inflammatory periapical diseases. Oral Microbiol Immunol. 2005;20:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Andrade AL, Nonaka CF, Gordón-Núñez MA, Freitas Rde A, Galvão HC. Immunoexpression of interleukin 17, transforming growth factor β1, and forkhead box P3 in periapical granulomas, radicular cysts, and residual radicular cysts. J Endod. 2013;39:990-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Marçal JR, Samuel RO, Fernandes D, de Araujo MS, Napimoga MH, Pereira SA, Clemente-Napimoga JT, Alves PM, Mattar R, Rodrigues V Jr, Rodrigues DB. T-helper cell type 17/regulatory T-cell immunoregulatory balance in human radicular cysts and periapical granulomas. J Endod. 2010;36:995-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 6. | Bracks IV, Armada L, Gonçalves LS, Pires FR. Distribution of mast cells and macrophages and expression of interleukin-6 in periapical cysts. J Endod. 2014;40:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Nair PN. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit Rev Oral Biol Med. 2004;15:348-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 648] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 8. | Tabassum S, Khan FR. Failure of endodontic treatment: The usual suspects. Eur J Dent. 2016;10:144-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 184] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 9. | Cavalla F, Letra A, Silva RM, Garlet GP. Determinants of Periodontal/Periapical Lesion Stability and Progression. J Dent Res. 2021;100:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 10. | Siqueira JF Jr, Rôças IN, Ricucci D, Hülsmann M. Causes and management of post-treatment apical periodontitis. Br Dent J. 2014;216:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 175] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 11. | Kopp CD. Brånemark osseointegration. Prognosis and treatment rationale. Dent Clin North Am. 1989;33:701-731. [PubMed] |
| 12. | Brånemark PI. Osseointegration and its experimental background. J Prosthet Dent. 1983;50:399-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1178] [Cited by in RCA: 1060] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 13. | Lazzara RJ, Testori T, Meltzer A, Misch C, Porter S, del Castillo R, Goené RJ. Immediate Occlusal Loading (IOL) of dental implants: predictable results through DIEM guidelines. Pract Proced Aesthet Dent. 2004;16:3-15. [PubMed] |
| 14. | Bianchi AE, Sanfilippo F. Single-tooth replacement by immediate implant and connective tissue graft: a 1-9-year clinical evaluation. Clin Oral Implants Res. 2004;15:269-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Schropp L, Wenzel A, Kostopoulos L, Karring T. Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int J Periodontics Restorative Dent. 2003;23:313-323. [PubMed] |
| 16. | Ragucci GM, Elnayef B, Criado-Cámara E, Del Amo FS, Hernández-Alfaro F. Immediate implant placement in molar extraction sockets: a systematic review and meta-analysis. Int J Implant Dent. 2020;6:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 17. | Pitman J, Seyssens L, Christiaens V, Cosyn J. Immediate implant placement with or without immediate provisionalization: A systematic review and meta-analysis. J Clin Periodontol. 2022;49:1012-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 18. | Hansson S, Ekestubbe A. Area moments of inertia as a measure of the mandible stiffness of the implant patient. Clin Oral Implants Res. 2004;15:450-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Ericsson I, Randow K, Nilner K, Peterson A. Early functional loading of Brånemark dental implants: 5-year clinical follow-up study. Clin Implant Dent Relat Res. 2000;2:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 20. | Blanco J, Carral C, Argibay O, Liñares A. Implant placement in fresh extraction sockets. Periodontol 2000. 2019;79:151-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 21. | Araújo MG, Wennström JL, Lindhe J. Modeling of the buccal and lingual bone walls of fresh extraction sites following implant installation. Clin Oral Implants Res. 2006;17:606-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 248] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 22. | Alsaadi G, Quirynen M, Komárek A, van Steenberghe D. Impact of local and systemic factors on the incidence of oral implant failures, up to abutment connection. J Clin Periodontol. 2007;34:610-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 241] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 23. | Quirynen M, Vogels R, Alsaadi G, Naert I, Jacobs R, van Steenberghe D. Predisposing conditions for retrograde peri-implantitis, and treatment suggestions. Clin Oral Implants Res. 2005;16:599-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 24. | Botticelli D, Berglundh T, Lindhe J. Resolution of bone defects of varying dimension and configuration in the marginal portion of the peri-implant bone. An experimental study in the dog. J Clin Periodontol. 2004;31:309-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Waasdorp JA, Evian CI, Mandracchia M. Immediate placement of implants into infected sites: a systematic review of the literature. J Periodontol. 2010;81:801-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med. 2016;15:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9979] [Cited by in RCA: 15920] [Article Influence: 1768.9] [Reference Citation Analysis (0)] |
| 27. | De Rouck T, Collys K, Cosyn J. Single-tooth replacement in the anterior maxilla by means of immediate implantation and provisionalization: a review. Int J Oral Maxillofac Implants. 2008;23:897-904. [PubMed] |
| 28. | Urban T, Kostopoulos L, Wenzel A. Immediate implant placement in molar regions: risk factors for early failure. Clin Oral Implants Res. 2012;23:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Chen H, Wang W, Gu X. Three-dimensional alveolar bone assessment of mandibular molars for immediate implant placement: a virtual implant placement study. BMC Oral Health. 2021;21:478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Albrektsson T, Wennerberg A. On osseointegration in relation to implant surfaces. Clin Implant Dent Relat Res. 2019;21 Suppl 1:4-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 183] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 31. | Buser D, Sennerby L, De Bruyn H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontol 2000. 2017;73:7-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 402] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 32. | Chen ST, Darby IB, Reynolds EC. A prospective clinical study of non-submerged immediate implants: clinical outcomes and esthetic results. Clin Oral Implants Res. 2007;18:552-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 261] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 33. | Zhang J, Huang X, Lu B, Zhang C, Cai Z. Can apical periodontitis affect serum levels of CRP, IL-2, and IL-6 as well as induce pathological changes in remote organs? Clin Oral Investig. 2016;20:1617-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 34. | Taheri M, Akbari S, Shamshiri AR, Shayesteh YS. Marginal bone loss around bone-level and tissue-level implants: A systematic review and meta-analysis. Ann Anat. 2020;231:151525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 35. | Kakar A, Kakar K, Leventis MD, Jain G. Immediate Implant Placement in Infected Sockets: A Consecutive Cohort Study. J Lasers Med Sci. 2020;11:167-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 36. | Hattingh A, De Bruyn H, Van Weehaeghe M, Hommez G, Vandeweghe S. Contour Changes Following Immediate Placement of Ultra-Wide Implants in Molar Extraction Sockets without Bone Grafting. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Naji BM, Abdelsameaa SS, Alqutaibi AY, Said Ahmed WM. Immediate dental implant placement with a horizontal gap more than two millimetres: a randomized clinical trial. Int J Oral Maxillofac Surg. 2021;50:683-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 38. | Botticelli D, Berglundh T, Buser D, Lindhe J. The jumping distance revisited: An experimental study in the dog. Clin Oral Implants Res. 2003;14:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 163] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 39. | Araujo-Pires AC, Francisconi CF, Biguetti CC, Cavalla F, Aranha AM, Letra A, Trombone AP, Faveri M, Silva RM, Garlet GP. Simultaneous analysis of T helper subsets (Th1, Th2, Th9, Th17, Th22, Tfh, Tr1 and Tregs) markers expression in periapical lesions reveals multiple cytokine clusters accountable for lesions activity and inactivity status. J Appl Oral Sci. 2014;22:336-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 40. | Maeda K, Kobayashi Y, Koide M, Uehara S, Okamoto M, Ishihara A, Kayama T, Saito M, Marumo K. The Regulation of Bone Metabolism and Disorders by Wnt Signaling. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 243] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 41. | Jakovljevic A, Knezevic A, Karalic D, Soldatovic I, Popovic B, Milasin J, Andric M. Pro-inflammatory cytokine levels in human apical periodontitis: Correlation with clinical and histological findings. Aust Endod J. 2015;41:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 42. | Larsen T, Fiehn NE. Dental biofilm infections - an update. APMIS. 2017;125:376-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 197] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 43. | Mertowska P, Mertowski S, Wojnicka J, Korona-Głowniak I, Grywalska E, Błażewicz A, Załuska W. A Link between Chronic Kidney Disease and Gut Microbiota in Immunological and Nutritional Aspects. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 44. | Carrillo C, Peñarrocha M, Vera F, Peñarrocha D. Immunohistochemical study of Langerhans cells in periapical lesions: correlation with inflammatory cell infiltration and epithelial cell proliferation. Med Oral Patol Oral Cir Bucal. 2010;15:e335-e339. [PubMed] |
| 45. | Oseko F, Yamamoto T, Akamatsu Y, Kanamura N, Iwakura Y, Imanishi J, Kita M. IL-17 is involved in bone resorption in mouse periapical lesions. Microbiol Immunol. 2009;53:287-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |