Published online Jul 16, 2023. doi: 10.12998/wjcc.v11.i20.4912
Peer-review started: March 12, 2023
First decision: May 8, 2023
Revised: May 21, 2023
Accepted: June 26, 2023
Article in press: June 26, 2023
Published online: July 16, 2023
Processing time: 122 Days and 2.7 Hours
The clinical symptoms and imaging manifestations of neurocysticercosis (NCC) are very different, and the difficulty and delay of clinical diagnoses may lead to an increase in mortality and disability. Rapid and accurate pathogen identification is important for the treatment of these patients. Metagenomic next-generation sequencing (mNGS) is a powerful tool to identify pathogens, especially in infections that are difficult to identify by conventional methods.
A 43-year-old male patient was admitted due to a recurrent headache for a few months. Imaging examinations showed hydrocephalus and cystic lesions, which were considered to be a central nervous system infection, but no etiology was found by routine examination. mNGS of the cerebrospinal fluid revealed high Taenia solium reads, and the positive results of a cysticercosis antibody test confirmed the infection. Combined with the patient’s clinical manifestations, the etiological evidence, and the imaging manifestation, the patient was finally diagnosed with NCC and he was prescribed dexamethasone, albendazole, neurotrophic drugs, and intracranial pressure reduction therapy. The headaches disappeared after anti-parasite treatment, and no associated symptoms recurred prior to the three- and six-month follow-up.
As an accurate and sensitivity detection method, mNGS can be a reliable approach for the diagnosis of NCC.
Core Tip: Neurocysticercosis (NCC) infection is rare, and the diagnostic methods are limited. Here we report a case of Taenia solium infection that was diagnosed by metagenomic next-generation sequencing (mNGS) using cerebrospinal fluid samples. Finally, after anti-parasite treatment, the headaches disappeared and the patient recovered well, and various indicators gradually returned to normal during the follow-up period. This indicates that mNGS can be a reliable approach for the diagnosis of NCC, due to its accuracy and sensitivity.
- Citation: Xu WB, Fu JJ, Yuan XJ, Xian QJ, Zhang LJ, Song PP, You ZQ, Wang CT, Zhao QG, Pang F. Metagenomic next-generation sequencing in the diagnosis of neurocysticercosis: A case report. World J Clin Cases 2023; 11(20): 4912-4919
- URL: https://www.wjgnet.com/2307-8960/full/v11/i20/4912.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i20.4912
Neurocysticercosis (NCC) is defined as an infection of the central nervous system (CNS) caused by Taenia solium (T. solium) eggs or the larval stage of T. solium[1]. The life cycle of T. solium involves pigs and humans as hosts[2], and NCC is common in most developing countries in Asia, Africa, and Latin America, but has also begun to appear in some developed countries in recent years, attracting increasing attention[3]. NCC is the most common cause of adult-acquired epilepsy in endemic areas[4]. Due to the differences of number, size, developmental stage, and location of the parasites in the nervous system, myriad clinical symptoms and neuroimaging manifestations appear when patients suffer from NCC[5]. Symptoms of NCC are varied, ranging from typical meningitis symptoms, such as headache, nausea, vomiting, and neck rigidity, to epilepsy, numbness, mental symptoms and occasional single clinical symptoms. Headaches associated with NCC are commonly linked to increased pressure caused by hydrocephalus, cysts in the subarachnoid or ventricular regions, or cysticercoticencephalitis[6]. However, the typical neuroimaging features can sometimes be imitated by other conditions, and the imaging results are often inconclusive[7]. Traditional diagnostic methods cannot identify the infection quickly and accurately, so proper and early diagnosis remains a challenge, especially in non-epidemic areas with low prevalence[8].
Cases of NCC infection are extremely rare in non-endemic areas, and physicians in general hospitals which do not specialize in infectious diseases often give little consideration to rare pathogens. Due to the different abilities and experience of physicians in the diagnosis of NCC, the infection may be missed and patient treatment may be delayed. Metagenomic next-generation sequencing (mNGS) is an unbiased sequencing method which can detect pathogen DNA or RNA in a sample with high sensitivity and specificity in a short time. The application of mNGS makes accurate and early diagnosis of unculturable, rare, and novel pathogens possible, and improves the diagnostic efficiency and accuracy of clinicians[9]. mNGS can assist in the diagnosis of NCC as it provides more sensitive findings in clinical conditions such as immunodeficiency syndrome, blood stream, respiratory, and doubtful infections. We aimed to report the case of a patient who was diagnosed with T. solium infection assisted by mNGS of cerebrospinal fluid (CSF) samples, resulting in the anti-parasite treatment and a favorable clinical outcome.
A 43-year-old man presented to our hospital complaining of recurrent headaches for 6 mo.
The headaches lasted from a day to a week with increasing severity and were occasionally accompanied by dizziness and nausea.
The patient had a history of hypertension, and amlodipine (5 mg/d) was regularly taken. Six months before admission, the patient developed a headache without any obvious cause, manifested as pulsatile pain at the top of the head in the occipital and bilateral temporal regions, and which was worse at night. When the headache started, the patient was weak all over, unable to walk, and sometimes suffered from eye swelling and pain lasting for about 10 min; there was no obvious abnormality after relief. Seven days before admission, the patient presented with the same symptoms; cerebral imaging examinations showed hydrocephalus and he was treated with a mannitol drip at a local hospital, which only slightly relieved the symptoms.
The patient had smoked for three decades, approximately 20 cigarettes per day. He also had a history of alcohol abuse for several decades. The patient was a farmer, living in a non-endemic area with contact with dogs and pigs. He had no history of raw meat consumption. Furthermore, he was not aware of any hereditary disease within his family.
The physical examination results included the following: Body temperature, 36.4 °C; blood pressure, 139/84 mmHg; heart rate, 86 beats/min; respiratory rate, 21 breaths/min; and neurological examination revealed cognitive decline.
On the day of admission, the routine laboratory tests were as follows: blood sample white blood cells were 11.75 × 109 cells/L; neutrophils, 8.23 × 109 cells/L; and basophils, 0.08 × 109 cells/L. The remaining erythrocyte sedimentation rate, C-reaction protein, serum amyloid A, and procalcitonin were in the normal range; urine samples revealed normal results; and a biochemical analysis showed normal results during the admission.
On the third day after admission, CSF was collected via standard procedures and an analysis revealed CSF pressure was 330 mmH2O, total protein level was 1 g/L (normal range, 0.15-0.40 g/L), and glucose level was 0.07 mmol/L (normal range, 2.5-4.4 mmol/L). The number of white blood cells in the CSF was 108 per microliter (normal range, < 8 per microliter) and the eosinophilic granulocytes were significantly increased in the sample. Gram staining for bacteria, Ziehl-Neelsen acid-faststainingfor mycobacterium, cryptococcus neoformans antigen test, CSF cultures, and polymerase chain reaction tests for syphilis and virus detection in the CSF were all negative.
The CSF was sent for mNGS. On the fourth day of the hospital stay, the serum test results for Toxoplasma, Herpes virus, Cytomegalovirus, and Rubella virus (TORCH) were all negative. In addition, no abnormalities were found in anti-O and rheumatoid factor, antinuclear antibody, tumor marker, and tuberculous infection of T cell spot tests.
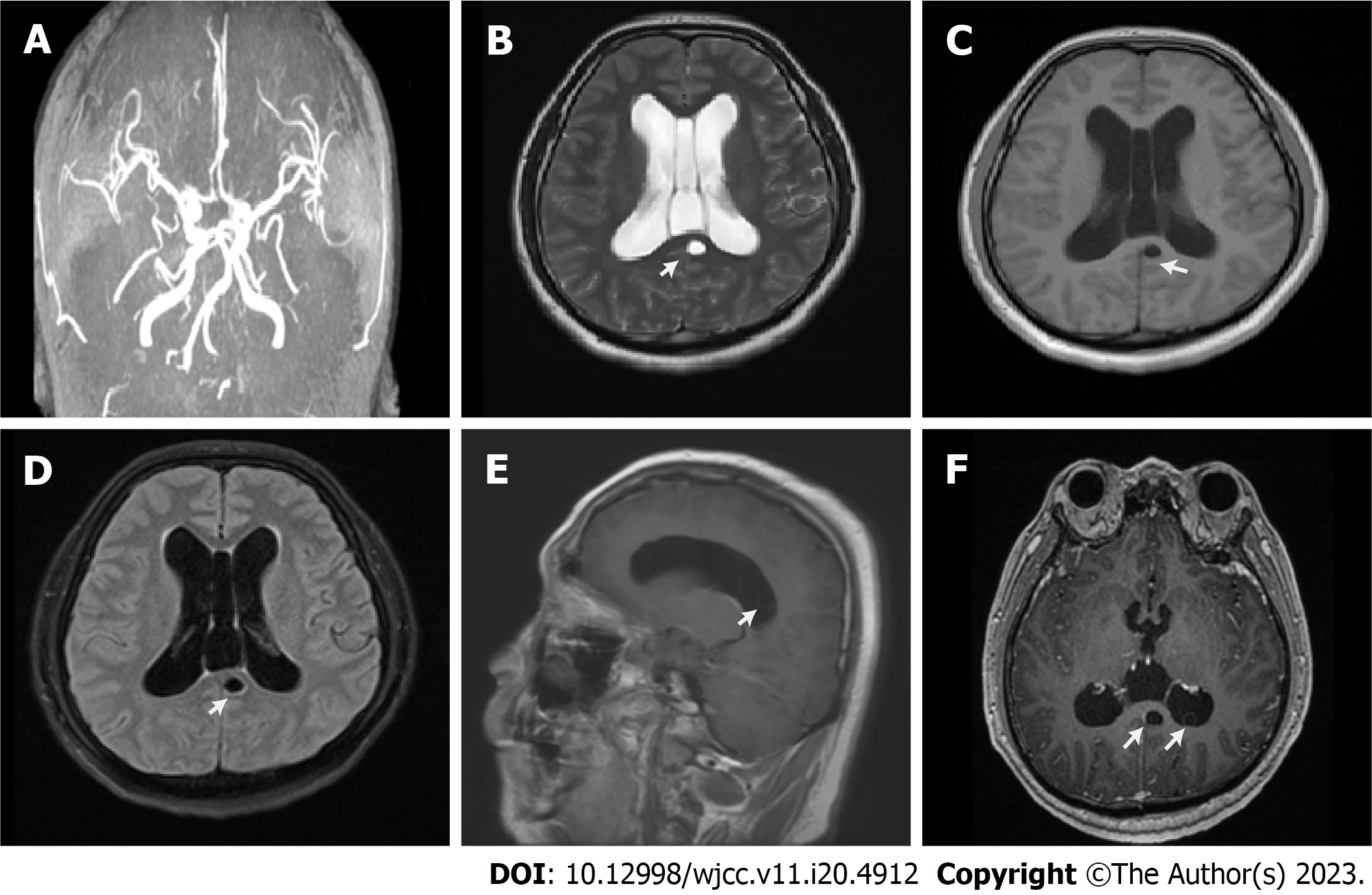
On the day of admission, magnetic resonance arteriography of the head and neck and magnetic resonance venography of the brain showed partial vascular stenosis and weakened blood flow signal. The next day, cerebral magnetic resonance imaging (MRI) results showed multiple cystic lesions in the sacculus of the hippocampus, behind the corpus callosum and the right cerebellum; moreover, an MRI revealed an expanded ventricular system and hydrocephalus, which was associated with headaches; cerebral MRI image with contrast showed several small hyperintense lesions. The doctors considered that the headache may be related to ventricular dilatation and hydrocephalus, perhaps caused by infection, but could not specify the etiology. The imaging examinations results are shown in Figure 1.
In total, 1.5 mL of CSF was collected via standard procedures and mNGS was conducted. DNA was extracted from 0.45 mL CSF samples using the TIANamp Micro DNA Kit (DP316, TIANGEN BIOTECH, Beijing, China) according to the manufacturer’s instructions. Then, DNA libraries were constructed via DNA fragmentation, end-repair, adapter-ligation, and polymerase chain reaction (PCR) amplification using the PMseqTM High throughput Gene detection kit for Infectious pathogens (Huada Biotechnology Co., Wuhan, Hubei, China). An Agilent 2100 Bio-analyzer was utilized for quality control of the DNA libraries. Qualified libraries (≥ 1 ng/μL) were pooled, and a DNA nanoball was generated and sequenced by the BGISEQ-50 platform.
High-quality sequencing data were generated by eliminating low-quality reads, and computational substraction of human host sequences mapped to the human reference genome (hg19) using a Burrows-Wheeler alignment tool. The remaining data, after removing low-complexity reads, were aligned with Pathogens Metagenomics Database, which is composed of bacteria, fungi, viruses, and parasites. Classification reference databases were sourced from NCBI (https://www.ncbi.nlm.nih.gov/). The threshold of the positive report was determined to be every 20 M of data volume, and more than 100 sequences.
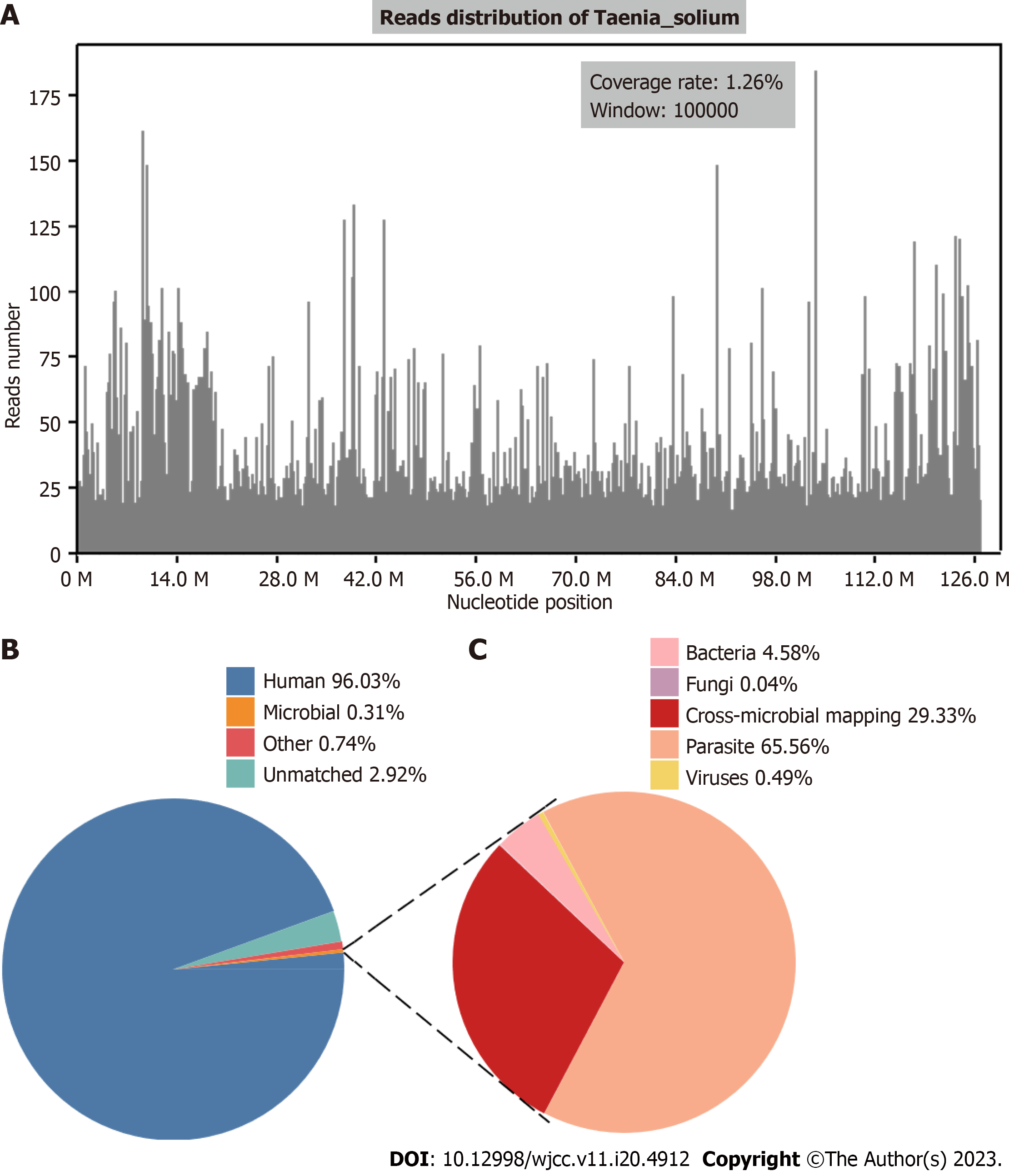
The result of mNGS showed that a total of 21507137 reads were generated from the next-generation sequencing. After analysis, most of the parasite reads (40710/43134; 94.38%) aligned to T.solium, with a genomic coverage of 1.26% and an average depth of 1.04 (Figure 2). Combined with the patient’s clinical manifestations, the etiology evidence, and the imaging manifestation, NCC was diagnosed.
In the days prior to mNGS, the patient received conventional treatments including anti-infection and intracranial pressure reducing medication, pain relief, blood circulation improvement, fluid balance maintaining, and physical detection treatments. Meanwhile, the headaches persisted.
After the mNGS result was obtained, the patient underwent antiparasitic treatment with albendazole (20 mg/kg/d, orally for 7 d in each course), corticosteroid anti-inflammatory therapy with dexamethasone (10 mg/d) to avoid potential inflammatory or anthelmintic administration complications, cranial pressure reduction therapy, and circulatory improvement therapy. All the symptoms were alleviated thereafter. Subsequently, the patient was discharged home and told to continue follow-up treatment.
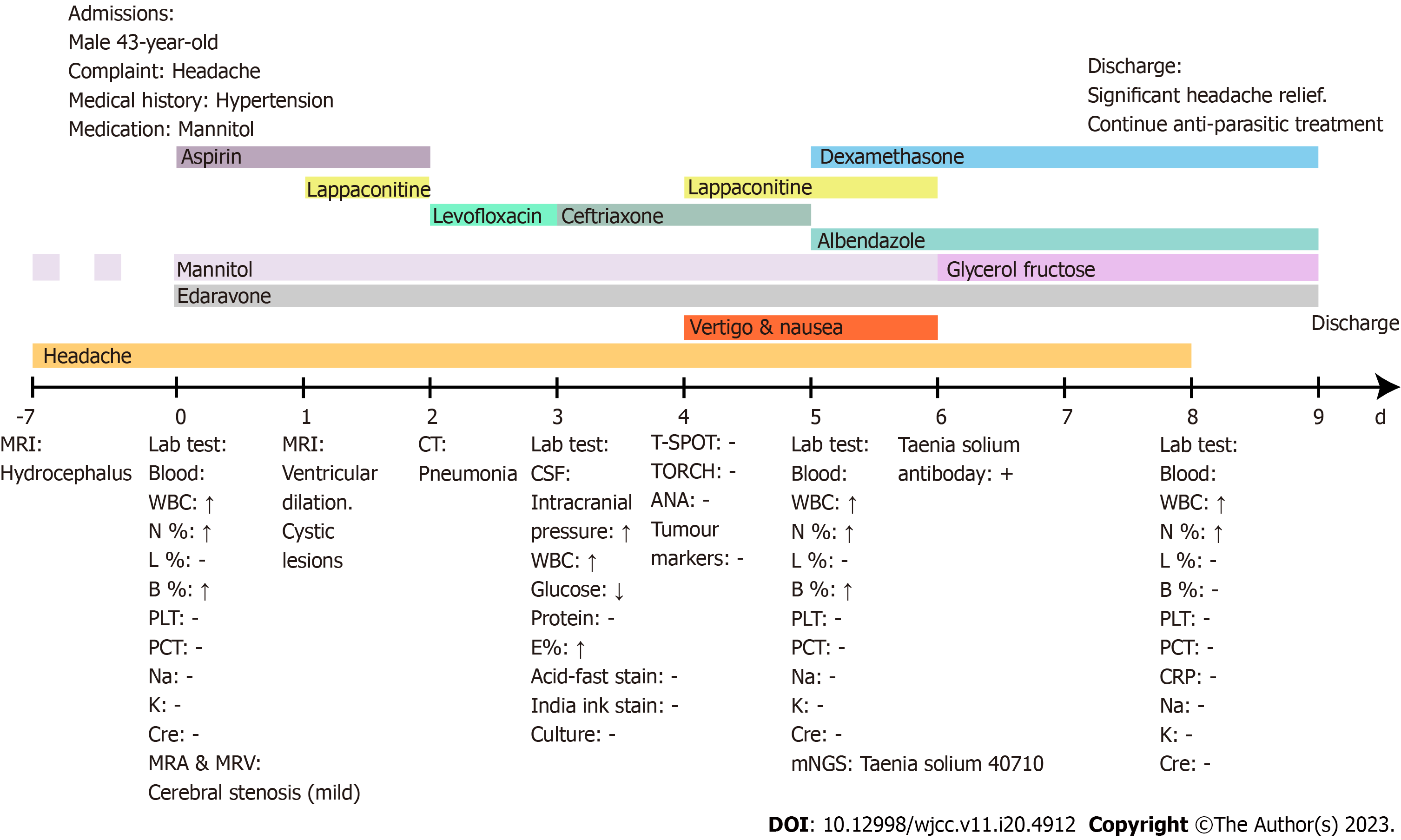
After a period of therapy in the hospital, the patient gradually recovered and continued to improve. He was satisfied with our treatment during the hospital stay. The symptoms did not reappear prior to the three-and six-month follow-up visits. The timeline of clinical events showing the laboratory results, symptoms, treatment, laboratory tests, and diagnosis are shown in Figure 3.
Cerebral cysticercosis is one of the most common CNS parasitic diseases in humans. According to the location of parasites in the CNS and psychiatric symptoms, NCC can be divided into parenchymal, meningeal, intraventricular and subarachnoid, and spinal forms. Parenchymal NCC is the most common type and has been widely studied. Contrastingly, the meningeal and spinal forms are uncommon. The intraventricular and subarachnoid forms cause acute ventriculomegaly episodes and are more serious than other forms[10]. We considered the NCC in our case to be the parenchymal and intraventricular forms of NCC.
For infections caused by parasites, the detection in tissues is the gold standard for the diagnosis thereof. However, for the diagnosis of NCC, it is obvious that in most cases the histological confirmation of parasites is impossible. Therefore, the diagnosis of NCC is usually based on neuroimaging and serological confirmation[4], or molecular approaches, most commonly via PCR. However, these traditional diagnostic techniques have some insurmountable limitations. A significant proportion of NCC has atypical clinical symptoms and nonspecific neuroimaging manifestations, so neuroimaging diagnosis alone is not conclusive. In addition, the specificity and sensitivity of immunodiagnostic tests are significantly reduced, especially in the early stages of the disease and in cases with viable cysticercosis, and cannot distinguish between a previous and an active infection, such as calcified cysticercus also producing a positive response[11]. In addition, antibody tests are not routine tests and physicians may not consider these tests in the first instance. O’Connell et al[12] have shown that the detection of T. solium DNA in CSF by conventional PCR has high sensitivity and specificity, but these methods require specific primers or probes, and the physician needs to anticipate the infection of T. solium in advance. This is sometimes difficult in non-endemic areas where the doctor may be inexperienced and the reagents are notroutinely available. mNGS can overcome some limitations of conservative approaches, and is a versatile technology which can identify pathogens more precisely and rapidly than traditional methods. mNGS can detect numerous pathogens in a run within 48 h and, compared to traditional detection methods which can only detect certain targeted pathogens, can identify all DNA or RNA of the sample and compare the data with the pathogen database to classify the pathogens. The pathogen profiles include almost all bacteria, fungi, viruses, and parasites that can infect patients. What’s more, as a result of unbiased sampling, mNGS can identify not only known pathogens but also unanticipated pathogens and novel organisms.
The application of mNGS is shifting from research to clinical laboratories on account of the proliferation of available sequencing instruments and substantial decreases in costs. This method is a needle-in-a-haystack endeavor; theoretically, all pathogens of a clinical sample can be characterized by mNGS in a single run. This breaks through the limitation of traditional diagnostic tests such as being culture-based, the quantitative restrictions of pathogens, or non-routine availability of reagents. Additionally, and most importantly, mNGS is hypothesis-free[13]. A limitation of mNGS, however, is that it cannot prove whether the identified microorganism is the causative agent or a contamination. Hence, a comprehensive diagnostic workup, a multidisciplinary approach by clinicians and microbiologists, and clinical imaging are required for unexplained brain lesions.
The treatment of NCC varies according to its stage. Patients with two or more cystic lesions have significantly higher rates of resolution with combination therapy with albendazole and praziquantel[14]; whereas patients with hydro
This study reported a case of CSF NCC using mNGS to detect T. solium, and to explore the value of mNGS in the diagnosis of NCC. Based on the mNGS results, we applied the Del Brutto et al[16] criteria to analyze the patient’s imaging findings and clinical symptoms, leading to the diagnosis of NCC. The targeted treatment yielded effective results. Our findings demonstrate that mNGS can serve as an effective adjunct to the standard diagnostic criteria. The patient visited our hospital due to a long history of repeated headaches. Subsequently, extensive clinical, laboratory, and imaging examinations were performed to address the cause of illness. However, the imaging examination was nonspecific and no etiology was found in other laboratory tests. Initially, based on the tests results, the patient received routine treatments which were of no significant effect. The exact cause of the headache was unclear until the mNGS was conducted. mNGS retrieved all the pathogens presented in the CSF samples in a short time and 94.38% of parasite reads were matched with T. solium. A positive serological test of CSF also supported the diagnosis of NCC. According to the results of mNGS, precise and effective therapy was carried out to relieve the clinical symptoms of the patient. After in-hospital treatment, the patient’s headache symptoms were significantly relieved, and there was no recurrence after three- and six-month follow-ups.
For patients with atypical clinical symptoms and nonspecific neuroimaging manifestations, and especially when the contact history is not clear and in the non-epidemic area of T. solium, NCC may be misdiagnosed. This may waste time for an NCC patient from onset to final correct diagnosis, which not only increases the economic burden of patients, but also wastes medical resources. Most data suggest that T. solium carriers can infect themselves with eggs or spread the ova to others[17]. Our case shows that mNGS is a powerful tool for the clinical diagnosis of NCC and can greatly shorten the diagnostic time of T. solium. mNGS is accurate and unbiased for pathogen identification, especially in rare infections that are difficult to diagnose by conventional methods.
mNGS provides a rapid and reliable diagnosis in challenging cases of NCC with high sensitivity, efficiency, and specificity, and may guide the management and treatment strategy and, therefore, benefit the patient and public health systems. This case may also remind clinicians to pay due attention to T. solium infection in CNS, as sporadic cases may occur in non-endemic areas.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Dauyey K, Kazakhstan; PittonRissardo J, Brazil S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Hamamoto Filho PT, Rodríguez-Rivas R, Fleury A. Neurocysticercosis: A Review into Treatment Options, Indications, and Their Efficacy. Res Rep Trop Med. 2022;13:67-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 2. | Li L, He W, Fan X, Liu M, Luo B, Yang F, Jiang N, Wang L, Zhou B. Proteomic analysis of Taenia solium cysticercus and adult stages. Front Vet Sci. 2022;9:934197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 3. | Ta R, Blond BN. The prevalence of and contributors to neurocysticercosis in endemic regions. J Neurol Sci. 2022;441:120393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 4. | Herrick JA, Bustos JA, Clapham P, Garcia HH, Loeb JA; Cysticercosis Working Group in Peru. Unique Characteristics of Epilepsy Development in Neurocysticercosis. Am J Trop Med Hyg. 2020;103:639-645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Arroyo G, Bustos JA, Lescano AG, Gonzales I, Saavedra H, Pretell EJ, Castillo Y, Perez E, Dorny P, Gilman RH, O'Neal SE, Gonzalez AE, Garcia HH; Cysticercosis Working Group in Peru (CWGP). Improved Diagnosis of Viable Parenchymal Neurocysticercosis by Combining Antibody Banding Patterns on Enzyme-Linked Immunoelectrotransfer Blot (EITB) with Antigen Enzyme-Linked Immunosorbent Assay (ELISA). J Clin Microbiol. 2022;60:e0155021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Del Brutto OH. Human Neurocysticercosis: An Overview. Pathogens. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 7. | Guzman C, Garcia HH; Cysticercosis Working Group in Peru. Current Diagnostic Criteria for Neurocysticercosis. Res Rep Trop Med. 2021;12:197-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Garcia HH, Gonzalez AE, Gilman RH. Taenia solium Cysticercosis and Its Impact in Neurological Disease. Clin Microbiol Rev. 2020;33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 9. | Liang Y, Feng Q, Wei K, Hou X, Song X, Li Y. Potential of metagenomic next-generation sequencing in detecting infections of ICU patients. Mol Cell Probes. 2023;68:101898. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | El-Kady AM, Allemailem KS, Almatroudi A, Abler B, Elsayed M. Psychiatric Disorders of Neurocysticercosis: Narrative Review. Neuropsychiatr Dis Treat. 2021;17:1599-1610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Fei X, Li C, Zhang Y, Zhang H, Liu X, Ji X, Shi Y, Liu N, Wu M, Du F, Yang Y, Dai W, Liu T, He Y, Bian T, Zhou H, An X, Cai Z, Shi J, Feng G, Shi M, Zhao G. Next-generation sequencing of cerebrospinal fluid for the diagnosis of neurocysticercosis. Clin Neurol Neurosurg. 2020;193:105752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | O'Connell EM, Harrison S, Dahlstrom E, Nash T, Nutman TB. A Novel, Highly Sensitive Quantitative Polymerase Chain Reaction Assay for the Diagnosis of Subarachnoid and Ventricular Neurocysticercosis and for Assessing Responses to Treatment. Clin Infect Dis. 2020;70:1875-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Fei X, Li C, Zhang Y, Zhang H, Liu X, Ji X, Shi Y, Liu N, Wu M, Du F, Yang Y, Dai W, Liu T, He Y, Bian T, Zhou H, An X, Cai Z, Shi J, Feng G, Shi M, Zhao G. Data relating to sequencing statistics and the reads and genomic coverage aligning to Taenia solium in the cerebrospinal fluid. Data Brief. 2020;31:105700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Garcia HH. Parasitic Infections of the Nervous System. Continuum (Minneap Minn). 2021;27:943-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Takayanagui OM, Haes TM. Update on the diagnosis and management of neurocysticercosis. Arq Neuropsiquiatr. 2022;80:296-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Del Brutto OH, Nash TE, White AC Jr, Rajshekhar V, Wilkins PP, Singh G, Vasquez CM, Salgado P, Gilman RH, Garcia HH. Revised diagnostic criteria for neurocysticercosis. J Neurol Sci. 2017;372:202-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 199] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 17. | Jansen F, Dorny P, Gabriël S, Dermauw V, Johansen MV, Trevisan C. The survival and dispersal of Taenia eggs in the environment: what are the implications for transmission? A systematic review. Parasit Vectors. 2021;14:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |