Published online Jun 26, 2023. doi: 10.12998/wjcc.v11.i18.4251
Peer-review started: March 26, 2023
First decision: May 12, 2023
Revised: May 19, 2023
Accepted: May 24, 2023
Article in press: May 24, 2023
Published online: June 26, 2023
Processing time: 85 Days and 0.1 Hours
Due to its prevalence of 0.5% to 2% in the general population, with a 75% predominance among men, bicuspid aortic valve is the most common congenital heart defect. It is frequently accompanied by other cardiac congenital anomalies, and clinical presentation can vary significantly, with stenosis being the most common manifestation, often resulting in mild to moderate concentric hypertrophy of the left ventricle. Echocardiography is the primary diagnostic modality utilized for establishing the diagnosis, and it is often the sole diagnostic tool relied upon by clinicians. However, due to the heterogeneous clinical presentation and possible associated anomalies (which are often overlooked in clinical practice), it is necessary to employ various diagnostic methods and persist in finding the accurate diagnosis if multiple inconsistencies exist. By employing this approach, we can effectively manage these patients and provide them with appropriate treatment. Through a clinical case from our practice, we provide an overview of the literature on bicuspid aortic valve with aortophaty and the possible association with hypertrophic cardiomyopathy, diagnostic methods, and treatment options. This review article highlights the critical significance of achieving an accurate diagnosis in patients with bicuspid aortic valve and significant left ventricular hypertrophy. It is crucial to exclude other possible causes of left ventricular outflow tract obstruction, such as sub- or supra-aortic obstructions, and hypertrophic cardiomyopathy.
Core Tip: Bicuspid aortic valve accompanied by significant left ventricular hypertrophy, with echocardiographic parameters suggesting possible obstruction of the left ventricular output tract, requires additional diagnostic workup to exclude obstruction other than at the aortic valve level, as well as possible associated hypertrophic cardiomyopathy. A multidisciplinary approach in the heart team and the utilization of multimodal imaging are imperative for the accurate diagnosis and appropriate management of such patients.
- Citation: Sopek Merkaš I, Lakušić N, Predrijevac M, Štambuk K, Hrabak Paar M. Bicuspid aortic valve with associated aortopathy, significant left ventricular hypertrophy or concomitant hypertrophic cardiomyopathy: A diagnostic and therapeutic challenge. World J Clin Cases 2023; 11(18): 4251-4257
- URL: https://www.wjgnet.com/2307-8960/full/v11/i18/4251.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i18.4251
Bicuspid aortic valve (BAV) is the most common congenital heart defect with prevalence of 0.5% to 2% in the general population, and 75% predominance among men[1]. It is often associated with other cardiac anomalies such as ventricular septal defect, patent ductus arteriosus, coarctation of the aorta, and the most frequent associated finding is dilation of the proximal ascending aorta[2]. The clinical presentation of patients with BAV can significantly vary from significant stenosis development already in childhood to milder degenerative changes and dysfunction in old age[2]. BAV is more prone to accelerated degeneration over time so the most common clinical presentation is in the form of stenosis with varying degree of insufficiency and typically results in mild to moderate concentric hypertrophy of the left ventricle[1,2]. In patients with symptomatic BAV, the treatment options are surgical or transcatheter aortic valve replacement (TAVR)[3], and depending on the diameter of the ascending aorta (> 45-55 mm) and associated comorbidities, replacement of the ascending aorta is recommended[4].
Hypertrophic cardiomyopathy (HCM) is the most common inherited cardiomyopathy, with a prevalence of 1 in every 500 individuals[5]. It is defined as a primary disorder of the heart muscle characterized by disproportionate asymmetric (in 95% of cases) or less commonly symmetric hypertrophy of the left ventricle (LV), without a clear cause to explain the observed hypertrophy (such as arterial hypertension, aortic stenosis, and others)[6]. HCM is mostly caused by autosomal dominant mutations in genes that encode sarcomere proteins (up to 60%) which control heart muscle growth and function[6]. Hypertrophy can also be caused by other genetic disorders such as neuromuscular diseases, chromosomal abnormalities, and genetic syndromes. These disorders may affect the myocardium and lead to abnormal ventricular hypertrophy or other structural abnormalities that can impede cardiac function[6]. Accurate diagnosis of the underlying etiology of hypertrophy is crucial for devising an appropriate therapeutic strategy and achieving optimal outcomes[6]. The disease is typically diagnosed in middle-aged and older adults between the third and fifth decade of life[7].
The onset of disease symptoms is attributed to diastolic dysfunction of the LV, which presents as fatigue, dyspnea on exertion, atypical chest pain, palpitations, and syncope. The symptoms and treatment of HCM depend on the presence of an obstructive gradient in the left ventricular outflow tract (LVOT). Asymptomatic patients without LVOT obstruction do not require active treatment, but they should undergo regular echocardiographic monitoring at one-year intervals[8]. The treatment options for symptomatic patients with HCM and obstructive gradient include medication such as non-vasodilating beta-blockers, verapamil, disopyramide, and more recently, mavacamten[9], while interventional/surgical options include surgical septal myectomy (Morrow’s operation) or percutaneous alcohol septal ablation. Dual-chamber pacing has not shown convincing benefits in treatment[8].
Coexistence of BAV and HCM has been reported with a prevalence of about 1%[10-14], however, its true prevalence remains unknown. The concomitance of BAV and severe left ventricular hypertrophy (LVH) can present a significant diagnostic and therapeutic challenge in everyday clinical practice.
The clinical presentation of patients with BAV and concomitant HCM can vary widely. Some patients may remain asymptomatic, while others may experience symptoms such as chest pain, shortness of breath, palpitations, or syncope. These symptoms may be related to the aortic valve stenosis or regurgitation, or they may be related to the HCM. In some cases, the LVH in patients with BAV and HCM can be so severe that it obstructs the LVOT, leading to symptoms of heart failure, such as fatigue, edema, and dyspnea on exertion[12,15].
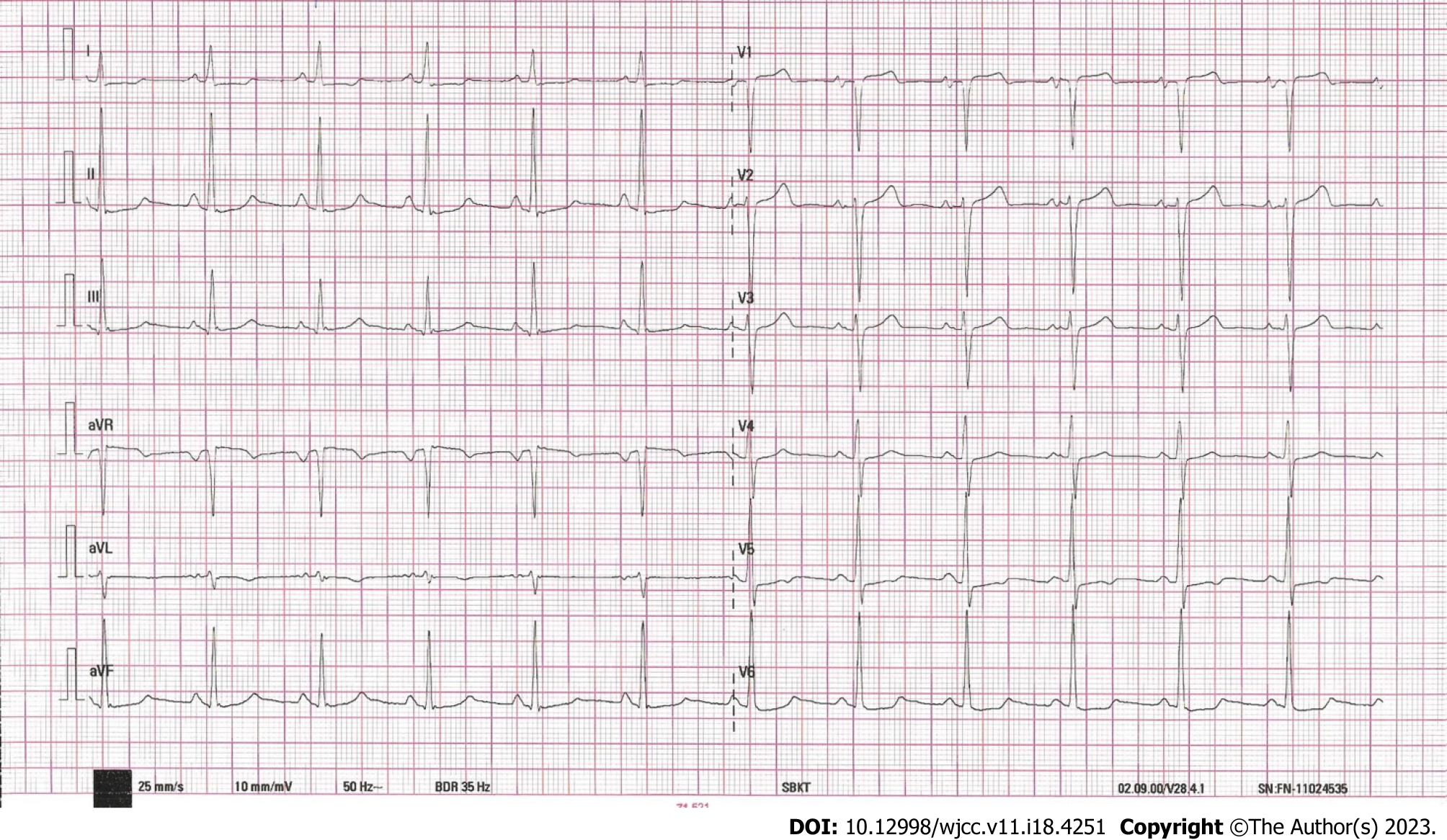
In case from clinical practice, a 39-year-old female patient who had previously (5 years before) been diagnosed with BAV presented to a cardiologist with symptoms consistent with heart failure with preserved ejection fraction. These symptoms included exertional intolerance, rapid fatigue, and occasional lower extremity edema. Upon evaluation, the patient’s N-terminal pro-B-type natriuretic peptide (NTproBNP) levels were found to be elevated, and there was LVH and left ventricular "strain" on electrocardiogram (Figure 1).
Transthoracic echocardiography (TTE) is the primary diagnostic modality utilized in the detection and longitudinal follow-up of patients with BAV or HCM. However, in cases where uncertainties or associated anomalies are suspected, additional diagnostic methods such as transesophageal echocardiography (TEE), magnetic resonance imaging (MRI), or computed tomography (CT) may be employed for diagnosing.
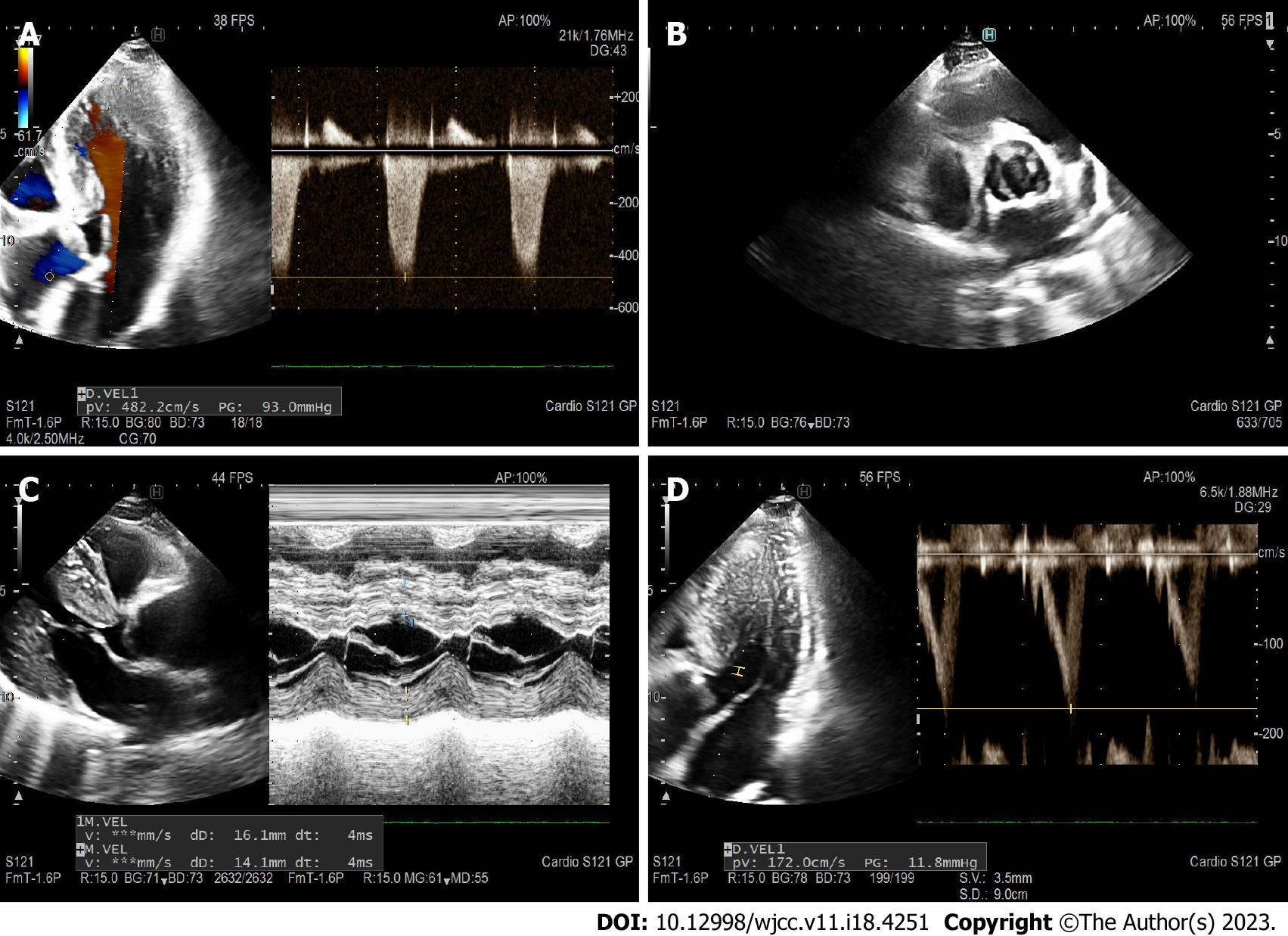
In this case, the TTE revealed a very high peak pressure gradient (up to 93 mmHg) (Figure 2A, Video 1) across the calcified BAV with mildly reduced orifice area (1.9 cm2) (Figure 2B, Video 1), and measured significant LVH (intraventricular septum 16-18 mm) (Figure 2C, Video 1), without typical dagger-shaped morphology on continuous Doppler at the level of the valve, and no systolic anterior motion. The peak gradient (PG) across the LVOT on pulse Doppler was 12 mmHg (Figure 2D), increasing to 35 mmHg with Valsalva maneuver. It became imperative to ascertain whether the obstruction was solely attributable to the altered valve, or if there were additional sub- or supra-aortic obstruction, and in combination with significant hypertrophy indicative of hypertrophic cardiomyopathy.
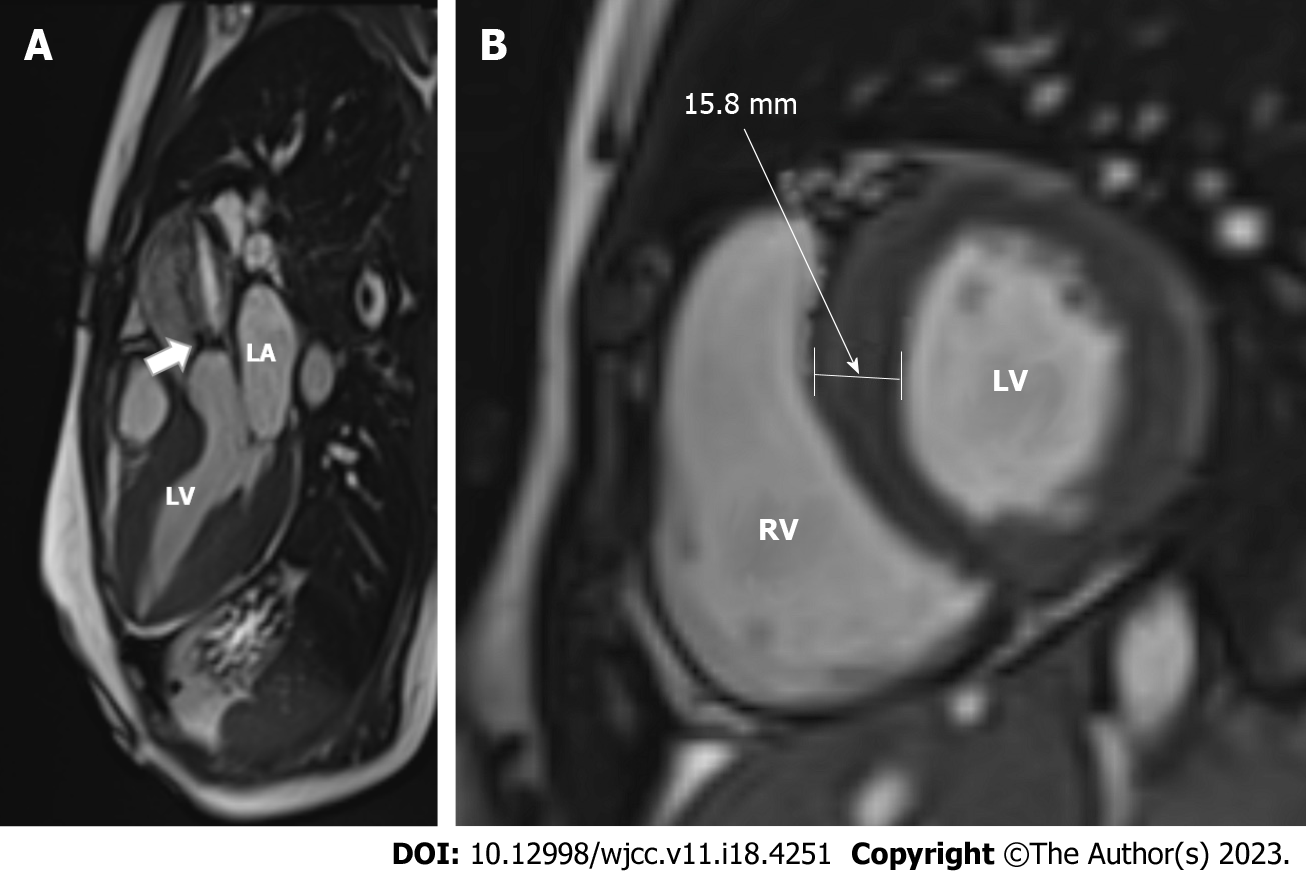
Next step was TEE, which confirmed the findings of the TTE. In order to rule out any associated anomalies, a CT angiography and aortography were conducted, which ruled out obstructive coronary artery disease and revealed the presence of a fusiform aneurysm in the ascending aorta measuring up to 45 mm, in addition to the previously identified BAV. Cardiac MRI confirmed the presence of BAV with clear obstruction at the valve level (Figure 3A) and LVH up to 16 mm (Figure 3B), with no indications of HCM, including no evidence of late gadolinium enhancement, and only mild diffuse fibrosis observed via borderline higher T1 values of the myocardium, with myocardial thickness appearing normal.
The diagnostic process for these patients is crucial and requires a multidisciplinary approach. A team of cardiologist, cardiac surgeon, and radiologist need to collaborate in order to evaluate the patient’s condition and determine the appropriate diagnostic methods for establishing an accurate diagnosis.
In patients with significant hypertrophy of LV and high doppler gradient localized on the aortic valve and in the LVOT, although bicuspid aortic valve and stenosis are known, it is important to exclude other possible causes of LVOT obstruction, possible at sub- or supra-aortic level, and such as hypertrophic cardiomyopathy. It is a particular challenge to make a diagnosis when the patient has certain elements that do not completely fit together. Except TTE and TEE examinations and their characteristics (e.g., systolic anterior movement, "dagger"-shape Doppler), additional imaging like cardiac MRI has a key role in establishing a diagnosis. Making the right diagnosis is crucial because depending on the diagnosis, there are many different treatment options (valve replacement – BAV without HCM, myectomy and valve replacement – stenotic BAV + HCM, alcohol septal ablation and later valve replacement – obstructive HCM with preserved BAV function). The multidisciplinary team recom
The choice of treatment will depend on individual patient characteristics and severity of the condition. In patients with a combined maximal instantaneous gradient of > 40 mmHg across the LVOT and aortic valve, and medically refractory symptoms despite maximal therapy, a surgical approach is the recommended course of action[10]. Surgical myectomy is favored over alcohol septal ablation due to its ability to simultaneously address aortic valve dysfunction and aortic pathology. However, if the patient is not a candidate for surgery and alcohol septal ablation is being considered, hemodynamic catheterization can provide valuable information as non-invasive quantitation of serial stenoses using doppler echocardiography has its limitations[10]. Additionally, innovative medicament treatment options are being used in this field. Such example are cardiac myosin inhibitors, a class of drugs that target the sarcomere, the basic contractile unit of cardiac muscle cells[6]. Mavacamten is a specific cardiac myosin inhibitor that has been approved by the FDA for the treatment of obstructive HCM in adult patients with New York Heart Association class II-III symptoms[6]. Mavacamten works by reducing the hypercontractility of the heart muscle, which can help to improve blood flow and alleviate symptoms in patients with obstructive HCM. It is taken orally and is usually prescribed in combination with other medications that help to manage symptoms and reduce the risk of complications[6].
The pathogenesis of BAV remains incompletely understood, but there is evidence of genetic predisposition, with several genes identified as potential contributors[16,17]. Additionally, the interplay between genetic factors and hemodynamic stresses has been proposed as a mechanism in the development of BAV[18]. BAV is often associated with aortopathy, a recent study shows up to 33%[19], suggesting that is a malformation involving the aorta[19,20]. The dilatation of the aortic root and ascending aorta is a common occurrence in individuals with BAV[21], particularly in certain morphology patterns [right-left vs right-noncoronary (RN) cusp fusion is associated with larger sinus of Valsalva diameter][22], and this condition can result in the formation of aneurysms, aortic dissection, or aortic rupture, even in the absence of significant valvular changes[23]. In the presence of root asymmetry (≥ 5 mm between cusp-to-cusp diameters), which is common in over 50% of BAV patients without raphe and over 40% of BAV-RN morphotype[24], it is possible to underestimate the diameter of the aortic root when measuring it using TTE with leading-leading edges. In such cases, it may be necessary to use more precise imaging techniques such as magnetic resonance imaging or CT to obtain the most accurate measurement of the largest aortic root diameter, especially in patients with BAV-RN and those without a raphe[25].
Research on BAV has not only focused on the valve itself, but also its effects on the left ventricle and associated remodeling[26-29]. Changes in left ventricular geometry and function, particularly impaired diastolic function, have been linked to symptom severity and poor clinical outcomes[29]. LV changes associated with BAV can include concomitant specific cardiomyopathies, as shown in a recent study with a prevalence of up to 5.6%[11]. It is important to consider this possibility as a potential associated anomaly in BAV patients, as we have highlighted.
Recent literature review[25] emphasizes the significance of utilizing various diagnostic techniques and a multimodality approach in the comprehensive evaluation of BAV morphology, identification of associated anomalies, assessment of LV, risk stratification, and therapy guidance[23,30].
In addition to classical, clear-cut cases of degenerative, stenotic BAV, and associated hypertrophy of the LV, there is a possibility of "entity overlap", where only a thorough multimodal diagnostic workup by the multidisciplinary team can lead to a clear diagnosis, enabling the planning of therapeutic options and appropriate management of such patients. Multimodality imaging is essential for accurate diagnosis, follow-up, and surgical management in patients with BAV, given the potential for associated aortic and LV anomalies that need to be ruled out or identified.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chauhan S, United States; Gupta P, United States S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Liu T, Xie M, Lv Q, Li Y, Fang L, Zhang L, Deng W, Wang J. Bicuspid Aortic Valve: An Update in Morphology, Genetics, Biomarker, Complications, Imaging Diagnosis and Treatment. Front Physiol. 2018;9:1921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 2. | Mordi I, Tzemos N. Bicuspid aortic valve disease: a comprehensive review. Cardiol Res Pract. 2012;2012:196037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 3. | Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, Delgado V, Freemantle N, Gilard M, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W; ESC/EACTS Scientific Document Group. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 3235] [Article Influence: 808.8] [Reference Citation Analysis (0)] |
| 4. | Borger MA, Fedak PWM, Stephens EH, Gleason TG, Girdauskas E, Ikonomidis JS, Khoynezhad A, Siu SC, Verma S, Hope MD, Cameron DE, Hammer DF, Coselli JS, Moon MR, Sundt TM, Barker AJ, Markl M, Della Corte A, Michelena HI, Elefteriades JA. The American Association for Thoracic Surgery consensus guidelines on bicuspid aortic valve-related aortopathy: Full online-only version. J Thorac Cardiovasc Surg. 2018;156:e41-e74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 183] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 5. | Butzner M, Leslie DL, Cuffee Y, Hollenbeak CS, Sciamanna C, Abraham T. Stable Rates of Obstructive Hypertrophic Cardiomyopathy in a Contemporary Era. Front Cardiovasc Med. 2021;8:765876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Packard E, de Feria A, Peshin S, Reza N, Owens AT. Contemporary Therapies and Future Directions in the Management of Hypertrophic Cardiomyopathy. Cardiol Ther. 2022;11:491-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Canepa M, Fumagalli C, Tini G, Vincent-Tompkins J, Day SM, Ashley EA, Mazzarotto F, Ware JS, Michels M, Jacoby D, Ho CY, Olivotto I; SHaRe Investigators. Temporal Trend of Age at Diagnosis in Hypertrophic Cardiomyopathy: An Analysis of the International Sarcomeric Human Cardiomyopathy Registry. Circ Heart Fail. 2020;13:e007230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 8. | Authors/Task Force members, Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2733-2779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2292] [Cited by in RCA: 3067] [Article Influence: 278.8] [Reference Citation Analysis (0)] |
| 9. | Reyes KRL, Bilgili G, Rader F. Mavacamten: A First-in-class Oral Modulator of Cardiac Myosin for the Treatment of Symptomatic Hypertrophic Obstructive Cardiomyopathy. Heart Int. 2022;16:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 10. | Padang R, Gersh BJ, Ommen SR, Geske JB. Prevalence and Impact of Coexistent Bicuspid Aortic Valve in Hypertrophic Cardiomyopathy. Heart Lung Circ. 2018;27:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Jeong H, Shim CY, Kim D, Choi JY, Choi KU, Lee SY, Hong GR, Ha JW. Prevalence, Characteristics, and Clinical Significance of Concomitant Cardiomyopathies in Subjects with Bicuspid Aortic Valves. Yonsei Med J. 2019;60:816-823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Brown PS Jr, Roberts CS, McIntosh CL, Roberts WC, Clark RE. Combined obstructive hypertrophic cardiomyopathy and stenotic congenitally bicuspid aortic valve. Am J Cardiol. 1990;66:1273-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Emanuel R, Withers R, O'Brien K, Ross P, Feizi O. Congenitally bicuspid aortic valves. Clinicogenetic study of 41 families. Br Heart J. 1978;40:1402-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 54] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Somerville J, McDonald L. Congenital anomalies in the heart with hypertrophic cardiomyopathy. Br Heart J. 1968;30:713-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Maron MS, Olivotto I, Zenovich AG, Link MS, Pandian NG, Kuvin JT, Nistri S, Cecchi F, Udelson JE, Maron BJ. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation. 2006;114:2232-2239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 732] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 16. | Martínez-Micaelo N, Beltrán-Debón R, Baiges I, Faiges M, Alegret JM. Specific circulating microRNA signature of bicuspid aortic valve disease. J Transl Med. 2017;15:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 17. | Andreassi MG, Della Corte A. Genetics of bicuspid aortic valve aortopathy. Curr Opin Cardiol. 2016;31:585-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Atkins SK, Sucosky P. Etiology of bicuspid aortic valve disease: Focus on hemodynamics. World J Cardiol. 2014;6:1227-1233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Sillesen AS, Vøgg O, Pihl C, Raja AA, Sundberg K, Vedel C, Zingenberg H, Jørgensen FS, Vejlstrup N, Iversen K, Bundgaard H. Prevalence of Bicuspid Aortic Valve and Associated Aortopathy in Newborns in Copenhagen, Denmark. JAMA. 2021;325:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 20. | Morosin M, Leonelli V, Piazza R, Cassin M, Neglia L, Leiballi E, Cervesato E, Barbati G, Sinagra G, Nicolosi GL. Clinical and echocardiographic predictors of long-term outcome of a large cohort of patients with bicuspid aortic valve. J Cardiovasc Med (Hagerstown). 2017;18:74-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Abdulkareem N, Smelt J, Jahangiri M. Bicuspid aortic valve aortopathy: genetics, pathophysiology and medical therapy. Interact Cardiovasc Thorac Surg. 2013;17:554-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Miśkowiec D, Lipiec P, Szymczyk E, Wejner-Mik P, Michalski B, Kupczyńska K, Wierzbowska-Drabik K, Kasprzak JD. Bicuspid aortic valve morphology and its impact on aortic diameters-A systematic review with meta-analysis and meta-regression. Echocardiography. 2018;35:667-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Verma R, Cohen G, Colbert J, Fedak PWM. Bicuspid aortic valve associated aortopathy: 2022 guideline update. Curr Opin Cardiol. 2023;38:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Vis JC, Rodríguez-Palomares JF, Teixidó-Tura G, Galian-Gay L, Granato C, Guala A, Sao-Aviles A, Gutiérrez L, González-Alujas T, García-Dorado D, Evangelista A. Implications of Asymmetry and Valvular Morphotype on Echocardiographic Measurements of the Aortic Root in Bicuspid Aortic Valve. J Am Soc Echocardiogr. 2019;32:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Evangelista Masip A, Galian-Gay L, Guala A, Lopez-Sainz A, Teixido-Turà G, Ruiz Muñoz A, Valente F, Gutierrez L, Fernandez-Galera R, Casas G, Panaro A, Marigliano A, Huguet M, González-Alujas T, Rodriguez-Palomares J. Unraveling Bicuspid Aortic Valve Enigmas by Multimodality Imaging: Clinical Implications. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Lee SY, Shim CY, Hong GR, Seo J, Cho I, Cho IJ, Chang HJ, Ha JW, Chung N. Association of aortic phenotypes and mechanical function with left ventricular diastolic function in subjects with normally functioning bicuspid aortic valves and comparison to subjects with tricuspid aortic valves. Am J Cardiol. 2015;116:1547-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Weismann CG, Lombardi KC, Grell BS, Northrup V, Sugeng L. Aortic stiffness and left ventricular diastolic function in children with well-functioning bicuspid aortic valves. Eur Heart J Cardiovasc Imaging. 2016;17:225-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Robicsek F, Thubrikar MJ, Cook JW, Fowler B. The congenitally bicuspid aortic valve: how does it function? Ann Thorac Surg. 2004;77:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 185] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 29. | Lee SY, Shim CY, Hong GR, Cho IJ, Chang HJ, Ha JW, Chung N. Determinants and Prognostic Significance of Symptomatic Status in Patients with Moderately Dysfunctional Bicuspid Aortic Valves. PLoS One. 2017;12:e0169285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Hardikar AA, Marwick TH. Surgical thresholds for bicuspid aortic valve associated aortopathy. JACC Cardiovasc Imaging. 2013;6:1311-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |