Published online Jan 16, 2023. doi: 10.12998/wjcc.v11.i2.316
Peer-review started: September 22, 2022
First decision: November 22, 2022
Revised: December 8, 2022
Accepted: January 5, 2023
Article in press: January 5, 2023
Published online: January 16, 2023
Processing time: 111 Days and 11.5 Hours
Coronavirus disease 2019 significantly impacted the liver transplant process worldwide. Consequently, it brought significant challenges and limitations to transplant policies and organ allocation forcing liver transplant centers to adjust their protocols to ensure maximum benefit and avoid harm to their patients. Our center, like many others, was obliged to adapt to the challenges. This paper provided an overview of the effects of coronavirus disease 2019 on liver transplantations and detailed our center’s experience and efforts during this unprecedented pandemic to serve as a guide for future public health crises.
Core Tip: The coronavirus disease 2019 pandemic gave rise to an exceptional situation for liver transplantation (LT) around the world, initially leading to a decline in LT followed by a rapid recovery. This robust response resulted from extensive efforts by various LT centers to offset these challenges in addition to emerging evidence and the provision of appropriate guidelines from major LT societies. It is of the utmost importance to share experiences among LT centers to improve outcomes and reduce graft loss.
- Citation: Khazaaleh S, Suarez ZK, Alomari M, Rashid MU, Handa A, Gonzalez AJ, Zervos XB, Kapila N. Liver transplantation amidst the COVID-19 era: Our center’s experience. World J Clin Cases 2023; 11(2): 316-321
- URL: https://www.wjgnet.com/2307-8960/full/v11/i2/316.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i2.316
The coronavirus (COVID-19) pandemic represented an unforeseen crisis to healthcare systems and transplant centers around the world, resulting in significant changes to solid organ transplant (SOT) practices[1,2]. At the beginning of the pandemic, a decrease in SOT was observed and attributed to multiple factors, which included a shortage of resources and staff, a saturation of critical care beds, changes to the donor pool, and uncertainty regarding optimal post-transplant immunosuppressive therapy. The stress on the health care system forced most of the transplant centers to reduce liver transplant (LT) activity to best utilize scarce resources.
LT centers that continued their activity had to adjust their policies and develop strategies that ensured the safety of their patients, including protocols regarding testing of donors and candidates, reorganization of clinical and isolation protocols to establish a coronavirus-free pathway, rearrangement of the waitlist based on priority, and promotion of telemedicine to minimize exposure to the virus[3-5]. The United States was not the exception, and the initial reduction in the number of LT performed at the beginning of 2020 was followed by a brisk comeback in the second half of 2020 and early 2021. The emergence of evidence and recommendations by the international transplant societies was crucial in guiding LT programs during these unprecedented times[6].
In this article, we discussed our center’s experience with COVID-19 regarding its effects on LT patients, including the pretransplant, perioperative and post-transplant periods.
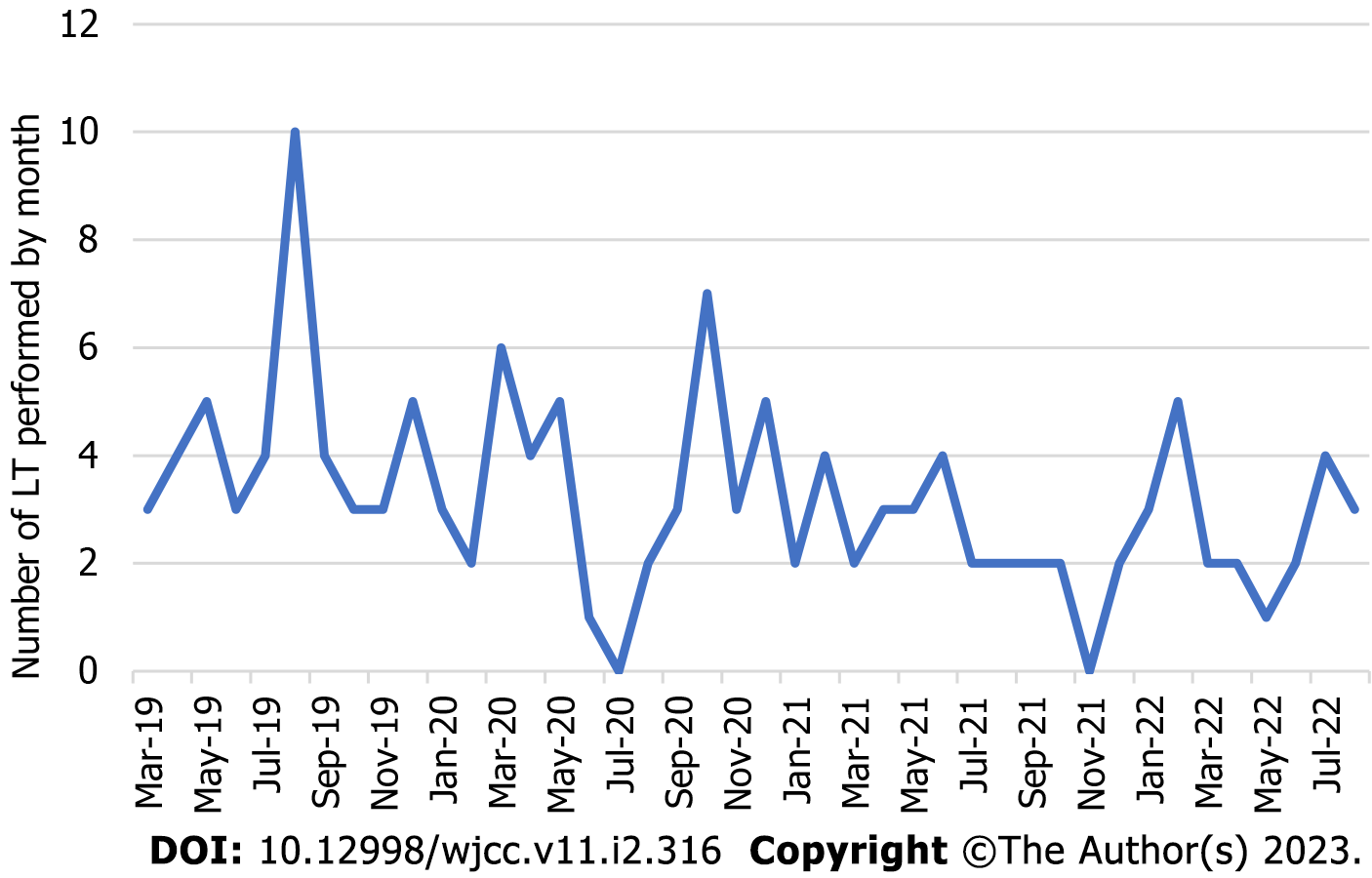
Soon after the pandemic was declared we observed a decline in the number of LT performed at our center, which was similar to other LT centers across the nation[7]. From March 2020 to February 2021, 42 LT were performed, representing a reduction of 14.28% of the cases when compared to the same period 1 year earlier. Subsequently, from March 2021 to February 2022, a 38.77% decrease in LT was noted when compared to the period between March 2019 and February 2020 and a reduction of 28.57% when compared to the period between March 2020 and February 2021 (Figure 1).
General COVID-19 preventive measures for LT candidates are similar to those established for the general public. We continue to recommend that LT recipients maintain personal measures to minimize exposure to COVID-19, such as social distancing, masking, and avoiding gatherings whenever possible, regardless of vaccination status.
LT centers faced significant ethical challenges of vaccine skepticism and uncertainty regarding if it should be mandatory for all candidates on the waitlist[8]. Based on guidance from international transplant societies, vaccination against COVID-19 was strongly recommended for patients with chronic liver disease[9]. However, SOT recipients were found to mount a lower humoral response to COVID-19 vaccination, for which the administration of booster doses was deemed necessary to achieve acceptable immunity[10,11].
On August 12th, 2021, the Food and Drug Administration approved a three-dose mRNA vaccine series for immunocompromised patients, including SOT recipients[12]. In addition, the Advisory Committee on Immunization Practices recommended that all immunocompromised adults should be vaccinated at least 3 mo after the third inoculation of the mRNA vaccine or 2 mo after the initial sequence of the Johnson and Johnson vaccine[13]. We agree with these recommendations, and in our center we highly recommend that all patients listed for LT receive at least three doses of an mRNA vaccine before transplant.
At the beginning of 2022, the Food and Drug Administration published an Emergency Use Authorization for tixagevimab/cilgavimab (Evusheld), a long-acting monoclonal antibody for pre-exposure prophylaxis of COVID-19 in patients with moderate to severe immunosuppression[14]. Evusheld became an attractive armamentarium for protection against COVID-19 infection in LT recipients who may not be able to mount an appropriate immune response to the vaccine[15]. In a retrospective study that followed 378 patients with hematologic malignancies, less than 60% of patients seroconverted after the third vaccine dose, regardless of the therapy used. Thirty-three patients (8.8%) eventually developed COVID-19 infection, and among them 3 patients died due to severe infection. Importantly, no deaths occurred among patients who achieved seroconversion, and none of the patients who received Evusheld (n = 25) developed COVID-19 infection[10]. Although data on the use of Evusheld may be lacking in the SOT population, at our center we adopted the use of Evusheld in all patients who undergo LT, irrespective of vaccination status.
Early case reports from Korea and India studied the viability of conducting an LT after 14-28 d of a positive COVID-19 infection in asymptomatic or minimally symptomatic patients[16,17]. In addition, a retrospective multicenter study from 11 European countries reported 26 patients who received an LT after a median of 78.5 d from an initial positive test for COVID-19. Even though LT candidates with symptomatic COVID-19 were at higher risk of early mortality when compared to counterparts with similar Model for End-stage Liver Disease scores, those who underwent LT had a favorable short-term survival of 96%. This study showed that once patients recover from COVID-19, LT is safe and encouraged[18].
In our center, patients on the waitlist who have a positive PCR test for COVID-19 are temporarily inactivated until becoming asymptomatic and 3 wk have elapsed since their initial positive test. Subsequently, if the patient had respiratory symptoms, we perform a contrast-enhanced computed tomography of the chest and pulmonary function tests before waitlist reactivation. Conversely, if the patient did not develop any respiratory symptoms, they are reactivated without further testing.
During the beginning of the pandemic, transplant societies recommended against SOT in situations where donors tested positive for COVID-19 due to the likelihood of developing complications, such as acute respiratory distress syndrome or thrombosis of the graft. However, as the pandemic continued, the Organ Procurement and Transplantation Network Ad Hoc Disease Transmission Advisory Committee recommended that the decision to transplant organs from donors with active infection of COVID-19 should take into consideration the likelihood of death of the recipient for delaying the procedure and the risk of transmission to members of the transplant team.
Nevertheless, studies revealed that, unlike lung transplant recipients, the chance of disseminating COVID-19 infection from the donor to the recipient was low in LT patients[19]. The degree of viral load in the blood is usually low, and therefore blood-borne transmission does not represent a significant risk[20]. In our center, we actively consider liver grafts from donors who are COVID-19 positive at the time of organ donation.
Initial reports suggested that LT recipients could be at an increased risk of acquiring severe COVID-19, given their immunosuppressed status, with the inherent risk of long-term viral shedding[21,22]. Although some studies showed an increased infection rate among SOT recipients, this was not associated with worse clinical outcomes. The Spanish Society for Liver Transplantation conducted a prospective nationwide study that included 111 LT recipients and concluded that these patients were twice as likely to be infected with COVID-19 compared to age- and sex-matched individuals (standardized incidence: 191.2; 95% confidence interval: 190.3-192.2)[23]. However, another prospective study from Italy followed 30 LT recipients with COVID-19 and suggested that LT recipients were more symptomatic yet with no increased risk of hospitalization or death[24].
The approach to management varies based on the severity of the COVID-19 infection and largely stems from experts’ opinions. It is generally advised to lower the cumulative dose of immunosuppression, particularly mycophenolate mofetil, if possible[23]. Immunosuppression was found to be an independent predictor of severe COVID-19 disease as it may interfere with mounting a humoral response to COVID-19 vaccination[25]. Commonly used agents for outpatient management of COVID-19 infection include oral antivirals such as molnupiravir and nirmatrelvir/ritonavir (Paxlovid). Molnupiravir appears to be effective, safe, and well-tolerated in LT patients[26]. Nonetheless, Paxlovid strongly interacts with calcineurin inhibitors, so concomitant use is contraindicated due to the potential for calcineurin inhibitor toxicity[27]. For those requiring inpatient management of COVID-19, the nucleotide analog remdesivir is our preferred therapeutic option. It has been shown to shorten the duration of illness and hospitalization, especially when given to patients on supplemental oxygen within 10 d of symptom onset[28].
We also use COVID-19-specific antibodies in LT recipients with COVID-19, mainly in the outpatient setting and selected patients in the inpatient setting. In a single-center, retrospective study that included liver and kidney transplant recipients, COVID-19 monoclonal antibody (casirivimab-imdevimab or bamlanivimab) reduced hospitalization from 32% to 15% (P = 0.045) with no mortality (13% vs 0%, P = 0.04)[29].
Healthcare providers are well-known to be at an additional risk of contracting COVID-19 when compared to the general population[30]. At our center, we adopted a strategy of decreasing interactions among team members to mitigate the risk of COVID-19 transmission. All meetings, including LT selection and multidisciplinary tumor boards, were transitioned to an online platform. A strict departmental protocol was implemented for caregivers who developed symptoms to allow them to undergo testing and appropriate isolation. Outpatient visits, when necessary, were shifted to an online platform to minimize unnecessary exposure and protect patients and staff members.
The COVID-19 pandemic has impacted transplant centers globally. Despite the burden, LT centers have been forced to adopt protocols to ensure patient and caregiver safety. A limitation of this review is that it only provides the experience of one LT center in the United States. In the future, emerging evidence will further guide LT centers toward the creation of contingency plans to provide optimal pretransplant, perioperative, and post-transplant care in future public health crises.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gupta MK, Germany; Jin Y, China S-Editor: Wang LL L-Editor: Filipodia P-Editor: Wang LL
| 1. | Kute VB, Tullius SG, Rane H, Chauhan S, Mishra V, Meshram HS. Global Impact of the COVID-19 Pandemic on Solid Organ Transplant. Transplant Proc. 2022;54:1412-1416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Loupy A, Aubert O, Reese PP, Bastien O, Bayer F, Jacquelinet C. Organ procurement and transplantation during the COVID-19 pandemic. Lancet. 2020;395:e95-e96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 209] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 3. | Fortunato AC, Pinheiro RS, Fernandes MR, Nacif LS, Arantes RM, Rocha-Santos V, Waisberg DR, De Martino RB, Ducatti L, Haddad LB, Song AT, Abdala E, Andraus W, Carneiro-D'Albuquerque LA. COVID-19 Pandemic Impact on Liver Donation in the Largest Brazilian Transplantation Center. Transplant Proc. 2022;54:1212-1214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 4. | da Silva Knihs N, da Silva AM, Dietrich MA, Rodrigues MC, Sens S, Wachholz LF, de Mello T, Bittencourt I, da Silva Martins M, Magalhães ALP, Amante LN. Technologies During the COVID-19 Pandemic: Teleconsultation in Care Management for Patients Undergoing Liver Transplant. Transplant Proc. 2022;54:1324-1328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 5. | De Carlis R, Vella I, Incarbone N, Centonze L, Buscemi V, Lauterio A, De Carlis L. Impact of the COVID-19 pandemic on liver donation and transplantation: A review of the literature. World J Gastroenterol. 2021;27:928-938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 6. | Organ transplants bounce back to near pre-COVID-19 Levels | UNOS. [cited 20 September 2022]. Available from: https://unos.org/news/transplants-bounce-back-to-near-pre-covid-19-levels. |
| 7. | Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91:157-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2616] [Reference Citation Analysis (0)] |
| 8. | Kates OS, Stock PG, Ison MG, Allen RDM, Burra P, Jeong JC, Kute V, Muller E, Nino-Murcia A, Wang H, Wall A. Ethical review of COVID-19 vaccination requirements for transplant center staff and patients. Am J Transplant. 2022;22:371-380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Hippen BE, Axelrod DA, Maher K, Li R, Kumar D, Caliskan Y, Alhamad T, Schnitzler M, Lentine KL. Survey of current transplant center practices regarding COVID-19 vaccine mandates in the United States. Am J Transplant. 2022;22:1705-1713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 10. | Ollila TA, Masel RH, Reagan JL, Lu S, Rogers RD, Paiva KJ, Taher R, Burguera-Couce E, Zayac AS, Yakirevich I, Niroula R, Barth P, Olszewski AJ. Seroconversion and outcomes after initial and booster COVID-19 vaccination in adults with hematologic malignancies. Cancer. 2022;128:3319-3329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 11. | Garcia-Beltran WF, St Denis KJ, Hoelzemer A, Lam EC, Nitido AD, Sheehan ML, Berrios C, Ofoman O, Chang CC, Hauser BM, Feldman J, Roederer AL, Gregory DJ, Poznansky MC, Schmidt AG, Iafrate AJ, Naranbhai V, Balazs AB. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022;185:457-466.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 832] [Cited by in RCA: 765] [Article Influence: 255.0] [Reference Citation Analysis (0)] |
| 12. | Coronavirus (COVID-19) Update: FDA Authorizes Additional Vaccine Dose for Certain Immunocompromised Individuals | FDA. [cited 20 September 2022]. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-vaccine-dose-certain-immunocompromised. |
| 13. | Joint Statement from HHS Public Health and Medical Experts on COVID-19 Booster Shots | CDC Online Newsroom | CDC. [cited 20 September 2022]. Available from: https://www.cdc.gov/media/releases/2021/s0818-covid-19-booster-shots.html. |
| 14. | Tixagevimab and Cilgavimab (Evusheld) for Pre-Exposure Prophylaxis of COVID-19. JAMA. 2022;327:384-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 96] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 15. | Pre-exposure Prophylaxis with Evusheld. [cited 20 September 2022]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/pre-exposure-prophylaxis.html. |
| 16. | Hong HL, Kim SH, Choi DL, Kwon HH. A case of coronavirus disease 2019-infected liver transplant donor. Am J Transplant. 2020;20:2938-2941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 17. | Kulkarni AV, Parthasarathy K, Kumar P, Sharma M, Reddy R, Chaitanya Akkaraju Venkata K, Gupta R, Gupta A, Swaroop S, Giri Vishwanathan P, Senapathy G, Menon PB, Reddy ND, Padaki NR. Early liver transplantation after COVID-19 infection: The first report. Am J Transplant. 2021;21:2279-2284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 18. | Belli LS, Duvoux C, Cortesi PA, Facchetti R, Iacob S, Perricone G, Radenne S, Conti S, Patrono D, Berlakovich G, Hann A, Pasulo L, Castells L, Faitot F, Detry O, Invernizzi F, Magini G, De Simone P, Kounis I, Morelli MC, Díaz Fontenla F, Ericzon BG, Loinaz C, Johnston C, Gheorghe L, Lesurtel M, Romagnoli R, Kollmann D, Perera MTP, Fagiuoli S, Mirza D, Coilly A, Toso C, Zieniewicz K, Elkrief L, Karam V, Adam R, den Hoed C, Merli M, Puoti M, De Carlis L, Oniscu GC, Piano S, Angeli P, Fondevila C, Polak WG; for all the centres contributing to the ELITA-ELTR COVID-19 Registry. COVID-19 in liver transplant candidates: pretransplant and post-transplant outcomes - an ELITA/ELTR multicentre cohort study. Gut. 2021;70:1914-1924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | Kute VB, Fleetwood VA, Meshram HS, Guenette A, Lentine KL. Use of Organs from SARS-CoV-2 Infected Donors: Is It Safe? Curr Transplant Rep. 2021;8:281-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 20. | Chang L, Yan Y, Wang L. Coronavirus Disease 2019: Coronaviruses and Blood Safety. Transfus Med Rev. 2020;34:75-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 447] [Cited by in RCA: 387] [Article Influence: 77.4] [Reference Citation Analysis (0)] |
| 21. | Pereira MR, Mohan S, Cohen DJ, Husain SA, Dube GK, Ratner LE, Arcasoy S, Aversa MM, Benvenuto LJ, Dadhania DM, Kapur S, Dove LM, Brown RS Jr, Rosenblatt RE, Samstein B, Uriel N, Farr MA, Satlin M, Small CB, Walsh TJ, Kodiyanplakkal RP, Miko BA, Aaron JG, Tsapepas DS, Emond JC, Verna EC. COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant. 2020;20:1800-1808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 541] [Cited by in RCA: 664] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 22. | Leung WF, Chorlton S, Tyson J, Al-Rawahi GN, Jassem AN, Prystajecky N, Masud S, Deans GD, Chapman MG, Mirzanejad Y, Murray MCM, Wong PHP. COVID-19 in an immunocompromised host: persistent shedding of viable SARS-CoV-2 and emergence of multiple mutations: a case report. Int J Infect Dis. 2022;114:178-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 23. | Colmenero J, Rodríguez-Perálvarez M, Salcedo M, Arias-Milla A, Muñoz-Serrano A, Graus J, Nuño J, Gastaca M, Bustamante-Schneider J, Cachero A, Lladó L, Caballero A, Fernández-Yunquera A, Loinaz C, Fernández I, Fondevila C, Navasa M, Iñarrairaegui M, Castells L, Pascual S, Ramírez P, Vinaixa C, González-Dieguez ML, González-Grande R, Hierro L, Nogueras F, Otero A, Álamo JM, Blanco-Fernández G, Fábrega E, García-Pajares F, Montero JL, Tomé S, De la Rosa G, Pons JA. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J Hepatol. 2021;74:148-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 269] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 24. | Guarino M, Cossiga V, Loperto I, Esposito I, Ortolani R, Fiorentino A, Pontillo G, De Coppi L, Cozza V, Galeota Lanza A, Di Costanzo GG, Picciotto FP, Morisco F. COVID-19 in liver transplant recipients: incidence, hospitalization and outcome in an Italian prospective double-centre study. Sci Rep. 2022;12:4831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 25. | Meunier L, Sanavio M, Dumortier J, Meszaros M, Faure S, Ursic Bedoya J, Echenne M, Boillot O, Debourdeau A, Pageaux GP. Mycophenolate mofetil decreases humoral responses to three doses of SARS-CoV-2 vaccine in liver transplant recipients. Liver Int. 2022;42:1872-1878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Sharma K. IL-18 attenuates experimental choroidal neovascularization as a potential therapy for wet age-related macular degeneration. Sci Transl Med 6, 230ra44 (2014). Ann Neurosci. 2014;21:150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Paxlovid Drug-Drug Interactions | COVID-19 Treatment Guidelines. [cited 20 September 2022]. Available from: https://www.covid19treatmentguidelines.nih.gov/therapies/antiviral-therapy/ritonavir-boosted-nirmatrelvir--paxlovid-/paxlovid-drug-drug-interactions/. |
| 28. | Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC; ACTT-1 Study Group Members. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020;383:1813-1826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5711] [Cited by in RCA: 5108] [Article Influence: 1021.6] [Reference Citation Analysis (0)] |
| 29. | Ahearn AJ, Thin Maw T, Mehta R, Emamaullee J, Kim J, Blodget E, Kahn J, Sher L, Genyk Y. A Programmatic Response, Including Bamlanivimab or Casirivimab-imdevimab Administration, Reduces Hospitalization and Death in COVID-19 Positive Abdominal Transplant Recipients. Transplantation. 2022;106:e153-e157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 30. | Andersen MP, Østergaard L, Phelps M, Butt JH, Køber L, Gislason G, Christensen HC, Torp-Pedersen C, Schou M, Fosbøl EL, Kragholm K. Risk of coronavirus disease 2019 (Covid-19) contraction and severe infection in home- or healthcare professionals. J Infect. 2021;83:e12-e14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |