Published online Jun 26, 2023. doi: 10.12998/wjcc.v11.i18.4341
Peer-review started: February 6, 2023
First decision: April 26, 2023
Revised: May 4, 2023
Accepted: May 22, 2023
Article in press: May 22, 2023
Published online: June 26, 2023
Processing time: 140 Days and 3.1 Hours
Synchronous endometrial and ovarian cancer (SEOC) is a rare genital tract tumor. Precise diagnosis is crucial for the disease management since prognosis and overall survival differ substantially between metastatic endometrial cancer (EC) or OC. In this review we present 2 cases of women who were diagnosed with SEOC, and discuss the clinical characteristic of SEOC, diagnostic and molecular profiling issues. Next generation sequencing of 10 gene panel was performed on cancerous tissue and uterine lavage samples.
In our report patients with SEOC had endometroid type histology with early stage and low-grade histology for both EC and OC. They underwent surgical treatment and staging. Next-generation sequencing of 10 gene-panel identified CTNNB1, PIK3CA, and PTEN gene mutations in ovarian tissue in one case, while none of these genes were mutated in other case. Literature review in support to our data suggest a good prognosis for SEOC diagnosed at early stage.
Accurate diagnosis of SEOC is essential for disease management and gene mutation analysis can be helpful as a complementary diagnostic and prognostic tool.
Core Tip: Synchronous gynecological tumors are a rare entity. The most common synchronous tumor is synchronous endometrial and ovarian cancer (SEOC). The importance of distinguishing SEOC from either isolated endometrium or ovarian cancer with metastasis is crucial, as it determines management and prognosis. When molecular testing was carried out for SEOC cases, a large proportion was found to be metastatic disease. Testing of vimentin, molecular analyses of gene mutation of CTNNB1, Paired box gene 8, β-catenin expression may be helpful to categorize SEOC in cases where clinical and pathological parameters are inconclusive.
- Citation: Žilovič D, Čiurlienė R, Šidlovska E, Vaicekauskaitė I, Sabaliauskaitė R, Jarmalaitė S. Synchronous endometrial and ovarian cancer: A case report. World J Clin Cases 2023; 11(18): 4341-4349
- URL: https://www.wjgnet.com/2307-8960/full/v11/i18/4341.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i18.4341
Synchronous gynecological malignant tumor is a rare entity. Synchronous endometrial and ovarian cancer (SEOC) is the most common, accounting for 50%-70% of all synchronous tumors. SEOC is described as the simultaneous presence of ovarian cancer (OC) and endometrial cancer (EC) at the same time of diagnosis. The rate of SEOC is approximately 3%-10%, 5% occur in patients with EC and 10% in patients with OC. Endometrioid histological type of EC is significantly associated with increased risk of secondary primary OC with endometrioid histology. Also, OC with endometroid histology diagnosed under the age of 50 can be associated with loss of mismatch repair genes expression, Lynch syndrome and other genetic mutations. In these patients, the risk of synchronous primary malignancy is higher. Up to 86% of SEOC have concordant endometrioid histology in the two cancer sites[1-4].
For more than 20 years histological criteria by Ulbright and Roth have been used to distinguish metastatic cancer from double primary cancer[5]. Criteria for SEOC diagnosis were first documented in 1981 and refined substantially in 1998[6,7]. The Scully criteria are based on histologic similarity, size, the presence of precursor lesions, location, and invasion pattern. Later, several authors have proposed methods of molecular analysis for precise SEOC diagnostic, but no consensus has been reached yet. Sometimes it is complicated to distinguish between SEOC and metastatic cancer (metastasis from endometrium to the ovary or ovary to the endometrium), but it is highly significant for proper treatment strategy selection. As SEOC is uncommon, it can be misdiagnosed as FIGO stage III of EC or FIGO stage II of OC. When histology of two tumors is discordant, the diagnosis of SEOC is less complicated, but dilemma occurs for concordant histologic types. The treatment management and overall survival differs significantly from SEOC and metastatic disease. Recent evidence has suggested that criteria by Ulbright and Roth for SEOC diagnostics may not always be applicable. When molecular testing was carried out for SEOC cases, a large proportion was found to be metastatic disease[8-12]. Molecular analyses of gene mutation of CTNNB1, TP53, KRAS, PIK3CA, and PTEN as well as microsatellite instability, β-catenin expression may be helpful to categorize SEOC in cases where clinical and pathological parameters are inconclusive[13]. In problematic cases molecular analysis of tumor foci may facilitate the precise diagnosis and identify the cell of origin, while mutation analysis in liquid biopsy has potential for early cancer detection.
In present study, next generation sequencing of 10 genes in DNA from uterine lavage and ovarian tissue samples from two cases with SEOC was performed aiming at improved understanding of the molecular profile of these rare tumors and evaluation of rationality of uterine lavage based genetic in SEOC diagnostics.
I clinical case: A 54-year-old, menopausal (gravida 0, para 0) woman presented with lower abdominal pain.
II clinical case: A 45-year-old woman (gravida 1, para 1) arrived to National Cancer Institute of Lithuania with histologically confirmed uterine cancer with no complaints.
I clinical case: The complaints lasted for last six months.
II clinical case: Hysteroscopy was performed because endometrial polyp was detected by ultrasound. Final histology confirmed well differentiated (G1) endometrioid endometrial cancer.
I cinical case: There were no other comorbidities, body mass index (BMI) 22.4 kg/m2.
II clinical case: There were no comorbidities, she had a BMI of 27.8 kg/m2.
I clinical case: Her medical and family histories were unremarkable for any type of cancer.
II clinical case: There were no history of malignancy in the family.
I clinical case: The clinical examination revealed mobile pelvic mass 15 cm in diameter which was extended till the umbilicus. Pelvic ultrasound examination identified: Thickened endometrium up to 11 mm and left adnexal multilocular cystic tumor with papillary structures. Computed tomography (CT) scan of the thorax, abdomen and pelvis revealed left cystic ovary mass approximately 136 mm × 114 mm × 114 mm in size with several septum and papillary component.
II clinical case: On clinical examination left adnexal mass was identified. Transvaginal ultrasound showed normal sized uterus with endometrial thickness of 6 mm, and 7 cm × 8 cm left adnexal solid mass with mixed echogenicity and cystic inclusion, right ovary cyst up to 3 cm is likely to be functional cyst. Magnetic resonance imaging of the abdominal and pelvis showed irregularly shaped, solid, left adnexal mass up to 60 mm × 77 mm × 80 mm, suspected paraaortic and iliac lymph nodes.
I clinical case: Cancer antigen 125 was evaluated 209 U/mL (normal range < 35 U/mL).
II clinical case: Cancer antigen 125 was not evaluated 32 U/mL (normal range < 35 U/mL).
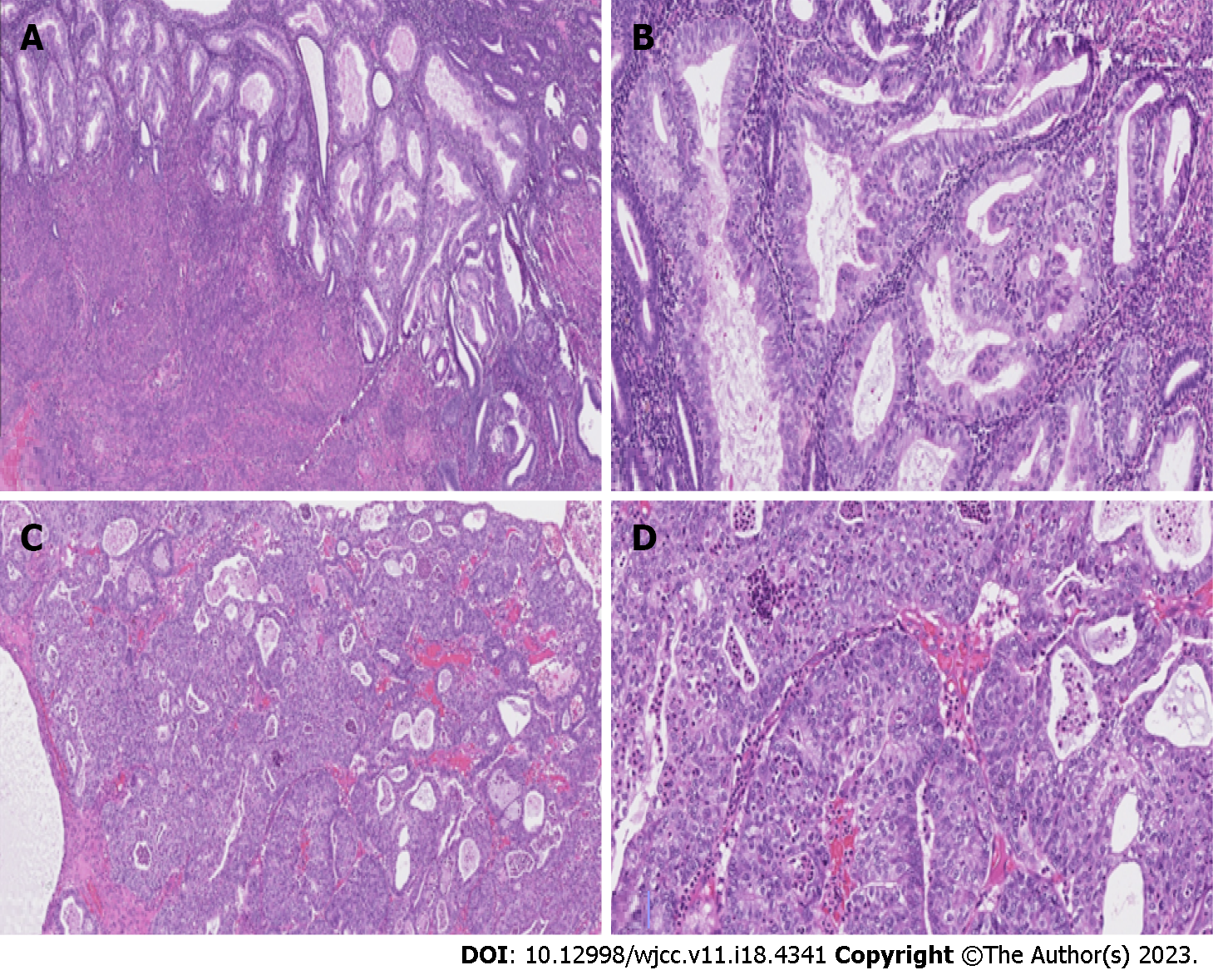
I clinical case: Figure 1 shows hematoxylin-eosin stains of the endometrial and Figure 2 shows ovarian tumor specimens. Peritoneal cytological washing and additional peritoneal biopsies, as well as lymph nodes, were negative for malignant cells. Haematoxylin and eosin (H&E)-stained slide at low power (lens objective × 4) shows closely packed irregular glandular structures. H&E-stained slide at medium power (lens objective × 14) shows columnar epithelium with oval, stratified nuclei and visible nucleoli (Figure 1A and B). H&E-stained slide at low power (lens objective × 4) shows closely packed irregular glandular structures and focal solid growth. H&E-stained slide at medium power (lens objective × 14) shows tumor composed of moderate amount of eosinophilic cytoplasm with slightly polymorphous nuclei and visible nucleoli (Figure 1C and D).
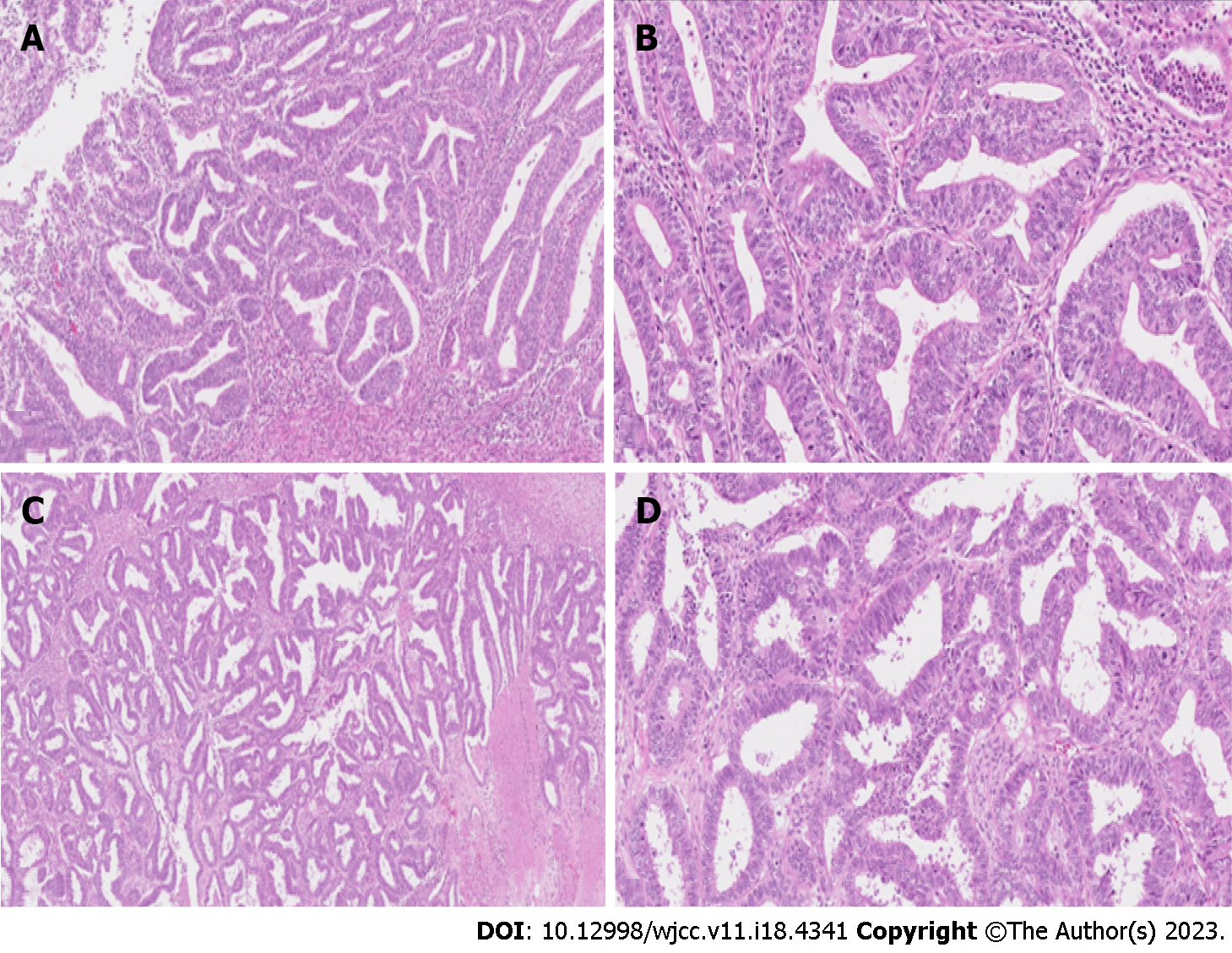
II clinical case: Figure 2A and B shows hematoxylin-eosin stains of the endometrial and Figure 3A and B shows ovarian tumor specimens. H&E-stained slide at low power (lens objective × 4) shows closely packed irregular glandular structures. H&E-stained slide at medium power (lens objective × 14) shows tall columnar epithelium with oval, stratified nuclei with visible nucleoli (Figure 2A and B).
H&E-stained slide at low power (lens objective × 4) shows closely packed irregular glandular structures with focal necrosis. H&E-stained slide at medium power (lens objective × 14) shows columnar epithelium with oval, stratified nuclei, and visible nucleoli (Figure 2C and D).
On final histological result: Pathological findings revealed a well-differentiated (G1) endometrioid carcinoma of the uterus without myometrial invasion and a moderately-differentiated (G2) endometrioid tumor limited to left ovary. Figure 1A and B shows hematoxylin-eosin stains of the endometrial and Figure 1C and D shows ovarian tumor specimens. Peritoneal cytological washing and additional peritoneal biopsies, as well as lymph nodes, were negative for malignant cells. Lymphovascular invasion was not seen. The final diagnosis of synchronous FIGO IA endometrioid grade 2 ovarian carcinoma and FIGO IA grade 1 endometrial carcinoma was made.
Histological examination confirmed well-differentiated endometrioid (G1) tumor limited to left ovary and a well-differentiated (G1) endometrioid carcinoma of the uterus without myometrial invasion. Figure 2A and B shows hematoxylin-eosin stains of the endometrial and Figure 2C and D shows ovarian tumor specimens. Peritoneal cytological washing and biopsies, as well as lymph nodes, were negative for malignant cells. The final diagnosis of synchronous FIGO IA endometrioid grade 1 ovarian carcinoma and FIGO IA grade 1 endometrial carcinoma was made. Lymphovascular invasion was not seen.
The patient underwent an exploratory laparotomy, frozen section of ovarian tumors was highly suggestive of malignancy. A total abdominal hysterectomy, bilateral salpingo-oophorectomy, pelvic and para-aortic lymph node dissection, omentectomy, selective peritonectomy were conducted.
The patient underwent surgical staging: Hysterectomy with bilateral salpingo-oophoarectomy, peritoneal biopsy, pelvic and para-aortic lymph node dissection.
To gain insight into the tumor biology, uterine lavage and ovarian tissue samples were sequenced using custom targeted 10 gene panel which included genes commonly associated with ovarian and endometrial cancers: TP53, BRCA1, BRCA2, PIK3CA, KRAS, PTEN, ARID1A, CTNNNB1, FBXW7, and PPP2R1A. No pathogenic mutations were detected in uterine lavage or tissue sample. After surgery, six cycles of chemotherapy of Carboplatin were performed. Patient was disease-free at 44 mo of follow-up.
As in previous case, uterine lavage and ovarian tissue samples were sequenced using custom targeted 10 gene panel. In ovarian tissue sample, somatic mutations of PIK3CA and PTEN were detected. Moreover, tumor sample had a mutation in β-catenin gene CTNNB1. No mutations were found in uterine lavage sample, showing low spread of cancerous cells. Mutation profile for both cases are shown in Table 1 No adjuvant treatment was considered. The patient is disease-free at 42 mo of follow-up.
| Gene | Uterine lavage mutation | Tissue mutation | dbSNP | Amino acid change |
| PIK3CA | - | c.1093G>A | rs1064793732 | p.Glu365Lys |
| PTEN | - | c.517C>T | rs121913293 | p.Arg173Cys |
| CTNNB1 | - | c.101G>A | rs28931589 | p.Gly34Glu |
SEOC is a rare variant of gynecological cancers. Usually, SEOC appears with different clinical characteristics compared to patients with EC or OC alone. SEOC is observed among the younger age women under 55 years and 40% of them are nulliparous. In most cases it can be diagnosed at an early stage and is associated with low-grade disease. The endometrioid subtype of the primary tumors is the most common histological finding with the rate of 50%-70% of cases. In our report, both women with SEOC had endometrioid histological type. The most common symptom of SEOC is abnormal uterine bleeding and pelvic pain due to the pelvic mass[9,11].
The importance of distinguishing SEOC from either isolated endometrium or ovarian cancer with metastasis is crucial, as it determines adjuvant treatment strategy and prognosis. The prognosis of patients with synchronous EC and OC is better than the patients with single-organ cancer with ovarian or endometrial spread. Median 5-year progression free survival rate is reported to be 65% for SEOC but is less than 50% for FIGO stage IIIA EC with ovarian spread. If SEOC is diagnosed in early stage the overall survival rate is excellent- up to 90%, in contrast to the poor prognosis noted in metastatic disease[1,2,4,13]. Both patients in our report were diagnosed with low grade endometrioid carcinomas of the ovary and uterus and are still alive more than 2 years after treatment. Based on histological findings alone, concurrent uterine and ovarian endometrioid carcinomas are considered synchronous primaries, when all of the following criteria are met: Both tumors are low grade (FIGO 1 or 2); less than 50% of myometrial invasion is present; ovarian tumor is unilateral, limited to parenchyma and no other site is involved in a malignant process; extensive lymphovascular invasion is absent at any location.
To determine cancer origin accurately, advancement of immunohistochemistry andmolecular testing is needed. According to literature it is suggested that immunohistochemistry testing of vimentin can be carried out. Desouki et al[14] suggested that a negative stain has a sensitivity and specificity to predict primary OC at 97% and 82%, while positive vimentin staining had an 82% sensitivity and 97% specificity in predicting EC. Another study used different antibodies (ER, PR, HER2, p53, and Ki-67) and they found that ER, PR, BCL2 showed different immunostaining patterns between EC and OC and suggested that they can be used as a surrogate marker in the distinction of these tumors[15]. However, the practical meaning of immunohistochemistry in this setting is still the matter of debate due to SEOC heterogeneity and clonality. Immunohistochemical analysis of hormone receptors and markers is not always helpful, because they did not allow a comprehensive evaluation and comparison of possible clonal origin.
Molecular studies of SEOC used variable approaches-mutation analysis of single or group of genes, microsatellite instability (MSI), loss of heterozygosity[16-20]. Clonality of SEOCs can be confirmed by sequencing different genes using next-generation sequencing. Paired box gene 8 (PAX8) is a marker that can be useful in distinguishing between EC and OC, because primary OC express PAX-8 but not EC metastases[18]. Nuclear localization of β-catenin and presence of CTNNB1 mutations are associated with SEOC. Irving et al[21] identified that CTNNB1 mutations were restricted to SEOC and were absent in all the metastatic tumors, providing direct evidence for a divergence of molecular oncogenetic mechanisms in the subset of SEOC. Genetic classification of SEOC vs metastatic tumors based on β-catenin expression/mutation correlates with clinical outcome, but screening of the tumor suppressor genes may be labor-intensive and expensive. Other studies analyze the DNA mismatch repair protein (MMR) expression in SEOC in comparison with the expression of these proteins in Lynch syndrome. The results of these studies showed that majority of SEOCs are sporadic cancers, but patients with endometrioid or clear cell OC under the age of 53 have higher risk for loss of MMR (MLH1, MSH2, MSH6) expression and Lynch syndrome[16,22]. Kobayashi et al[23] reported that the frequency of MSI in SEO was 24%, which is comparable to the frequency of endometrial cancer. In our report, none of the patients had a medical history of Lynch syndrome or other hereditary cancers.
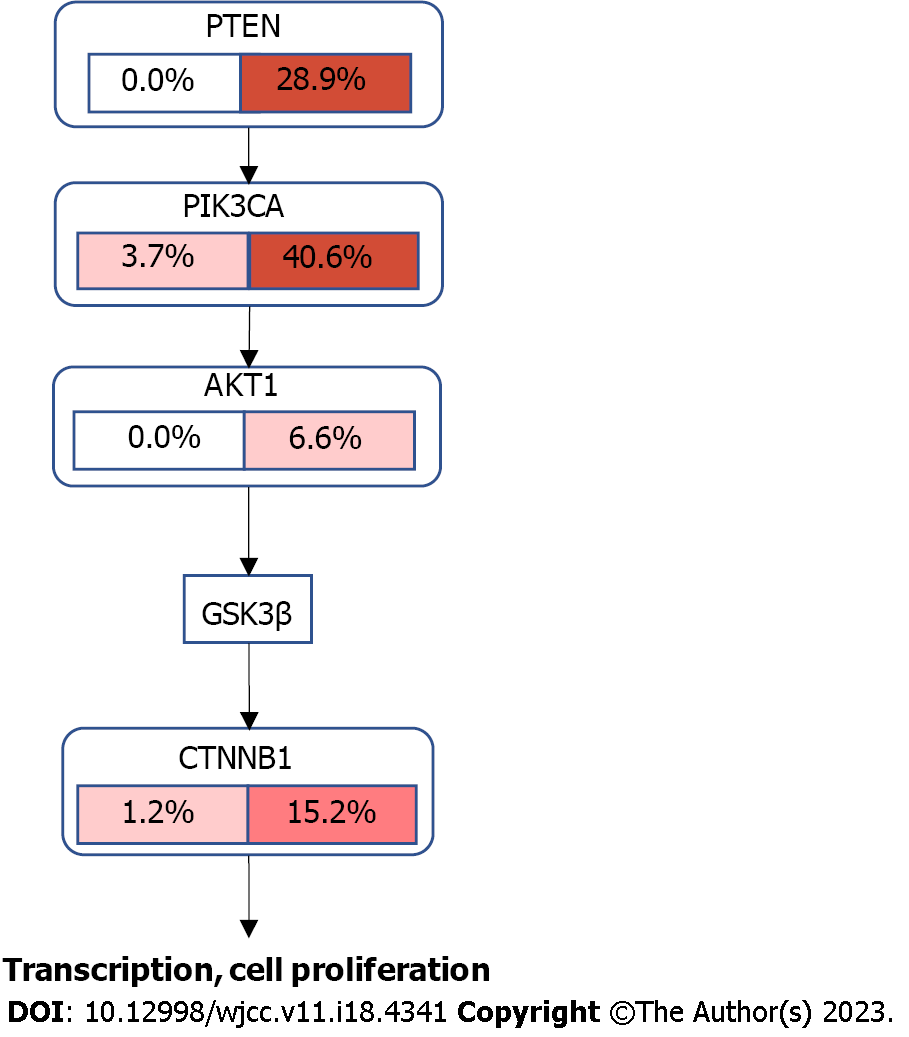
Molecular profiling also can be beneficial for prognostic and predictive meaning. Studies analyzing SEOC mutational profiles identified PI3KCA, PTEN, KRAS, CTNNB1, and ARID1A gene mutations can be helpful for diagnostic of SEOC[16,22-28]. The study by Reijnen et al[22] comparing 50 SEOC cases with metastatic EC, OC and TCGA mutation data, showed that mutational profile of SEOC harbored a profoundly different molecular profile compared to metastatic disease. SEOC were enriched for PTEN and CTNNB1 mutations and TP53 was mutated less frequently in SEO than in ovarian metastatic cases. Although, SEOC closely resembles endometrial EC, but with slightly less frequent ARID1A mutations. In the endometrioid SEO subgroup, PTEN mutations were identified in 72% of ovarian tissue and in 75% of endometrial tissue. In contrast, PTEN mutations are less common in endometrioid OC, with prevalence only 17%. In endometrioid endometrial carcinomas PTEN mutation was found in 67% of cases[22]. Other studies also found frequent mutation of CTNNB1 in SEOC cases[22]. In our molecular analysis, one case was identified with mutations of the CTNNB1, PIK3CA, and PTEN genes, which are frequently detected in SEOC. Other case Mutation frequency from TCGA database and the crosstalk between PI3K/AKT and WNT pathways are visualized in Figure 3, showing possible molecular mechanism of SEOC development. No mutations were identified in other SEOC case in our study, showing further need of genome-wide analysis for improved understanding of molecular origins of these rare tumors.
SEOC predominantly occurs in younger premenopausal women. The identification of SEOC or metastatic endometrium/ovarian disease have great clinical significance, as the disease management, prognosis and overall survival differ. Precise diagnosis of SEOC may require additional molecular or IHC testing in addition to routine histopathologic assessment. According to literature review testing of vimentin, molecular analyses of gene mutation of CTNNB1, PAX8, and β-catenin expression may be helpful to categorize SEOC in cases where clinical and pathological parameters are inconclusive. Our two cases do not show that uterine lavage was suitable diagnostic test for SEOC, but further investigation is needed, and additional disease-specific biomarkers need to be discovered.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Lithuania
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Imai Y, Japan S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Zaino R, Whitney C, Brady MF, DeGeest K, Burger RA, Buller RE. Simultaneously detected endometrial and ovarian carcinomas--a prospective clinicopathologic study of 74 cases: a gynecologic oncology group study. Gynecol Oncol. 2001;83:355-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 191] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 2. | Soliman PT, Slomovitz BM, Broaddus RR, Sun CC, Oh JC, Eifel PJ, Gershenson DM, Lu KH. Synchronous primary cancers of the endometrium and ovary: a single institution review of 84 cases. Gynecol Oncol. 2004;94:456-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 158] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Chen T, Brenner H, Fallah M, Jansen L, Castro FA, Geiss K, Holleczek B, Katalinic A, Luttmann S, Sundquist K, Ressing M, Xu L, Hemminki K; GEKID Cancer Survival Working Group+. Risk of second primary cancers in women diagnosed with endometrial cancer in German and Swedish cancer registries. Int J Cancer. 2017;141:2270-2280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Matsuo K, Machida H, Blake EA, Holman LL, Rimel BJ, Roman LD, Wright JD. Trends and outcomes of women with synchronous endometrial and ovarian cancer. Oncotarget. 2018;9:28757-28771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Ulbright TM, Roth LM. Metastatic and independent cancers of the endometrium and ovary: a clinicopathologic study of 34 cases. Hum Pathol. 1985;16:28-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 170] [Article Influence: 4.3] [Reference Citation Analysis (2)] |
| 6. | Selvaggi SM. Tumors of the ovary, maldeveloped gonads, fallopian tube, and broad ligament. Arch Pathol Lab Med. 2000;124:477. [PubMed] |
| 7. | Scully RE, Young RH. Metastatic Tumors of the Ovary. In: Kurman RJ, Hedrick Ellenson L, Ronnett BM, editors. Blaustein’s Pathology of the Female Genital Tract. Cham: Springer International Publishing; 2002: 987-90. [DOI] [Full Text] |
| 8. | Song T, Seong SJ, Bae DS, Suh DH, Kim DY, Lee KH, Lim MC, Lee TS. Synchronous primary cancers of the endometrium and ovary in young women: a Korean Gynecologic Oncology Group Study. Gynecol Oncol. 2013;131:624-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Shin W, Park SY, Kang S, Lim MC, Seo SS. How to manage synchronous endometrial and ovarian cancer patients? BMC Cancer. 2021;21:489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Matias-Guiu X, Stewart CJR. Endometriosis-associated ovarian neoplasia. Pathology. 2018;50:190-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 11. | Amaral PI, Silva A, Lacerda A, Barros C. Synchronous endometrioid endometrial and ovarian cancer in a 34-year-old woman. BMJ Case Rep. 2015;2015. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Anglesio MS, Wang YK, Maassen M, Horlings HM, Bashashati A, Senz J, Mackenzie R, Grewal DS, Li-Chang H, Karnezis AN, Sheffield BS, McConechy MK, Kommoss F, Taran FA, Staebler A, Shah SP, Wallwiener D, Brucker S, Gilks CB, Kommoss S, Huntsman DG. Synchronous Endometrial and Ovarian Carcinomas: Evidence of Clonality. J Natl Cancer Inst. 2016;108:djv428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 13. | Singh N. Synchronous tumours of the female genital tract. Histopathology. 2010;56:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Desouki MM, Kallas SJ, Khabele D, Crispens MA, Hameed O, Fadare O. Differential vimentin expression in ovarian and uterine corpus endometrioid adenocarcinomas: diagnostic utility in distinguishing double primaries from metastatic tumors. Int J Gynecol Pathol. 2014;33:274-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Halperin R, Zehavi S, Hadas E, Habler L, Bukovsky I, Schneider D. Simultaneous carcinoma of the endometrium and ovary vs endometrial carcinoma with ovarian metastases: a clinical and immunohistochemical determination. Int J Gynecol Cancer. 2003;13:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Hájková N, Tichá I, Hojný J, Němejcová K, Bártů M, Michálková R, Zikán M, Cibula D, Laco J, Geryk T, Méhes G, Dundr P. Synchronous endometrioid endometrial and ovarian carcinomas are biologically related: A clinico-pathological and molecular (next generation sequencing) study of 22 cases. Oncol Lett. 2019;17:2207-2214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | AlHilli MM, Dowdy SC, Weaver AL, St Sauver JL, Keeney GL, Mariani A, Podratz KC, Bakkum-Gamez JN. Incidence and factors associated with synchronous ovarian and endometrial cancer: a population-based case-control study. Gynecol Oncol. 2012;125:109-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Schultheis AM, Ng CK, De Filippo MR, Piscuoglio S, Macedo GS, Gatius S, Perez Mies B, Soslow RA, Lim RS, Viale A, Huberman KH, Palacios JC, Reis-Filho JS, Matias-Guiu X, Weigelt B. Massively Parallel Sequencing-Based Clonality Analysis of Synchronous Endometrioid Endometrial and Ovarian Carcinomas. J Natl Cancer Inst. 2016;108:djv427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 149] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 19. | Castro IM, Connell PP, Waggoner S, Rotmensch J, Mundt AJ. Synchronous ovarian and endometrial malignancies. Am J Clin Oncol. 2000;23:521-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Guerra F, Girolimetti G, Perrone AM, Procaccini M, Kurelac I, Ceccarelli C, De Biase D, Caprara G, Zamagni C, De Iaco P, Santini D, Gasparre G. Mitochondrial DNA genotyping efficiently reveals clonality of synchronous endometrial and ovarian cancers. Mod Pathol. 2014;27:1412-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Irving JA, Catasús L, Gallardo A, Bussaglia E, Romero M, Matias-Guiu X, Prat J. Synchronous endometrioid carcinomas of the uterine corpus and ovary: alterations in the beta-catenin (CTNNB1) pathway are associated with independent primary tumors and favorable prognosis. Hum Pathol. 2005;36:605-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Reijnen C, Küsters-Vandevelde HVN, Ligtenberg MJL, Bulten J, Oosterwegel M, Snijders MPLM, Sweegers S, de Hullu JA, Vos MC, van der Wurff AAM, van Altena AM, Eijkelenboom A, Pijnenborg JMA. Molecular profiling identifies synchronous endometrial and ovarian cancers as metastatic endometrial cancer with favorable clinical outcome. Int J Cancer. 2020;147:478-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Kobayashi Y, Nakamura K, Banno K, Aoki D. Current status of diagnosis for synchronous endometrial and ovarian carcinomas by molecular biological approach. Eur J Gynaecol Oncol. 2021;42:1300-1302. |
| 24. | Valtcheva N, Lang FM, Noske A, Samartzis EP, Schmidt AM, Bellini E, Fink D, Moch H, Rechsteiner M, Dedes KJ, Wild PJ. Tracking the origin of simultaneous endometrial and ovarian cancer by next-generation sequencing-a case report. BMC Cancer. 2017;17:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Bahceci I, Dogrusoz U, La KC, Babur Ö, Gao J, Schultz N. PathwayMapper: a collaborative visual web editor for cancer pathways and genomic data. Bioinformatics. 2017;33:2238-2240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 26. | Chao A, Wu RC, Jung SM, Lee YS, Chen SJ, Lu YL, Tsai CL, Lin CY, Tang YH, Chen MY, Huang HJ, Chou HH, Huang KG, Chang TC, Wang TH, Lai CH. Implication of genomic characterization in synchronous endometrial and ovarian cancers of endometrioid histology. Gynecol Oncol. 2016;143:60-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Ishikawa M, Nakayama K, Nakamura K, Ono R, Yamashita H, Ishibashi T, Minamoto T, Iida K, Razia S, Ishikawa N, Kyo S. High frequency of POLE mutations in synchronous endometrial and ovarian carcinoma. Hum Pathol. 2019;85:92-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Matsubayashi H, Higashigawa S, Kiyozumi Y, Horiuchi Y, Hirashima Y, Kado N, Abe M, Ohishi T, Ohnami S, Urakami K, Yamaguchi K. Metachronous ovarian endometrioid carcinomas in a patient with a PTEN variant: case report of incidentally detected Cowden syndrome. BMC Cancer. 2019;19:1014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |