Published online Jun 26, 2023. doi: 10.12998/wjcc.v11.i18.4318
Peer-review started: February 2, 2023
First decision: March 24, 2023
Revised: April 15, 2023
Accepted: May 23, 2023
Article in press: May 23, 2023
Published online: June 26, 2023
Processing time: 144 Days and 13.2 Hours
Hepatic inflammatory myofibroblastic tumor (HIMT) is a rare type of hepatic tumor. It is always misdiagnosed and mistreated because it is primarily found with no obvious specific manifestation, and its imaging findings are diverse.
Here, we report a case of HIMT that was initially diagnosed as liver malignancy but was confirmed as HIMT by histopathology after hepatectomy. Mostly, HIMTs are infiltrated with plasma cells and stain positively for anaplastic lymphoma kinase on immunohistochemistry as well as for some other kinases.
HIMT can be treated with single nonsteroidal anti-inflammatory drugs and without surgery when it is diagnosed accurately. Because the etiology of HIMT is unknown and the diagnosis is difficult, the pathogenesis and clinical process need to be further studied.
Core Tip: Hepatic inflammatory myofibroblastic tumor (HIMT) is a rare type of hepatic tumor. HIMT are found during physical examination with no obvious specific manifestation. The imaging manifestations of HIMT are diverse, making diagnosis through imaging examination difficult. Accurate diagnosis of HIMT is obtained by histopathology of the resected/biopsied specimen. We reported a case of HIMT initially diagnosed as liver malignancy. HIMT can be treated with single nonsteroidal anti-inflammatory drugs when it is diagnosed accurate. Through this case analysis and literature review, further data was provided to aid in the early diagnosis and initial treatment of this tumor.
- Citation: Tong M, Zhang BC, Jia FY, Wang J, Liu JH. Hepatic inflammatory myofibroblastic tumor: A case report. World J Clin Cases 2023; 11(18): 4318-4325
- URL: https://www.wjgnet.com/2307-8960/full/v11/i18/4318.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i18.4318
Inflammatory myofibroblastic tumors (IMTs) are rare tumors that usually affect children and young adults and are slightly more common among females than males. The cause of IMT is unknown. IMT is more frequently encountered in anatomical sites in the gastrointestinal tract[1].
Hepatic inflammatory myofibroblastic tumor (HIMT) is a rare type of hepatic tumor, with a complex etiology. The mechanism of the carcinogenesis process remains unclear, and it is always misdiagnosed and mistreated because it accounts for less than 1% of liver tumors[2]. Most cases of HIMT are found on physical examination with no obvious specific clinical manifestations. The imaging manifestations of HIMT are diverse, and it is difficult to obtain a clear diagnosis through imaging examination. Therefore, the only way to accurately diagnose HIMT is histopathology of a resected or biopsied specimen. In this paper, we reported a case of HIMT in a 65-year-old female that was incidentally identified during a routine physical examination and initially diagnosed as liver malignancy. The patient underwent laparoscopic hepatectomy and was confirmed to have HIMT on histopathological examination. No evidence of recurrence or metastasis was noticed in the recent follow-up, and the patient remains under clinical surveillance.
The purpose of this study was to review and summarize the special and common features of this rare case of HIMT through a literature review to provide clinicians with ways to diagnose the condition through its characteristics, explore different treatment methods, and enable patients to obtain the maximum benefit and a good prognosis.
A 65-year-old female patient was admitted to Linyi People’s Hospital due to a space-occupying lesion in her liver that was found by a routine physical examination in July 2022. However, the patient had no symptoms, such as pain, fever, nausea and vomiting.
There was no history of other underlying diseases except a history of hysteromyomectomy.
After admission, the serum levels of red blood cells, white blood cells, platelets, alpha-fetoprotein, carcinoembryonic antigen, cancer antigen 199, total bilirubin, direct bilirubin, indirect bilirubin, alanine aminotransferase, aspartate aminotransferase, gamma glutamyl transferase, alkaline phosphatase and hepatitis B surface antigen were all within the normal range.
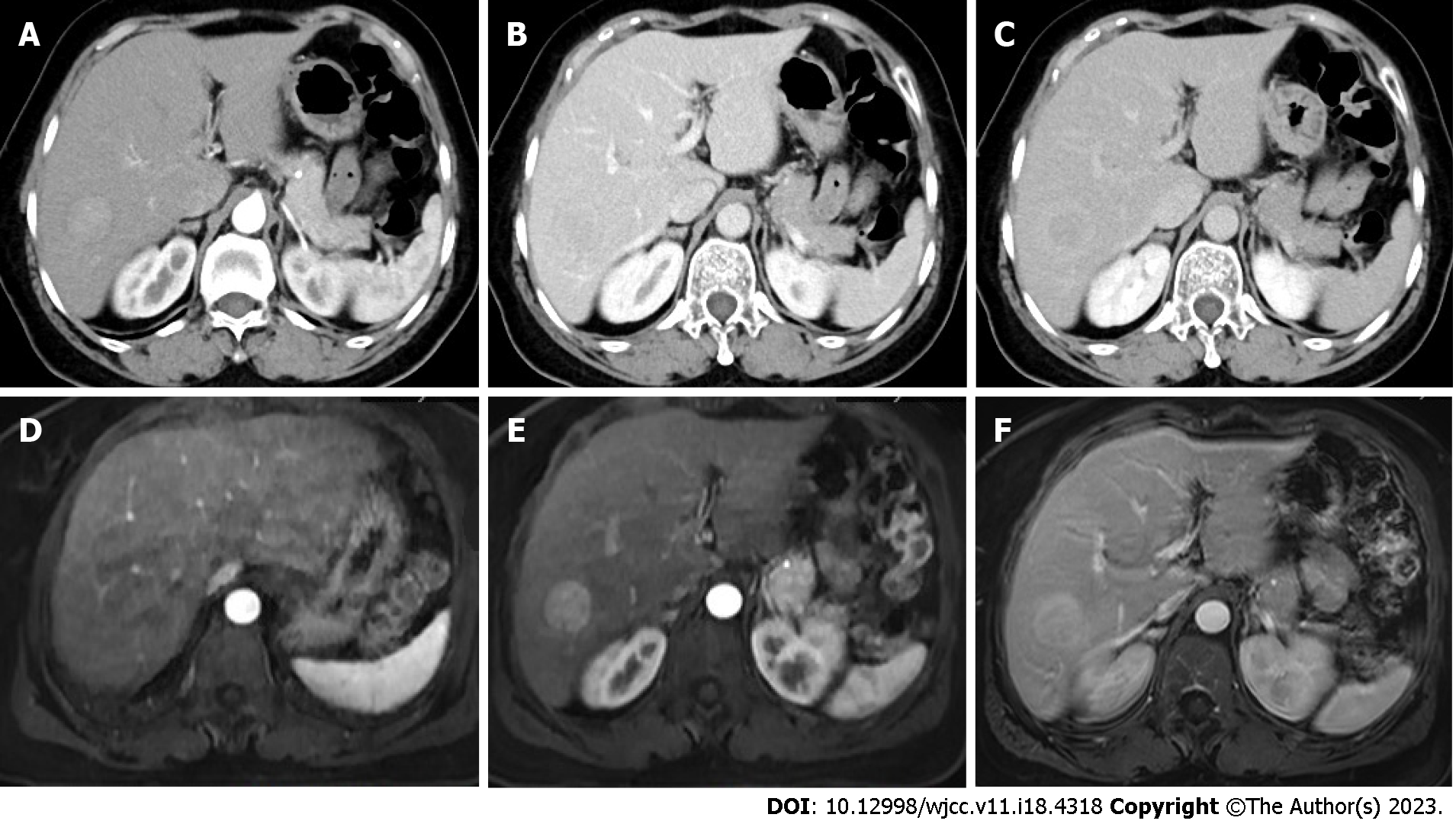
Enhanced computed tomography and magnetic resonance imaging revealed a right lower posterior lobe vascular enhancement mass, which was suspected to be a malignant tumor (Figure 1).
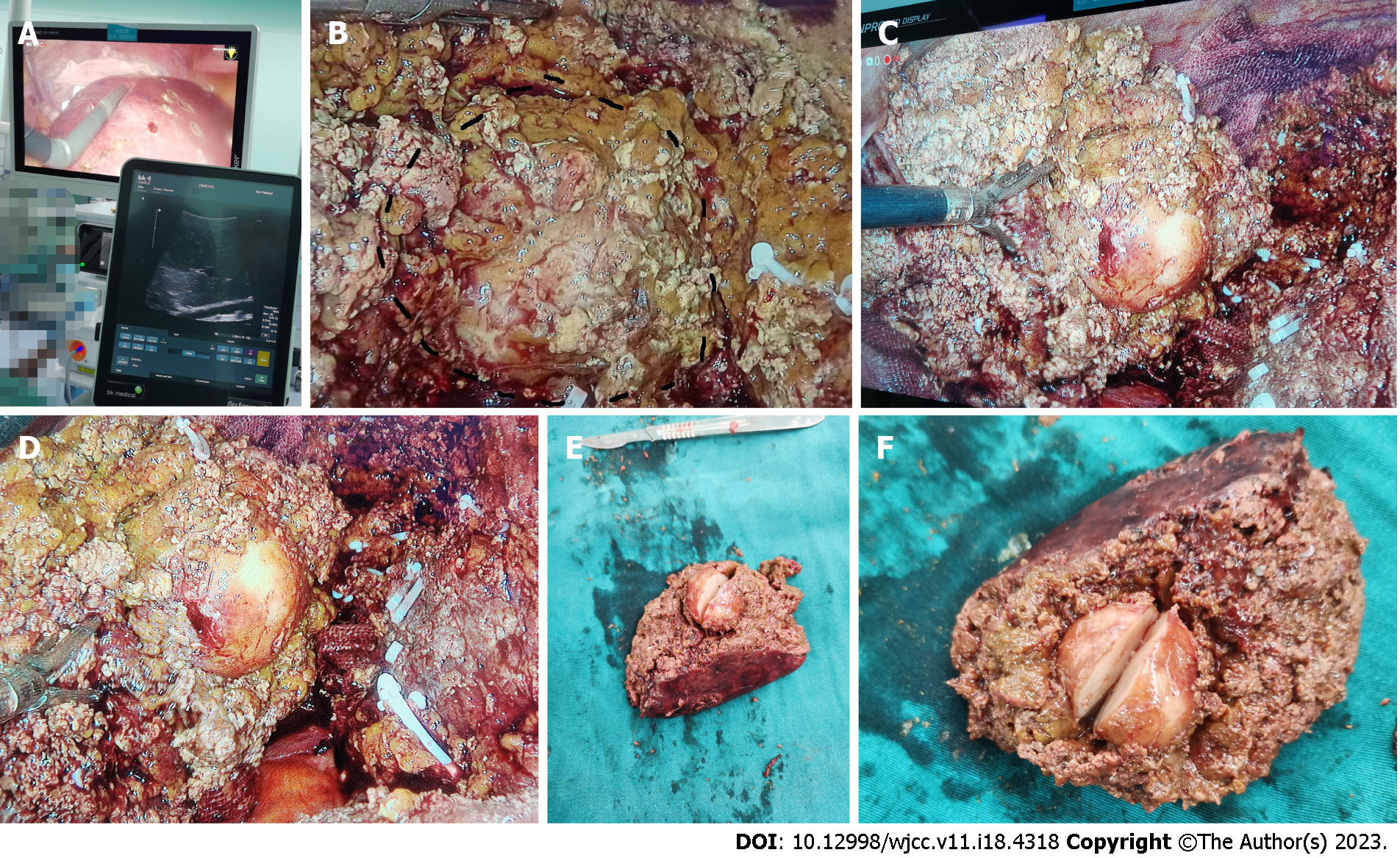
After a multidisciplinary team discussion, a suspicion of hepatocellular carcinoma was made. With the assistance of intraoperative ultrasound localization, laparoscopic segment VI hepatectomy was performed successfully.
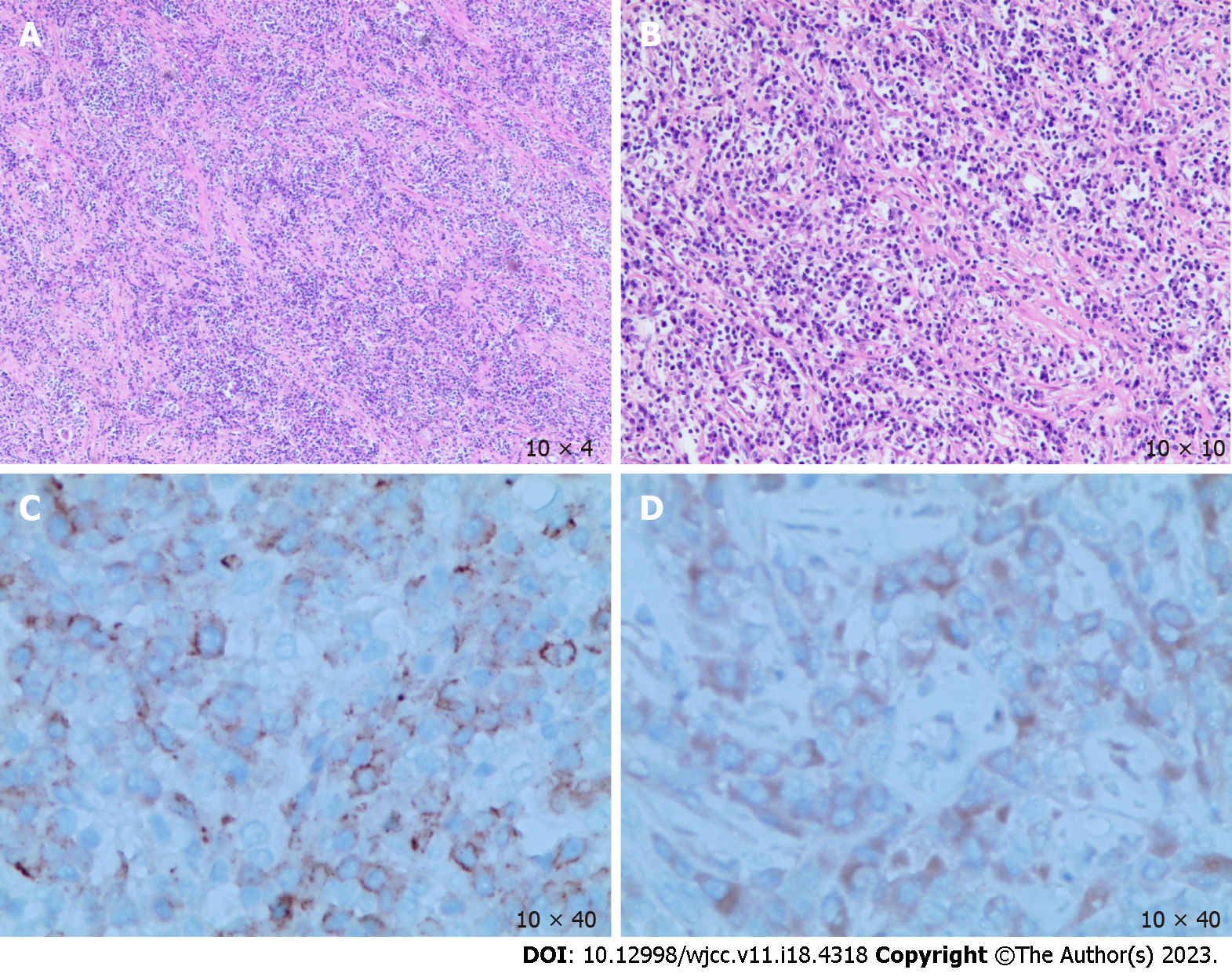
The postoperative pathological results revealed the following: (1) The macroscopic view (Figure 2) showed that the size of the liver tissue was 10.0 cm × 5.5 cm × 4.0 cm; the size of the liver neoplasm was 3.0 cm × 3.0 cm × 2.5 cm, with a clear boundary with the surroundings and partial exposure to the liver fracture. The mass showed a grayish-red color mixed with some yellowish tinge, and it was slightly brittle; (2) Microscopic view (Figure 3A and B) showed spindle cell proliferation and inflammatory cell infiltration in the neoplasm; and (3) Immunohistochemistry (IHC) showed CK (W+), CD3 (T-cell +), CD20 (B-cell +), CD38 (plasma cell+), CD138 (plasma cell+, Figure 3C), Kappa (plasma cell+), Lambda (plasma cell+), IgG (plasma cell+), IgG4 (plasma cell+), ALK-1A4/1H7 (W+, Figure 3D) and Ki67-MIB1 (20%).
Combined with morphology and IHC, the pathologist diagnosed it as HIMT.
Laparoscopic segment VI hepatectomy was performed successfully. The liver had massively nodular cirrhosis, and the tumor was found at the junction of segments VI and VII with complete tumor encapsulation and an estimated diameter of 3 cm and 1-2 cm underneath the liver surface with intraoperative ultrasound location (Figure 2A). The tumor was spherical and hard in texture, grayish-white in color, with complete capsule isolation from surrounding tissues and without obvious blood supply vessels. The operation was successful, and the tumor was excised with 1 cm margins (Figure 2B-D).
The patient recovered well without bleeding, bile leakage or ascites and was discharged on day 5 after the operation. No evidence of recurrence or metastasis was noticed in the recent follow-up, and the patient remains under clinical surveillance.
IMTs are rare tumors generated by a reactive or inflammatory state and usually affect children and young adults. They can occur in almost all age groups and are slightly more common among females than males[3]. It can occur in any tissue or organ but usually occurs in the abdominal soft tissues and lungs[4]. IMTs are known as inflammatory pseudotumors. The World Health Organization classification of soft tissue tumors now considers it an intermediate malignancy with less than a 5% risk of metastatic spread, and it was officially named IMT in 2002[5]. In the 2020 fifth edition of the World Health Organization classification of soft tissue and bone tumors, IMTs were defined as a distinctive, rarely metastasizing neoplasm composed of myofibroblastic and fibroblastic spindle cells accompanied by an inflammatory infiltrate of plasma cells, lymphocytes and/or eosinophils[6]. The cause of IMT is unknown, but cytogenetic changes suggest that these lesions may be of clonal origin, usually accompanied by an inflammatory infiltrate of plasma cells, lymphocytes and eosinophils. HIMT accounts for 1% of liver tumors, and its pathogenesis may be related to liver cells, bile duct epithelial cells or portal vein infection[2].
There are different theories regarding the etiology of IMTs including trauma, surgery, hemangioma hemorrhage or rupture, bacterial or viral infection, immune disorders and tumor processes[7-10]. Viruses, including HIV, human herpesvirus-8, and Epstein-Barr virus, are presumed to influence the development of IMT[11-14], and there is even a case report of secondary actinomycosis[15]. However, the actual pathogenesis remains unclear[16]. Due to the lack of specific symptoms and manifestations in imaging, they are usually found by accident and often misdiagnosed as malignant tumors.
Because the clinical manifestations lack specificity and the imaging characteristics are diverse, HIMT is easily misdiagnosed clinically and should be differentiated from benign and malignant diseases such as undifferentiated embryonic sarcoma of the liver, primary liver cancer, cholangiocarcinoma, inflammatory malignant fibrous histiocytoma, liver metastasis and liver abscess[17].
For clinicians, pathological examination of surgical specimens seems to be the only way to diagnose it accurately. During the macroscopic examination of excised specimens, most IMTs appear as well-defined, nonenveloped yellow-white neoplasms. The patient in this case had no genetic disease and no history of liver disease. Computed tomography and magnetic resonance imaging showed similar characteristic images of hepatocellular carcinoma, but there was no history of hepatitis B virus/hepatitis C virus, and tumor markers and the relevant laboratory examination were all within the normal range. Under macroscopic examination, the specimen had no envelope but a clear boundary with the surrounding liver tissue, with a brittle section mixed with grayish-red material that was mixed with yellowish material. Combined with the characteristic spindle cell and inflammatory cell infiltration observed under the microscope, the diagnosis of IMT was finally established, and malignancy was excluded.
Based on the major component in microscopy, IMT is divided into three histopathological subtypes including xanthogranuloma type, plasma cell granuloma type and sclerosing pseudotumor[15]. Histologically, IMTs are characterized by spindle cells proliferating in a myxoid-to-collagen matrix with a prominent inflammatory infiltrate mainly composed of plasma cells and lymphocytes, and it is occasionally admixed with eosinophils and neutrophils. Approximately 71% of IMTs stained ALK positive on IHC[18]. The background lymphocytes were composed of CD3(+) or CD20(+) cells, and there were several CD68(+) cells. However, IHC staining for myogenin, myohemoglobin, S100, CD21 and CD35 are negative[16]. Approximately 50% of patients with IMT demonstrate clonal chromosomal rearrangements at band 2p23, the site of the ALK-1 gene in the tyrosine kinase locus. Mutations at the ALK site have been associated with constitutive overexpression of ALK and oncogenesis. ALK-1 expression is highly specific for IMT, with sensitivity varying depending on the histogenesis[19].
In a recent study, crizotinib, a competitive tyrosine kinase inhibitor of ALK, induced a sustained partial response in a patient with ALK-translocated IMT. This observation may offer a new therapeutic strategy for the subset of IMT patients with ALK mutation phenotypes[20]. With molecular techniques, Cheng et al[21] showed that all thoracic IMTs harbored a tyrosine kinase abnormality, with 30% involving a kinase gene other than ALK, including ROS1, NTRK3 and RET gene fusions. At present, the pathogenesis of IMT tumors with negative ALK expression is still unclear. However, by using the next-generation sequencing technique, six out of nine ALK-negative IMT tumors showed the presence of fusions involving ROS1 or platelet-derived growth factor receptor beta genes, suggesting that IMT is largely a kinase fusion-driven neoplasm[22]. There is no consensus about reliable pathologic predictors of IMTs, although the presence of ganglion-like cells, p53 expression and aneuploidy have been associated with more aggressive behavior[23].
The presence of human herpesvirus-8 DNA sequences and the overexpression of interleukin (IL)-6 and cyclin D1 have also been reported in IMTs[24]. IL-6 promotes fibroblast proliferation. Furthermore, the major origins of IL-6 are monocytes and macrophages, which are the constant components of IMT[19,25]. IMT cells are characteristically positive for vimentin and CD117(-)/CD34(-). The cells are positive for smooth muscle actin with/without desmin expression and S100 positivity. Their mitotic activity is low (0 to 2 mitoses/10 high power fields), and atypical mitoses are rare. Necrosis and vascular invasion have been reported in typical IMT, and they have been shown to express chromosomal rearrangements explaining their locally aggressive character[26].
According to the macroscopic view and hematoxylin-eosin and IHC staining of the tumor, the final and accurate diagnosis of this patient was achieved. Surgical resection is usually recommended for the treatment of IMTs so that pathological specimens can be obtained to achieve a precise diagnosis, relieve symptoms, cure the disease and exclude malignant tumors. Due to disease progression, lack of alternative therapeutic regimens and recurrence, almost all patients with respectable IMT have been managed with radical surgical resection or single nonsteroidal anti-inflammatory drugs (NSAIDs)[27]. Nonsurgical management of IMT includes anti-inflammatory agents (such as NSAIDs or corticosteroids) and chemotherapeutic agents (such as cyclosporine, methotrexate, azathioprine and cyclophosphamide)[28]. Conservative treatments with NSAIDs, corticosteroids or chemotherapeutic agents cannot be started in many cases due to the lack of a definitive diagnosis of the neoplasm preoperatively.
The outcome of IMT depends on its behavior, which ranges from entirely benign to malignant with fatal outcomes[29]. IMT may have a local recurrence, but local invasive or malignant lesions with distant metastasis are rare[30]. Incomplete resection, such as adhesion of essential structures and multifocality, may be a significant cause of IMT recurrence[31]. IMT that occurs in the abdomen or retroperitoneum tends to be more aggressive and more prone to multiple recurrences and distant metastases[30]. A study in 2007 showed that ALK-negative IMT might be more likely to metastasize[19].
Surgical resection should be considered only for patients with ineffective medical treatment, with an unclear diagnosis or in whom malignant tumors cannot be excluded. Complete resection of the tumor should be emphasized during surgery; otherwise, local recurrence may occur. HIMT has a good prognosis, and patients can survive for a long time[32].
In summary, because the etiology of HIMT is unknown, the diagnosis is difficult, and the pathogenesis needs to be further studied. Complete resection was the first choice to obtain a diagnosis via hematoxylin-eosin and IHC staining. Regular follow-up is required for monitoring tumor recurrence and metastasis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Brillanti S, Italy; Maffeis V, Italy S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Makhlouf HR, Sobin LH. Inflammatory myofibroblastic tumors (inflammatory pseudotumors) of the gastrointestinal tract: how closely are they related to inflammatory fibroid polyps? Hum Pathol. 2002;33:307-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 94] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Filips A, Maurer MH, Montani M, Beldi G, Lachenmayer A. Inflammatory myofibroblastic tumor of the liver: A case report and review of literature. World J Hepatol. 2020;12:170-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Yagmur Y, Akbulut S, Gumus S. Mesenteric inflammatory pseudotumor: a case report and comprehensive literature review. J Gastrointest Cancer. 2014;45:414-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Liu Z, Li G, Gou A, Xiao Z, Xu Y, Song S, Guo K, Ma G. Inflammatory myofibroblastic tumor in the pancreatic neck: a rare case report and literature review. Gland Surg. 2021;10:1832-1839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Siemion K, Reszec-Gielazyn J, Kisluk J, Roszkowiak L, Zak J, Korzynska A. What do we know about inflammatory myofibroblastic tumors? - A systematic review. Adv Med Sci. 2022;67:129-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 6. | International Agency for Research on Cancer. Publication of the WHO Classification of Tumours, 5th edition, 3: Soft Tissue and Bone Tumours. 2020. WHO. Available from: https://www.iarc.who.int/news-events/publication-of-the-who-classification-of-tumours-5th-edition-volume-3-soft-tissue-and-bone-tumours/. |
| 7. | Rajabi P, Noorollahi H, Hani M, Bagheri M. Inflammatory pseudotumor of spleen. Adv Biomed Res. 2014;3:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Ugalde P, García Bernardo C, Granero P, Miyar A, González C, González-Pinto I, Barneo L, Vazquez L. Inflammatory pseudotumor of spleen: a case report. Int J Surg Case Rep. 2015;7C:145-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Chen WC, Jiang ZY, Zhou F, Wu ZR, Jiang GX, Zhang BY, Cao LP. A large inflammatory myofibroblastic tumor involving both stomach and spleen: A case report and review of the literature. Oncol Lett. 2015;9:811-815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Ma ZH, Tian XF, Ma J, Zhao YF. Inflammatory pseudotumor of the spleen: A case report and review of published cases. Oncol Lett. 2013;5:1955-1957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Falk GA, Nooli NP, Morris-Stiff G, Plesec TP, Rosenblatt S. Sclerosing Angiomatoid Nodular Transformation (SANT) of the spleen: Case report and review of the literature. Int J Surg Case Rep. 2012;3:492-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Gómez-Román JJ, Sánchez-Velasco P, Ocejo-Vinyals G, Hernández-Nieto E, Leyva-Cobián F, Val-Bernal JF. Human herpesvirus-8 genes are expressed in pulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). Am J Surg Pathol. 2001;25:624-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 105] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Gonzalez-Duarte A, Sullivan S, Sips GJ, Naidich T, Kleinman G, Murray J, Morgello S, Germano I, Mullen M, Simpson D. Inflammatory pseudotumor associated with HIV, JCV, and immune reconstitution syndrome. Neurology. 2009;72:289-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Kiryu S, Takeuchi K, Shibahara J, Uozaki H, Fukayama M, Tanaka H, Maeda E, Akahane M, Ohtomo K. Epstein-Barr virus-positive inflammatory pseudotumour and inflammatory pseudotumour-like follicular dendritic cell tumour. Br J Radiol. 2009;82:e67-e71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Akbulut S, Yagmur Y, Gumus S, Sogutcu N, Demircan F. Actinomyces-induced inflammatory myofibroblastic tumor of the colon: A rare cause of an abdominal mass: Akbulut et al. inflammatory myofibroblastictumor due to actinomyces spp. Int J Surg Case Rep. 2015;9: 15-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Wang B, Xu X, Li YC. Inflammatory myofibroblastic tumor of the spleen: a case report and review of the literature. Int J Clin Exp Pathol. 2019;12:1795-1800. [PubMed] |
| 17. | Yang ZL, Zhu F, and Meng Q. A misdiagnosis of inflammatory myofibroblastoma of the liver and reviewed in literature. Linchuang Wuzhen Wuzhi. 2018;1:47-51. |
| 18. | Oeconomopoulou A, de Verney Y, Kanavaki K, Stefanaki K, Pavlakis K, Salakos C. Inflammatory myofibroblastic tumor of the small intestine mimicking acute appendicitis: a case report and review of the literature. J Med Case Rep. 2016;10:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol. 2007;31:509-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 621] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 20. | Butrynski JE, D'Adamo DR, Hornick JL, Dal Cin P, Antonescu CR, Jhanwar SC, Ladanyi M, Capelletti M, Rodig SJ, Ramaiya N, Kwak EL, Clark JW, Wilner KD, Christensen JG, Jänne PA, Maki RG, Demetri GD, Shapiro GI. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med. 2010;363:1727-1733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 727] [Cited by in RCA: 650] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 21. | Chang JC, Zhang L, Drilon AE, Chi P, Alaggio R, Borsu L, Benayed R, Travis WD, Ladanyi M, Antonescu CR. Expanding the Molecular Characterization of Thoracic Inflammatory Myofibroblastic Tumors beyond ALK Gene Rearrangements. J Thorac Oncol. 2019;14:825-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 22. | Lovly CM, Gupta A, Lipson D, Otto G, Brennan T, Chung CT, Borinstein SC, Ross JS, Stephens PJ, Miller VA, Coffin CM. Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discov. 2014;4:889-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 320] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 23. | Surabhi VR, Chua S, Patel RP, Takahashi N, Lalwani N, Prasad SR. Inflammatory Myofibroblastic Tumors: Current Update. Radiol Clin North Am. 2016;54:553-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 24. | Gómez-Román JJ, Ocejo-Vinyals G, Sánchez-Velasco P, Nieto EH, Leyva-Cobián F, Val-Bernal JF. Presence of human herpesvirus-8 DNA sequences and overexpression of human IL-6 and cyclin D1 in inflammatory myofibroblastic tumor (inflammatory pseudotumor). Lab Invest. 2000;80:1121-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 85] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Karimi M, Tizmaghz A, Shabestanipour G. An interesting case of inflammatory myofibroblastic tumor presenting as cholangiocarcinoma. Int J Surg Case Rep. 2018;47:38-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Bettach H, Alami B, Boubbou M, Chbani L, Maâroufi M, Lamrani MA. Inflammatory myofibroblastic tumor of the spleen: a case report. Radiol Case Rep. 2021;16:3117-3119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Palaskar S, Koshti S, Maralingannavar M, Bartake A. Inflammatory myofibroblastic tumor. Contemp Clin Dent. 2011;2:274-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Tao YL, Wang ZJ, Han JG, Wei P. Inflammatory myofibroblastic tumor successfully treated with chemotherapy and nonsteroidals: a case report. World J Gastroenterol. 2012;18:7100-7103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Shek AW, Wu PC, Samman N. Inflammatory pseudotumour of the mouth and maxilla. J Clin Pathol. 1996;49:164-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Difiore JW, Goldblum JR. Inflammatory myofibroblastic tumor of the small intestine. J Am Coll Surg. 2002;194:502-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995;19:859-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1029] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 32. | Lorenzi L, Cigognetti M, Medicina D, Pellegrini V, Balzarini P, Cestari R, Facchetti F. ALK-positive inflammatory myofibroblastic tumor of the abdomen with widespread microscopic multifocality. Int J Surg Pathol. 2014;22:640-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |