Published online Jun 26, 2023. doi: 10.12998/wjcc.v11.i18.4277
Peer-review started: May 6, 2023
First decision: May 19, 2023
Revised: May 22, 2023
Accepted: May 26, 2023
Article in press: May 26, 2023
Published online: June 26, 2023
Processing time: 51 Days and 17.4 Hours
This study aimed to analyze the predictive value of multi-slice spiral computed tomography (CT) perfusion imaging for upper gastrointestinal bleeding in patients with cirrhotic portal hypertension. A total of 62 patients with cirrhotic portal hypertension and 28 healthy individuals were included. The results showed that multi-slice spiral CT perfusion imaging had a significant predictive value for upper gastrointestinal bleeding in patients with cirrhotic portal hypertension. The vascular area, number of vascular cross-sections, and gastric coronary vein diameter (GCVD) showed high predictive values, with the vascular area having the best predictive value.
To investigate the predictive accuracy of multi-slice spiral CT perfusion imaging for upper gastrointestinal bleeding in patients with cirrhosis and portal hypertension.
This study included 62 patients with cirrhotic portal hypertension (disease group) and 28 healthy individuals (control group). The disease group was further divided into two subgroups: Group A (n = 27, bleeding) and group B (n = 35, no bleeding). All patients underwent multi-slice spiral CT perfusion imaging at our hospital, and we compared various parameters such as liver blood flow, vein size, number of blood vessels, and blood vessel area between the two groups. We employed statistical analysis to identify factors associated with upper gastrointestinal bleeding and created a graph comparing the predictive value of different factors for bleeding.
We found no difference in hepatic artery (HAP) levels among the three groups (all P > 0.05). The portal vein levels in groups A and B were much lower than in the control group; group A was much lower than group B (all P < 0.05). The HAP perfusion index levels in groups A and B were much higher than in the control group; group A was much higher than group B (all P < 0.05). The portal vein diameter, splenic vein diameter, and GCVD levels in groups A and B were much higher than in the control group; those in group A were much higher than those in group B (all P < 0.05). The number of blood vessels and blood vessel area in groups A and B were much higher than in the control group; those in group A were much higher than those in group B (all P < 0.05). The statistical method showed a strong link between GCVD, number of blood vessels, blood vessel area, and upper gastrointestinal bleeding (odds ratio = 1.275, 1.346, 1.397, P < 0.05). The graph showed that GCVD, number of blood vessels, and blood vessel area could predict bleeding well, with blood vessel area having the best prediction power.
That multi-slice spiral CT perfusion imaging can predict upper gastrointestinal bleeding well in patients with cirrhosis and high blood pressure in the portal vein. GCVD, number of blood vessels, and blood vessel area had high prediction power. The blood vessel area had the best prediction power, with an area under the curve of 0.831.
Core Tip: Multi-slice spiral computed tomography (CT) perfusion imaging can predict the occurrence of upper gastrointestinal bleeding in patients with cirrhotic portal hypertension. This study found that gastric coronary vein diameter, number of vascular cross-sections, and vascular area were significantly correlated with the occurrence of bleeding. The vascular area had the best predictive value, with an area under the curve of 0.831. These findings suggest that multi-slice spiral CT perfusion imaging can be a useful tool for early identification of high-risk patients and prompt management to prevent or manage bleeding events in patients with cirrhotic portal hypertension.
- Citation: Song XJ, Liu JL, Jia SY, Zhang K. Portal vein computed tomography imaging characteristics and their relationship with bleeding risk in patients with liver cirrhosis undergoing interventional therapy. World J Clin Cases 2023; 11(18): 4277-4286
- URL: https://www.wjgnet.com/2307-8960/full/v11/i18/4277.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i18.4277
Cirrhosis is a common chronic digestive system disease induced by single or multiple etiologies[1]. Clinical and pathological features include progressive, chronic, and diffuse liver fibrosis, pseudolobule formation, and nodular regeneration[2]. Intrahepatic fibrosis can increase the flow resistance of the posterior sinus portal vein in patients with cirrhosis, resulting in abnormal changes in the shape and size of the portal vein system, leading to cirrhotic portal hypertension[3]. According to a previous study[4], several lethal complications and increased mortality are associated with cirrhotic portal hypertension. Upper gastrointestinal hemorrhage is one of the most frequent and fatal outcomes. Relevant studies show that the incidence of upper gastrointestinal bleeding in patients with cirrhosis portal hypertension is as high as 29.7%-38.6%, and the mortality is as high as 24.3%-48.9%, with a risk of multiple occurrences[5]. Therefore, the early prediction of upper gastrointestinal bleeding in patients with cirrhosis and portal hypertension plays an important role in ensuring safety.
Previously, gastroscopy and B-scan ultrasonography were commonly used in clinical practice to predict upper gastrointestinal bleeding in patients with cirrhotic portal hypertension. However, these methods are invasive, incomplete, and have significant limitations in clinical applications[6]. In recent years, multi-slice spiral computed tomography (CT) has been widely used in the clinical evaluation of collateral circulation in portal hypertension owing to its advantages of simple operation, objectivity, and non-invasion[7]. However, there have been few clinical studies on multi-slice spiral CT for predicting upper gastrointestinal bleeding in patients with cirrhotic portal hypertension. In this study, we collected and compared liver perfusion parameters, venous diameters, and vascular-related CT data in patients with or without bleeding and healthy individuals using multi-slice spiral CT to analyze the predictive value of multi-slice spiral CT perfusion imaging for upper gastrointestinal bleeding in patients with cirrhotic portal hypertension.
Sixty-two patients with cirrhosis portal hypertension (disease group) and 28 healthy individuals (control group) in our hospital were selected for this study. Patients with cirrhotic portal hypertension were further divided into groups A (bleeding, n = 27) and B (no bleeding, n = 35) based on whether upper gastrointestinal bleeding occurred. All patients met the inclusion criteria and underwent multi-slice spiral CT perfusion imaging at our hospital. This study was approved by the Ethics Committee of our hospital, and all patients provided written informed consent.
Inclusion criteria: (1) Diagnosis of cirrhosis portal hypertension through clinically relevant tests; (2) whether the included patients had upper gastrointestinal bleeding depending on the results of gastroscopy; (3) adults; (4) cooperation to complete multi-slice spiral CT perfusion imaging detection; and (5) informed of the contents of this study and voluntarily agreed to participate.
Exclusion criteria: (1) Patients with a history of gastrointestinal bleeding; (2) organ dysfunction; (3) individuals with relevant contraindications to the research modes and methods used in this study; (4) coagulation dysfunction, cognitive dysfunction, and mental illness; and (5) unable to fully cooperate with the researcher for various reasons.
Multi-slice spiral CT perfusion imaging detection method: Before starting the test, routine fasting and drinking bans were required. Ten minutes prior to the start of the test, participants consumed 2 L of water. A LightSpeed 16-row spiral CT scanner was used. The scanning parameters were as follows: Voltage, 120 kV; current, 250 mA; slice thickness, 10 mm; pitch, 0.983; matrix, 512 × 512 pixels. Patients were instructed to assume a supine position; the spleen and liver were initially scanned, and thereafter, the first hepatic portal was used as the target plane for CT perfusion scanning. Using a high-pressure syringe, 40 mL of nonionic contrast I/s was injected into the median vein of the elbow at a rate of 3.5 mL/s. After a delay of 5 s, a scan was performed with an inter-scan gap of 1 s. Forty scans were performed. During this period, relevant personnel informed the patients to keep their mouth closed and breathing stable. Perfusion scanning parameters: Voltage, 84 kV; current, 50 mA; collimation, 1.5 mm; scan time, 0.5 s; slice thickness, 0.75-12.00 mm. Enhanced scanning was performed 5 min after perfusion scanning with 80 mL of injected contrast. Thirty seconds after contrast injection, arterial phase scanning was performed, and 65 s thereafter, portal phase scanning was performed.
Image processing: The collected images were transferred to the AEW 4.0 workstation for processing. The maximum intensity projection algorithm was used to reconstruct the portal vein, portal vein diameter (PVD), splenic vein diameter (SVD), gastric coronary vein diameter (GCVD), number of vascular cross-sections, and vascular area in the portal region of the stomach fundus spleen. Liver perfusion parameters were measured using Basama perfusion software. The threshold was set to 0-400 Hu, and the end time of the arterial phase and start time of the portal phase were based on the peak enhancement time of the liver. The abdominal aorta was the input artery and the portal vein was the input portal vein. The computer automatically calculated the hepatic artery (HAP), portal vein (PVP), and hepatic artery perfusion index (HPI).
The liver perfusion parameters (HAP, PVP, and HPI), internal diameters of the veins (PVD, SVD, and GCVD), number of vascular cross-sections, and vascular area in the fundus spleen portal region of the three groups were compared. These indicators were included in the multivariate logistic regression analysis to screen for factors that significantly correlated with the occurrence of upper gastrointestinal bleeding. Subsequently, a receiver operating characteristic (ROC) curve was created, and the area under the curve (AUC) of different indicators was compared. The higher the AUC value, the better the predictive value of an indicator for upper gastrointestinal bleeding in patients with cirrhotic portal hypertension.
The images were processed using GraphPad Prism 8 and the data were analyzed using SPSS Statistics version 26.0 (IBM Copr., Armonk, NY, United States). The counting data are represented as n (%), and the χ2 test was used to determine whether there was any statistical difference. The measurement data is represented by (mean ± SD), and t-test was used to determine whether there was any statistical difference. Significant correlation between the index and the occurrence of upper gastrointestinal bleeding was assessed using logistic multivariate regression analysis. ROC curves were used to evaluate the value of different indices in predicting upper gastrointestinal bleeding caused by portal hypertension in patients with liver cirrhosis. Statistical significance was set at P < 0.05.
The 62 patients in the disease group comprised 41 males and 21 females, aged 34-71 years, with an average age of 52.37 years ± 8.45 years. Types of cirrhosis: Alcoholic in eight cases, hepatitis B in 38 cases, hepatitis C in nine cases, and primary biliary in seven cases. The Child-Pugh classification included26 cases of grade A, 20 cases of grade B, and 16 cases of grade C. The control group comprised 19 males and nine females, aged 35-70 years, with an average age of 51.87 years ± 8.62 years. General information, such as sex and age of the participants in the two groups were comparable. There were no significant differences between the groups (P > 0.05) (Table 1).
| Disease group (n = 62) | Control group (n = 28) | t/χ2 | P value | |
| Sex | 0.026 | 0.872 | ||
| Male | 41 | 19 | ||
| Female | 21 | 9 | ||
| Age (yr) | 34-71 | 35-70 | ||
| Average age (years) | 52.37 ± 8.45 | 51.87 ± 8.62 | 0.258 | 0.797 |
| Types of cirrhosis | - | - | - | |
| Alcoholic | 8 | |||
| Hepatitis B | 38 | |||
| Hepatitis C | 9 | |||
| Primary biliary | 7 | |||
| Child-Pugh classification | - | - | - | |
| A | 26 | |||
| B | 20 | |||
| C | 16 |
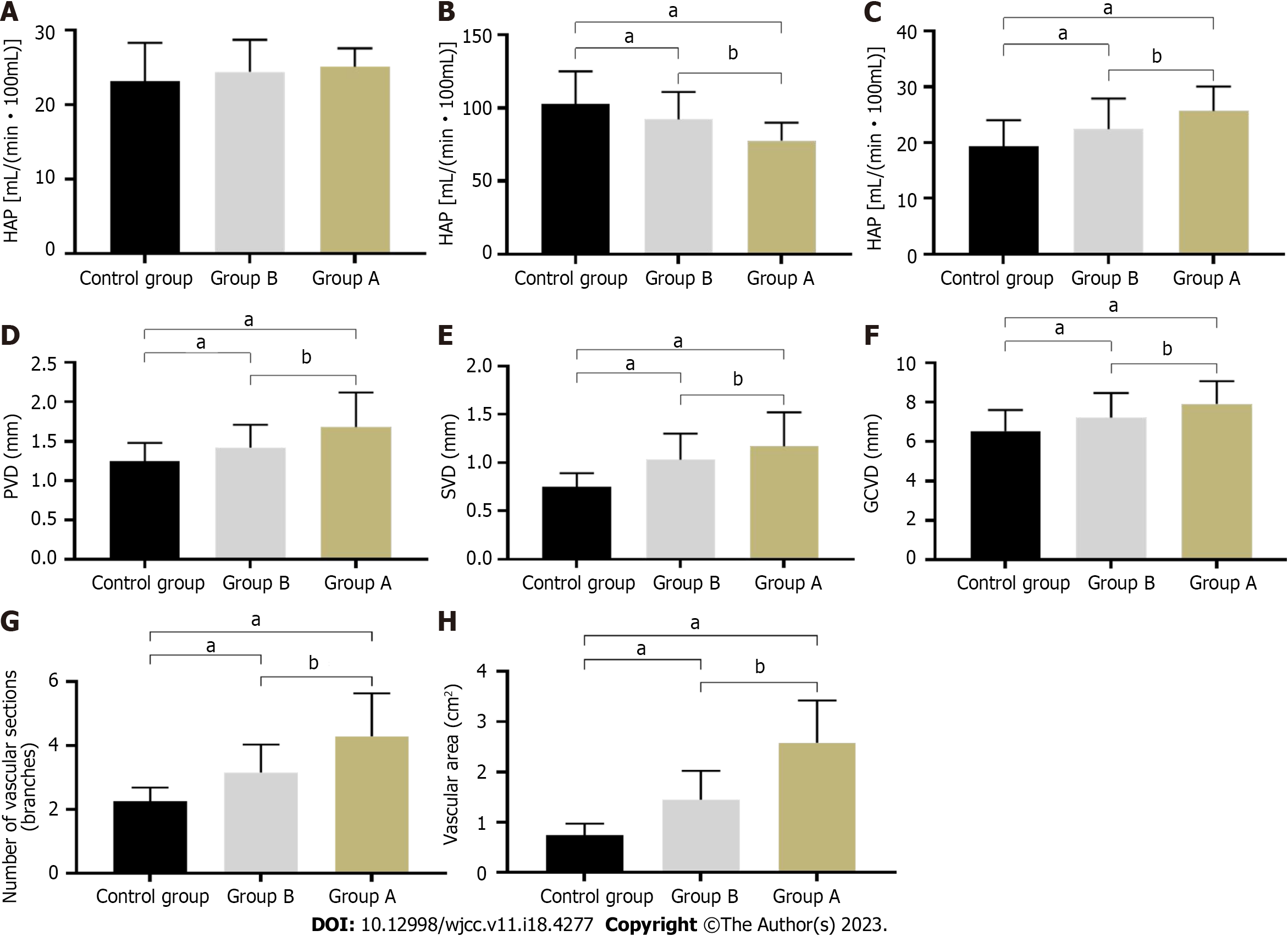
As shown in Figure 1A-C, the HAP of the control, A, and B groups were 23.14 ± 5.16, 25.10 ± 2.49, and 24.45 ± 4.28, respectively. The PVP were 102.66 ± 22.41, 77.53 ± 12.39, and 92.35 ± 18.54, respectively. The HPI were 19.27 ± 4.72, 25.65 ± 4.41, and 22.38 ± 5.49, respectively. There was no significant difference in HAP levels among the three groups (all P > 0.05). PVP levels in groups A and B were significantly lower than those in the control group, and group A was significantly lower than group B (all P < 0.05). HPI levels in groups A and B were significantly higher than those in the control group, and group A was significantly higher than group B (all P < 0.05).
As shown in Figure 1D-F, the PVD of the control, A, and B groups were 1.25 ± 0.23, 1.68 ± 0.44, and 1.42 ± 0.29, respectively. The SVD were 0.75 ± 0.14, 1.17 ± 0.35, and 1.03 ± 0.27, respectively. The GCVD were 6.53 ± 1.07, 7.91 ± 1.15, and 7.22 ± 1.24, respectively. The PVD, SVD, and GCVD levels in groups A and B were significantly higher than those in the control group, and those in group A were significantly higher than those in group B (all P < 0.05).
As shown in Figure 1G and H, the number of vascular cross-sections in the control, A, and B groups were 2.25 ± 0.43, 4.29 ± 1.34, and 3.15 ± 0.88, respectively. The vascular area is 0.74 ± 0.23, 2.58 ± 0.84, and 1.45 ± 0.57, respectively. The number of vascular cross-sections and vascular area levels in groups A and B were significantly higher than those in the control group, and those in group A were significantly higher than those in group B (all P < 0.05).
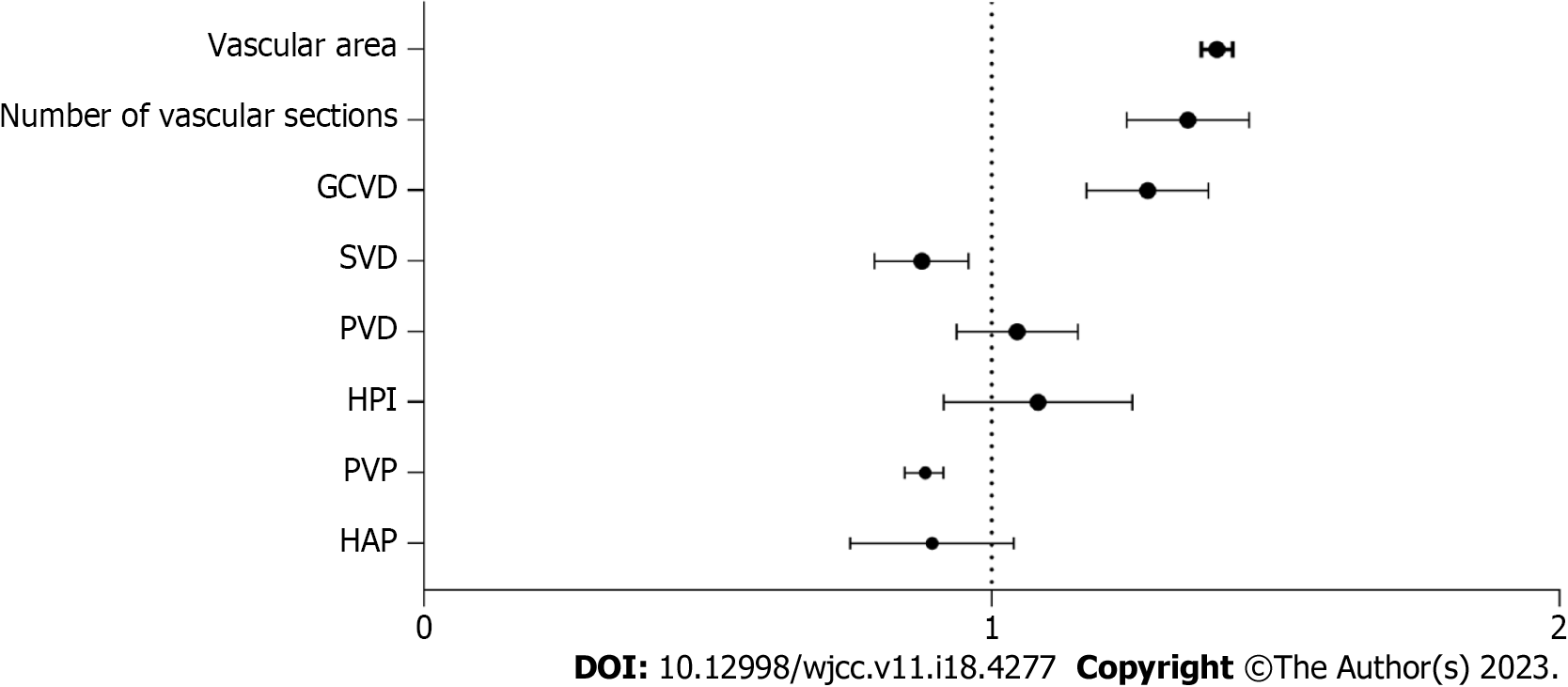
Logistic multivariate regression analysis showed that GCVD, number of vascular cross-sections, and vascular area were significantly associated with upper gastrointestinal bleeding [odds ratio (OR) = 1.275, 1.346, 1.397, P < 0.05] (Table 2, Figure 2).
| Variable | Regression coefficient | Standard error | P value | OR | 95%CI |
| HAP | 0.002 | 0.018 | 0.964 | 0.895 | 0.751-1.039 |
| PVP | 0.004 | 0.033 | 0.872 | 0.883 | 0.847-0.915 |
| HPI | 0.049 | 0.037 | 0.286 | 1.082 | 0.916-1.248 |
| PVD | 0.053 | 0.029 | 0.347 | 1.045 | 0.938-1.152 |
| SVD | 0.067 | 0.036 | 0.767 | 0.877 | 0.794-0.959 |
| GCVD | 0.577 | 0.276 | 0.009 | 1.275 | 1.167-1.382 |
| Number of vascular cross-sections | 0.217 | 0.041 | 0.001 | 1.346 | 1.238-1.454 |
| Vascular area | 0.235 | 0.064 | 0.001 | 1.397 | 1.369-1.425 |
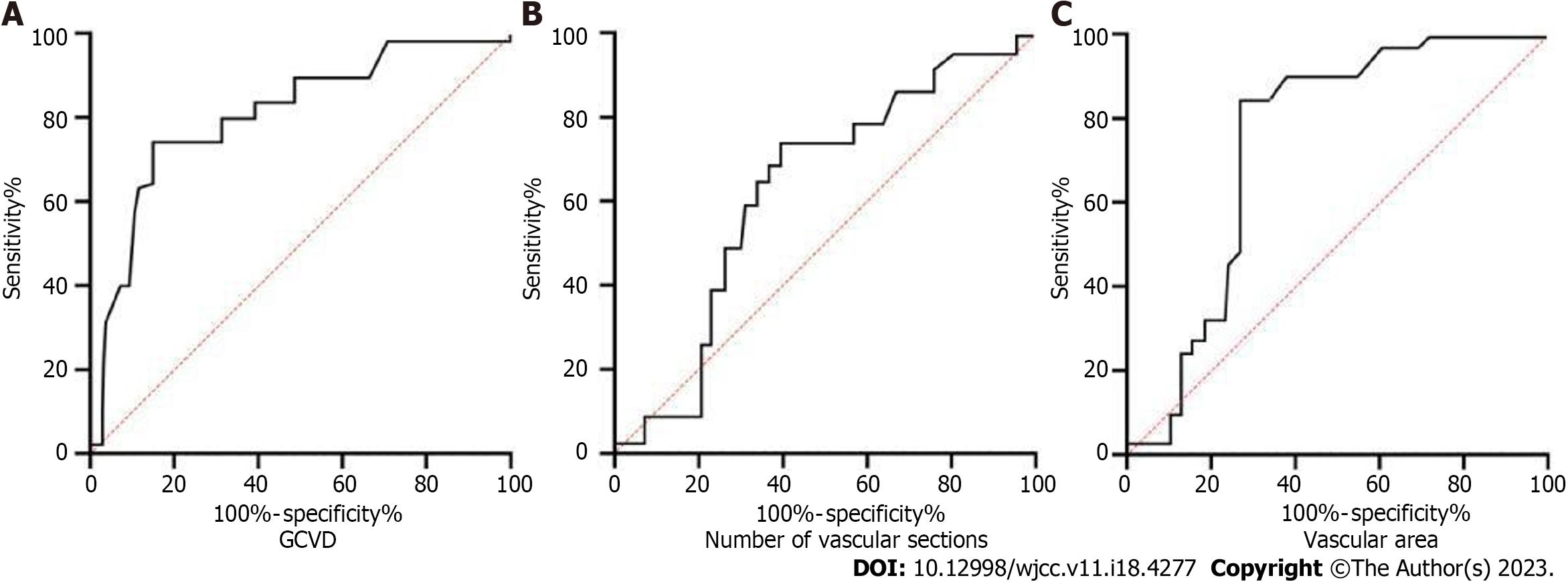
The AUC values for GCVD, number of vascular cross-sections, and vascular area were 0.772, 0.704, and 0.831, respectively (Figure 3). The sensitivities of GCVD, number of vascular cross-sections, and vascular area were 70.85, 75.14, and 87.62, respectively. The specificities for GCVD, number of vascular cross-sections, and vascular area were 87.50, 67.82, and 82.44, respectively. The AUC value of the vascular area was significantly higher than that of GCVD and the number of vascular cross-sections, indicating that it had the best predictive value for upper gastrointestinal bleeding in patients with cirrhotic portal hypertension.
Long-term cirrhosis leads to the accumulation of a large amount of scar tissue, increased blood circulation resistance in the liver, and portal hypertension[8]. To relieve pressure on the portal vein, the liver usually produces collateral vessels. Because of their poor toughness, venous vessels are prone to rupture and bleeding in the upper digestive tract, which, in severe cases, can lead to death[9]. Currently, the main clinical detection methods for upper gastrointestinal bleeding in patients with cirrhotic portal hypertension include gastroscopy and B-scan ultrasonography[10]. Gastroscopy is regarded as the gold standard in clinical practice for detecting upper gastrointestinal bleeding in patients with cirrhosis and portal hypertension. However, owing to the invasive nature of gastroscopy, it can cause significant pain, and some patients cannot tolerate it. Moreover, gastroscopy may induce varicose vein bleeding, and its many shortcomings limit its clinical application[11]. Although B-scan ultrasonography is not harmful to patients, it has not been clinically recognized because of its low efficacy and partial collateral vessels[12]. Therefore, it is important to identify a safer and more efficient detection and imaging index for predicting upper gastrointestinal bleeding caused by cirrhotic portal hypertension.
The human liver is mainly supplied with blood via the HAP and portal vein. In patients with severe cirrhosis, most liver cells are deformed, and hepatic lobular fibers are damaged and divided, leading to blood flow disorder in the liver[13]. A relevant diagnostic study showed that portal venous blood flow and speed are reduced after liver damage, but the impact on the HAP is small[14]. Therefore, a reduction in portal venous blood flow will further increase the velocity of HAP blood flow. Related studies have shown that when upper gastrointestinal bleeding occurs in patients with cirrhotic portal hypertension, the PVP significantly decreases, while the HPI significantly increases. In this study, the PVP level in groups A and B were significantly lower than that in the control group, and group A was significantly lower than group B (all P < 0.05), while the HPI level of groups A and B were significantly higher than that in the control group, and group A was significantly higher than group B (all P < 0.05). This result is consistent with those of previous studies[15], confirming that long-term cirrhosis can alter liver hemodynamics in patients and suggesting that PVP and HPI can serve as predictive indicators of upper gastrointestinal bleeding in cirrhotic portal hypertension. Patients with cirrhotic portal hypertension often experience vascular dilation, increased blood volume, and increased vascular diameter owing to factors such as low blood flow velocity and obstruction of blood flow return[16]. Fejfar et al[17] reported that PVD and SVD levels are closely related to pressure on the portal vein. Therefore, these indicators could be used for bleeding in patients with cirrhosis. In this study, the PVD and SVD levels of groups A and B were significantly higher than those of the control group, and those of group A were significantly higher than those of group B (all P < 0.05). This result is consistent with that of Fejfar et al[17], confirming that changes in PVD and SVD levels in patients are closely related to upper gastrointestinal bleeding. The portal and gastric coronary veins are the most common collateral circulations in patients with cirrhotic portal hypertension and are prone to dilation. Multiple studies have found that the occurrence of upper gastrointestinal bleeding is significantly and positively correlated with the number of vascular cross-sections and vascular areas in the stomach fundus spleen portal region in patients with cirrhosis and portal hypertension. Patients with upper gastrointestinal bleeding have a significantly increased number of vascular cross-sections and vascular areas compared with those without bleeding and healthy individuals[18-20]. Another study indicated that an increase in GCVD levels is also an important reason for the increase in vascular cross-sectional area[21]. In this study, the number of vascular cross-sections and vascular areas in groups A and B were significantly higher than those in the control group, and those in group A were significantly higher than those in group B (all P < 0.05), which is consistent with previous studies[22].
These indicators were included in the logistic multivariate regression analysis, and the results showed that GCVD, number of vascular cross-sections, and vascular area were significantly associated with the occurrence of upper gastrointestinal bleeding. In the subsequent ROC curve analysis, the vascular area had the highest predictive value for upper gastrointestinal bleeding in patients with cirrhotic portal hypertension, with an AUC value of 0.831. Several clinical studies on GCVD, number of venous cross sections, and areas of blood vessels have been conducted to predict upper gastrointestinal bleeding[23-25]. Many reports have noted that GCVD levels can effectively reflect the severity of portal hypertension in patients with cirrhosis and that it is significantly positively correlated with bleeding and has a significant predictive value for bleeding. Galante et al[26] also showed that when the GCVD level exceeded 6 mm, the predictive value for upper gastrointestinal bleeding in cirrhotic portal hypertension was ideal. However, in this study, although GCVD can be used as an indicator for predicting upper gastrointestinal bleeding in patients with cirrhosis and portal hypertension and its effectiveness is relatively ideal, its AUC value (0.772) was lower than that of the vascular area (0.831). This result is not consistent with some previous research results; this difference may be related to the different anatomical structures of the coronary veins in different patients as well as the possible influence of other collateral circulation factors.
This study has limitations, such as the bleeding patients in the study usually only underwent multi-slice spiral CT perfusion imaging detection after hemostasis intervention, but the hemodynamic and other indicators may have changed at this time. Moreover, the number of samples included in this study is relatively small, which may cause bias in the accuracy of the results. Therefore, in future research, we will make improvements to the above shortcomings and continue to expand the sample size to further explore and correct the accuracy of this conclusion.
The background of this study is the high prevalence of upper gastrointestinal bleeding in patients with cirrhotic portal hypertension. This can lead to significant morbidity and mortality, making early detection and prevention crucial. Multi-slice spiral computed tomography (CT) perfusion imaging is a non-invasive diagnostic tool that can provide detailed information on blood flow and vessel characteristics in the liver and portal vein. However, its predictive value for upper gastrointestinal bleeding in patients with cirrhotic portal hypertension has not been fully explored. The present status of the study is to investigate the predictive accuracy of multi-slice spiral CT perfusion imaging for upper gas
The motivation for this research is the high prevalence of upper gastrointestinal bleeding in patients with cirrhotic portal hypertension, which can lead to significant morbidity and mortality. Early detection and prevention are crucial for improving patient outcomes, but current diagnostic tools have limitations. Multi-slice spiral CT perfusion imaging is a non-invasive diagnostic tool that offers detailed information on blood flow and vessel characteristics in the liver and portal vein. However, its predictive value for upper gastrointestinal bleeding in patients with cirrhotic portal hypertension has not been fully explored. Therefore, this study aims to investigate the predictive accuracy of multi-slice spiral CT perfusion imaging and identify specific parameters that may have high predictive values. The results of this study could improve early detection and prevention of upper gastrointestinal bleeding in patients with cirrhotic portal hypertension, ultimately leading to better patient outcomes and quality of life.
To investigate the predictive accuracy of multi-slice spiral CT perfusion imaging for upper gastro
The research methods of this study involved a retrospective analysis of 62 patients with cirrhotic portal hypertension and 28 healthy individuals who served as controls. The disease group was further divided into two subgroups: Group A (n = 27, bleeding) and group B (n = 35, no bleeding). All participants underwent multi-slice spiral CT perfusion imaging at the hospital. The researchers compared various parameters such as liver blood flow, vein size, number of blood vessels, and blood vessel area between the two groups using statistical analysis. They calculated the OR to determine the strength of the association between these parameters and upper gastrointestinal bleeding. They also created a graph to compare the predictive value of different factors for bleeding. The research methods used in this study allowed the researchers to identify factors associated with bleeding and evaluate the potential of multi-slice spiral CT perfusion imaging for predicting upper gastrointestinal bleeding in patients with cirrhosis and portal hypertension.
The research results showed that multi-slice spiral CT perfusion imaging had a significant predictive value for upper gastrointestinal bleeding in patients with cirrhotic portal hypertension. The researchers found that the vascular area, number of vascular cross-sections, and GCVD had high predictive values for bleeding, with the vascular area having the best predictive power. They also identified several factors associated with upper gastrointestinal bleeding, including portal vein levels, hepatic artery perfusion index levels, portal vein diameter, splenic vein diameter, and the number and area of blood vessels. The statistical analysis revealed a strong link between GCVD, number of blood vessels, blood vessel area, and upper gastrointestinal bleeding. Overall, the study suggests that multi-slice spiral CT perfusion imaging can help predict upper gastrointestinal bleeding in patients with cirrhosis and portal hypertension, and blood vessel area may be the strongest predictor among the evaluated parameters.
The research conclusions of this study suggest that multi-slice spiral CT perfusion imaging can predict upper gastrointestinal bleeding well in patients with cirrhosis and portal hypertension. The study found that the vascular area, number of vascular cross-sections, and GCVD had high predictive values for bleeding, with the vascular area having the best predictive power. The researchers also identified several factors associated with upper gastrointestinal bleeding in this patient population. Overall, the study highlights the potential of multi-slice spiral CT perfusion imaging as a useful tool for predicting upper gastrointestinal bleeding in patients with cirrhosis and portal hypertension. These findings may inform clinical decision-making and improve patient outcomes in this population.
That future research can build upon these findings to further validate the use of multi-slice spiral CT perfusion imaging for predicting upper gastrointestinal bleeding in patients with cirrhosis and portal hypertension. Future studies can explore the optimal cutoff values and diagnostic performance of different parameters identified in this study. Additionally, studies can investigate the potential of other imaging modalities or biomarkers for predicting upper gastrointestinal bleeding in this patient population. These efforts may further improve the management and outcomes of patients with cirrhosis and portal hypertension who are at risk of upper gastrointestinal bleeding.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nio M, Japan; Yoon HM, South Korea S-Editor: Chen YL L-Editor: A P-Editor: Zhang XD
| 1. | Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398:1359-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 852] [Article Influence: 213.0] [Reference Citation Analysis (1)] |
| 2. | Arab JP, Martin-Mateos RM, Shah VH. Gut-liver axis, cirrhosis and portal hypertension: the chicken and the egg. Hepatol Int. 2018;12:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 155] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 3. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1482] [Article Influence: 494.0] [Reference Citation Analysis (2)] |
| 4. | Simonetto DA, Liu M, Kamath PS. Portal Hypertension and Related Complications: Diagnosis and Management. Mayo Clin Proc. 2019;94:714-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 128] [Article Influence: 21.3] [Reference Citation Analysis (34)] |
| 5. | Gioia S, Nardelli S, Ridola L, Riggio O. Causes and Management of Non-cirrhotic Portal Hypertension. Curr Gastroenterol Rep. 2020;22:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 6. | Gunarathne LS, Rajapaksha H, Shackel N, Angus PW, Herath CB. Cirrhotic portal hypertension: From pathophysiology to novel therapeutics. World J Gastroenterol. 2020;26:6111-6140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (6)] |
| 7. | Pham JT, Kalantari J, Ji C, Chang JH, Kiang SC, Jin DH, Tomihama RT. Quantitative CT Predictors of Portal Venous Intervention in Uncontrolled Variceal Bleeding. AJR Am J Roentgenol. 2020;215:1247-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Reiberger T. The Value of Liver and Spleen Stiffness for Evaluation of Portal Hypertension in Compensated Cirrhosis. Hepatol Commun. 2022;6:950-964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 85] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 9. | Lunova M, Frankova S, Gottfriedova H, Senkerikova R, Neroldova M, Kovac J, Kieslichova E, Lanska V, Sticova E, Spicak J, Jirsa M, Sperl J. Portal hypertension is the main driver of liver stiffness in advanced liver cirrhosis. Physiol Res. 2021;70:563-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Park HJ, Park B, Lee SS. Radiomics and Deep Learning: Hepatic Applications. Korean J Radiol. 2020;21:387-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 11. | Nett A, Binmoeller KF. Endoscopic Management of Portal Hypertension-related Bleeding. Gastrointest Endosc Clin N Am. 2019;29:321-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Hectors SJ, Bane O, Kennedy P, Cuevas J, Thung S, Fischman A, Friedman SL, Schiano TD, Taouli B. Noninvasive diagnosis of portal hypertension using gadoxetate DCE-MRI of the liver and spleen. Eur Radiol. 2021;31:4804-4812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Lesmana CRA, Raharjo M, Gani RA. Managing liver cirrhotic complications: Overview of esophageal and gastric varices. Clin Mol Hepatol. 2020;26:444-460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 14. | Gelman S, Sakalauskas A, Zykus R, Pranculis A, Jurkonis R, Kuliavienė I, Lukoševičius A, Kupčinskas L, Kupčinskas J. Endogenous motion of liver correlates to the severity of portal hypertension. World J Gastroenterol. 2020;26:5836-5848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Li H. Angiogenesis in the progression from liver fibrosis to cirrhosis and hepatocelluar carcinoma. Expert Rev Gastroenterol Hepatol. 2021;15:217-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 16. | Friedman SL, Pinzani M. Hepatic fibrosis 2022: Unmet needs and a blueprint for the future. Hepatology. 2022;75:473-488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 286] [Cited by in RCA: 259] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 17. | Fejfar T, Vaňásek T, Hůlek P. Bleeding in portal hypertension. Vnitr Lek. 2020;66:32-41. [PubMed] [DOI] [Full Text] |
| 18. | Villanueva C, Albillos A, Genescà J, Garcia-Pagan JC, Calleja JL, Aracil C, Bañares R, Morillas RM, Poca M, Peñas B, Augustin S, Abraldes JG, Alvarado E, Torres F, Bosch J. β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2019;393:1597-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 451] [Article Influence: 75.2] [Reference Citation Analysis (0)] |
| 19. | Henry Z, Patel K, Patton H, Saad W. AGA Clinical Practice Update on Management of Bleeding Gastric Varices: Expert Review. Clin Gastroenterol Hepatol. 2021;19:1098-1107.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 20. | Alqahtani SA, Jang S. Pathophysiology and Management of Variceal Bleeding. Drugs. 2021;81:647-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Sheng JY, Liu S, Yang YS, Zhang XW. [The progress in management of esophagogastric variceal bleeding in cirrhotic portal hypertension]. Zhonghua Wai Ke Za Zhi. 2020;58:808-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 22. | Crismale JF, Friedman SL. Acute Liver Injury and Decompensated Cirrhosis. Med Clin North Am. 2020;104:647-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Zanetto A, Garcia-Tsao G. Management of acute variceal hemorrhage. F1000Res. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Philips CA, Ahamed R, Rajesh S, George T, Mohanan M, Augustine P. Beyond the scope and the glue: update on evaluation and management of gastric varices. BMC Gastroenterol. 2020;20:361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | Jakab SS, Garcia-Tsao G. Evaluation and Management of Esophageal and Gastric Varices in Patients with Cirrhosis. Clin Liver Dis. 2020;24:335-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 26. | Galante A, De Gottardi A. Portal vein thrombosis: an overview of current treatment options. Acta Gastroenterol Belg. 2021;84:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |