Published online Jun 16, 2023. doi: 10.12998/wjcc.v11.i17.4179
Peer-review started: April 5, 2023
First decision: April 28, 2023
Revised: May 6, 2023
Accepted: May 19, 2023
Article in press: May 19, 2023
Published online: June 16, 2023
Processing time: 67 Days and 19.4 Hours
Patients with chronic inflammatory disorders are at a higher risk of developing aggressive Merkel cell carcinoma (MCC). Diabetes is a common chronic inflammatory disease that is possibly associated with MCC; however, there are still no reports on the association between hepatitis B virus (HBV) infection and MCC. Whether there is an association between these three diseases and the specific mechanisms behind their effects is worth further research in the future.
We herein report a rare case of MCC with extracutaneous and nodal invasion in an Asian individual with type 2 diabetes mellitus and chronic HBV infection, but no immunosuppression or other malignancies. Such cases are uncommon and have rarely been reported in the literature. A 56-year-old Asian male presented with a significant mass on his right cheek and underwent extensive resection combined with parotidectomy, neck lymphadenectomy, and split-thickness skin grafting. Based on the histopathological findings, a diagnosis of MCC involving the adipose tissue, muscle, nerve, and parotid gland with lymphovascular invasion was made. Subsequently, he received radiotherapy with no adverse reactions.
MCC is a rare, aggressive skin cancer with frequent local recurrence, nodal inv
Core Tip: Merkel cell carcinoma in a person of the yellow race with type 2 diabetes mellitus and chronic hepatitis B virus infection but no immunosuppression or other malignancies is a rare case and has rarely been reported in the literature.
- Citation: Ren MY, Shi YJ, Lu W, Fan SS, Tao XH, Ding Y. Facial Merkel cell carcinoma in a patient with diabetes and hepatitis B: A case report. World J Clin Cases 2023; 11(17): 4179-4186
- URL: https://www.wjgnet.com/2307-8960/full/v11/i17/4179.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i17.4179
Merkel cell carcinoma (MCC) is an aggressive cutaneous neuroendocrine cancer with frequent local recurrence, nodal invasion, and metastasis, with an increasing annual incidence of 0.6 cases per 100000. By 2025, the United States is expected to have 3000 cases per year[1]. Moreover, the incidence of MCC is highest among individuals of the white race than among those of other races. MCC affects men more frequently than women in Northern Europe. However, due to the limited number of reported cases, there is a lack of epidemiological research on MCC in Africa and Asia. The case reported here was a Chinese male with MCC who did not receive any immunosuppressive treatment and had type 2 diabetes mellitus (T2DM) and chronic hepatitis B virus (HBV) infection, which may help address the lack of MCC data in Asian patients.
MCC usually manifests as a single asymptomatic red-to-purple nodule on sun-exposed areas and predominantly affects older adults with white skin. We reported the case of an East Asian with MCC with a 4.5-cm diameter mass that invaded the subcutaneous adipose tissue, muscle, nerve, and parotid gland.
A 56-year-old man presented with a painless, solitary, firm skin neoplasm on the right cheek.
The lesion initially presented as a small pimple 7 mo earlier but rapidly grew to a 4.5-cm diameter mass over the last 2 mo with malodorous discharge and exudation.
The patient denied any history of trauma at the site. His medical history included hypertension, T2DM, and chronic HBV infection with irregular medication. His blood glucose was notably poorly controlled; the postprandial blood glucose level fluctuated at 15–20 mmol/L. He also had hepatic insufficiency; his HBV-DNA level was 7.2 × 104 IU/mL.
There were no obvious abnormalities in the patient’s personal and family history.
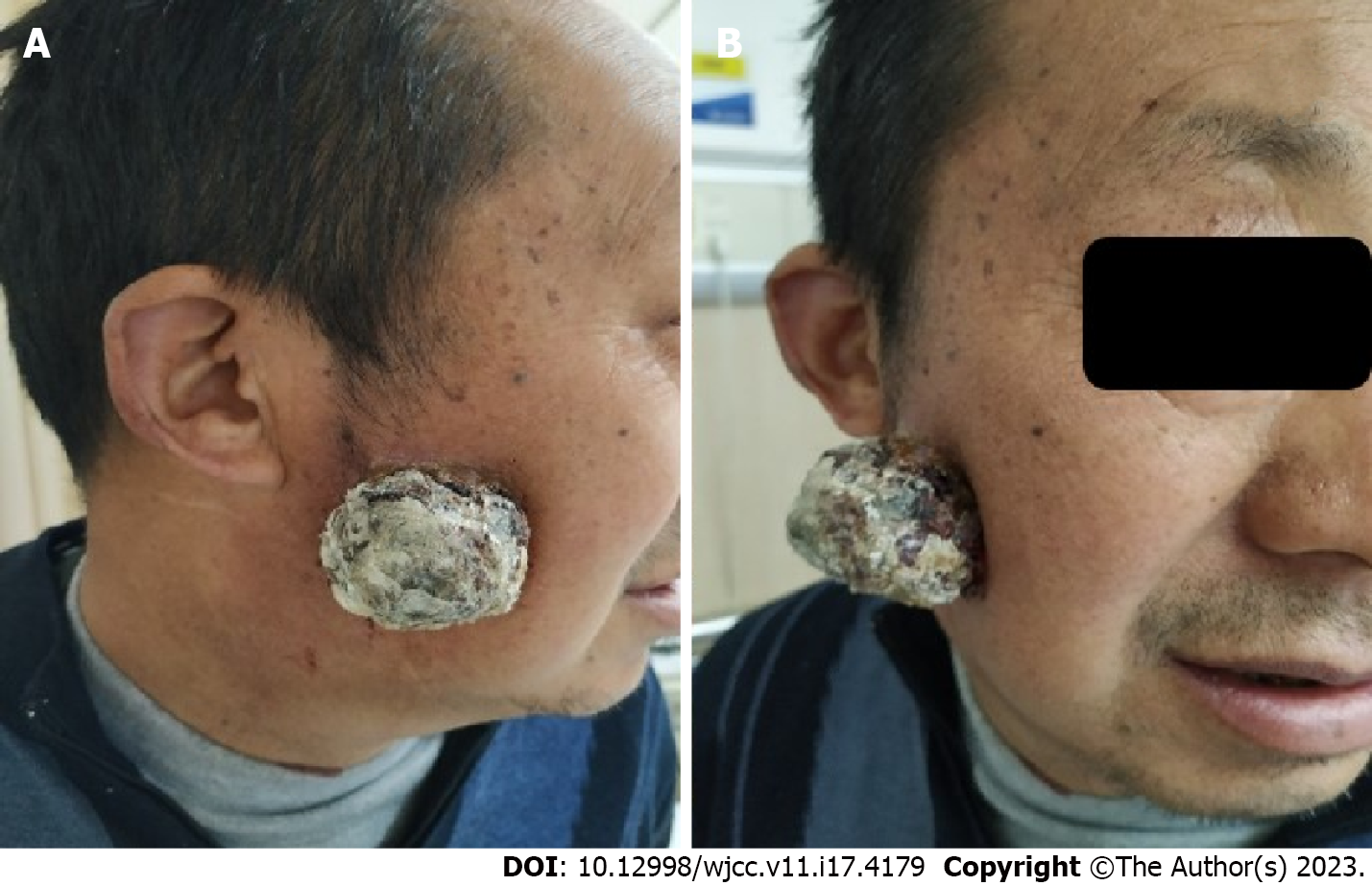
His physical examination revealed a 4.5 cm × 4.5 cm mass with an irregular surface and foul-smelling discharge on the right cheek (Figure 1).
The patient had T2DM and chronic HBV, infection; however, no obvious abnormalities were found in other laboratory examinations. Subsequently, the mass was subjected to fine-needle aspiration cytology, which revealed a substantial amount of poorly differentiated tumor cells (Figure 2).
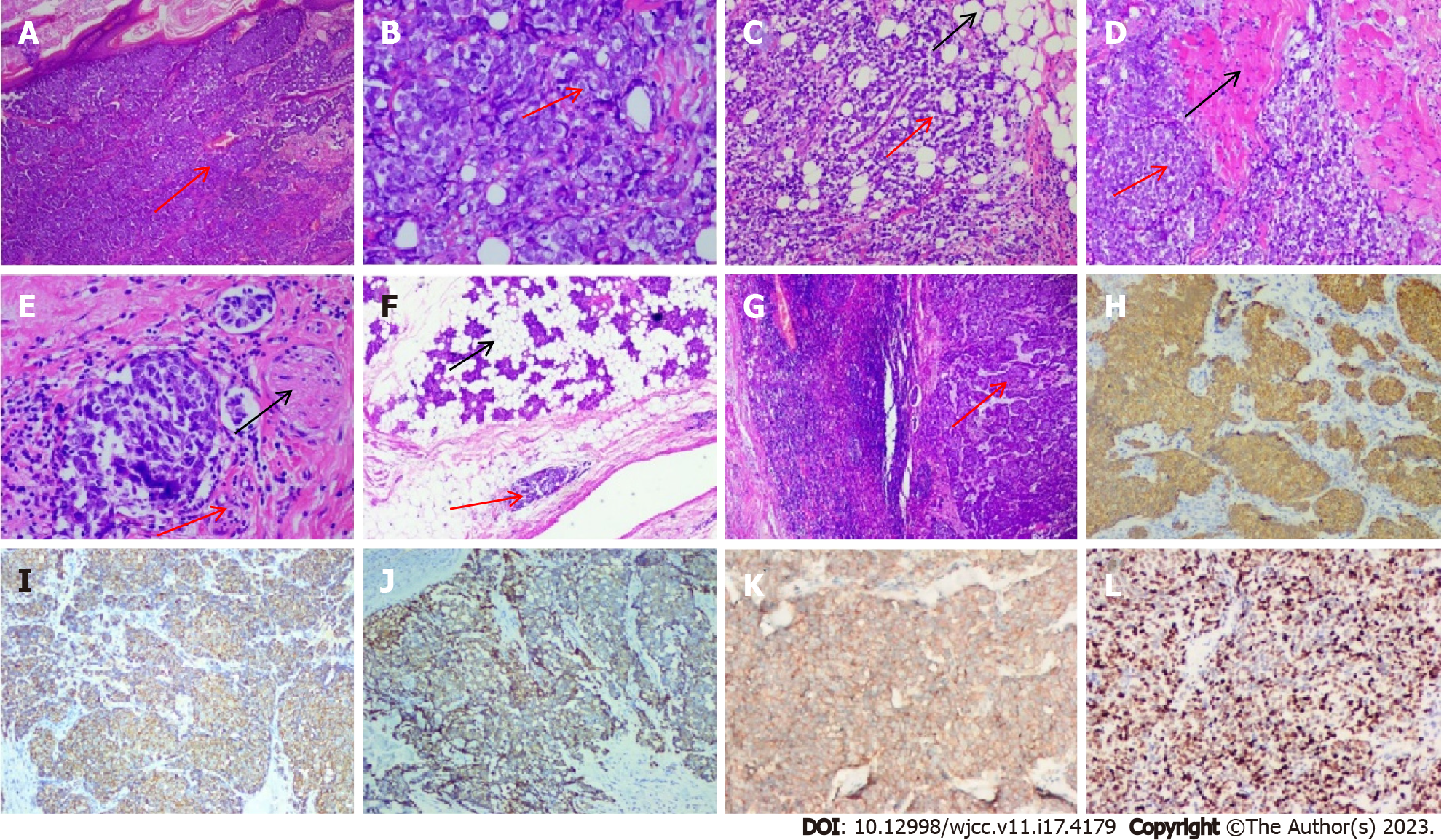
Intraoperative frozen and rapid paraffin sections displayed small round blue cell tumors, consistent with MCC, which invaded the adipose tissue, muscle, nerve, and parotid gland, with negative margins and positive lymphovascular invasion (Figure 3). Therefore, the right facial mass was believed to be a malignant tumor, a poorly differentiated carcinoma. The postoperative examination of lymph nodes in zones 1–5 and submental and submandibular regions revealed that the submandibular lymph node (9/18) had metastatic cancer, whereas the “lymph node in zone 1” (0/1), “lymph node in zone 2” (0/13), “lymph node in zone 3” (0/1), “lymph node in zone 4” (0/7), “lymph node in zone 5” (0/9), and “submental lymph node” (0/1) showed no evidence of cancer metastasis. Considering the detection of cancer, the fact that the patient had simultaneous regional lymph node metastasis (stage III) and that pathological TNM suggested T2NIM0, we did not test this patient for Merkel cell polyomavirus due to limited resources.
Immunohistochemical staining revealed that the cancer cells were positive for cytokeratin 20 (CK20), CK (Pan), CD56, CgA, and Syn (cytokeratin, a cluster of differentiation, chromogranin, synaptophysin for CK, CD, CgA, and Syn) (Figure 3). The proliferative activity of Ki-67 reached approximately 90%.
Computed tomography (CT) scans of the head and neck demonstrated a bulky right mass with central necrosis and cervical lymphadenopathy (Figure 4). Further CT scans of the chest and abdomen revealed no evidence of distant metastases. Enhanced magnetic resonance imaging of the face and the cervical region was also performed, and postoperative changes were noted. The CT scans of the chest and abdomen revealed no distant metastasis. Unfortunately, the patient refused chemotherapy for financial reasons.
MCC, T2DM, chronic HBV infection, hypertension.
Extensive resection combined with parotidectomy, cervical lymphadenectomy, and split-thickness skin grafting was planned. At 6 wk postoperatively, the surgical area received 6 MV electronic wire radiotherapy (50 Gy/25 fractions). We actively controlled the patient’s blood sugar level and protected liver function simultaneously. The patient did not complain of discomfort during the radiation treatment.
The patient was followed up 4 mo postoperatively at the hospital. The wound appeared to heal well. The patient is still under careful follow-up. Until now, he has been in good health and did not report any adverse effects.
MCC is an aggressive neuroendocrine skin cancer commonly found in the head and neck, which is very rare clinically, with 98% of MCCs occurring in people of the white race; 81% of cases occur in skin exposed to the sun. Light-colored skin is a strong risk factor for the development of MCC; thus, skin pigmentation appears to be a protective factor for MCC, as people of the Black, Asian, and Hispanic race are at a lower risk than those of the white race[2].
MCC cases in Asian people have been infrequently reported previously. MCC often presents as a rapidly growing, painless, solitary, intracutaneous nodule with a hard texture on sun-exposed skin among older people. The mass typically develops in the reticular dermis or subcutis and rarely involves the papillary dermis or epidermis[3]. The size of the mass ranges from < 1 cm to > 2 cm; ulceration and crusting are uncommon. However, epidermotropism (invasion of the epidermis by tumor cells) with a tumor diameter of > 2 cm and rough surface has neem reported in 10% of cases[4].
Merkel cell polyomavirus, ultraviolet radiation, and immunosuppression (including organ trans
Vascular endothelial growth factors (VEGF) are specific mitogens for endothelial cells that play a critical role in angiogenesis and lymphangiogenesis. Moreover, increased expression of intratumoral VEGF encourages angiogenesis within the MCC environment, resulting in a higher risk of metastasis and worse recurrence and overall survival[13]. Similarly, the VEGF levels are significantly elevated in patients with T2DM.
Toll-like receptors (TLRs) are important components of the innate immune system, acting as the first line of defense against pathogens. TLRs are expressed in several innate immune cells and other tissues. Alterations in TLR expression are associated with clinicopathological and prognostic markers of MCCs[14]. Moreover, TLRs are associated with the pathogenesis of diabetes.
Chronic inflammation may play an immunomodulatory role in the pathogenesis of MCC and increase susceptibility to oncogenesis, possibly via tumor surveillance mechanisms. Diabetes is a common chronic inflammatory disease, and diabetes is possibly associated with MCC[15]. This association could explain the recent rise in MCC rates; however, the studies have only focused on people of the white race. Patients with diabetes have a lower risk of cutaneous cancer than those without diabetes[16], which contradicts the previously established theory. Although there are recent reports on the association between diabetes and MCC, there are none on that between HBV infection and MCC. Whether there is an association between these three diseases and the specific mechanisms behind their effects is worth further research in the future.
Both microscopic examinations and immunohistochemical profiles are usually required to form a diagnosis. MCC is composed of monotonously uniform, small, round, blue cells, containing large hyperchromatic nuclei with powdery chromatin, multiple small nucleoli, and sparse cytoplasm. However, the MCC lesions are asymptomatic red-to-purple nodules that may be clinically misinterpreted as benign lesions (e.g., cysts or infectious or inflammatory lesions) or other malignant lesions (e.g., cutaneous squamous cell carcinoma, lymphoma, or metastases)[17].
The clinical diagnosis of MCC is often delayed due to the non-specificity of the clinical presentation. The acronym AEIOU (asymptomatic/lack of tenderness, expanding rapidly, immune suppression, older than 50 years, and ultraviolet-exposed site on a person with fair skin) has been used to recall the associated clinical features of MCC in patients[17].
The diagnosis of MCC can be confirmed by immunohistochemical examination. CK20 is a comparatively specific and sensitive indicator of MCC, whereas low-differentiated MCCs can lack the CK20 marker. Negative thyroid transcription factor-1 stains and positive chromogranin A, synaptophysin, or CD56 (nerve cell adhesion molecules) stains distinguish MCCs from small-cell carcinoma[18].
For the treatment of local/regional MCC, excision of the primary tumor should be preferred. To stage and guide subsequent treatment, sentinel lymph node biopsy (SLNB) should be performed at the time of surgery. Negative SLNB, radiation to the primary site, and the regional lymph node basin should be followed. Positive SLNB, lymphadenectomy, and/or radiotherapy should be performed[19], as this neuroendocrine tumor is highly radio-sensitive. Radiotherapy has been shown to improve locoregional control and reduce recurrence rates, although it may not have a significant effect on overall survival[20]. In cases where a tumor cannot be surgically removed, radiotherapy alone can still be effective in achieving locoregional control[21].
No curative treatment has been found yet for metastatic MCC. Although MCC is sensitive to chemotherapy, it is a short-term control method and the response is not durable. Furthermore, the relevant toxicity may reduce overall survival[22].
Immune checkpoint blockade therapy is the latest, rapidly expanding treatment for advanced MCC. Immune checkpoint inhibitors can attain durable responses by reactivating antitumor cellular immune responses[23]. Anti-programmed death-1 antibodies and anti-programmed death ligand-1 monoclonal antibodies have demonstrated potential in the treatment of advanced-stage or chemotherapy-resistant metastatic MCC[24,25]. However, previous studies have revealed that the anti-programmed death-1 antibodies and anti-programmed death ligand-1 monoclonal antibodies therapy can result in immune-related adverse effects, which may be fatal[26,27]. Therefore, there is a critical and unmet requirement to validate biomarkers that can predict the response to treatment in MCC.
Due to the rarity and lack of research regarding diabetes mellitus, HBV infection, and MCC, there is no certain prognosis and agreement on optimal treatment. Furthermore, whether patients with diabetes mellitus or chronic HBV infection can tolerate radiotherapy is unknown. However, the case presented in this report tolerated radiation well and is in good general health currently.
MCC is a rare, aggressive skin cancer with frequent local recurrence, nodal invasion, and metastasis, which usually arises in older people of the white race. Patients with chronic inflammatory disorders are at a higher risk of developing aggressive MCC. The diagnosis can be confirmed with histology and immunohistochemistry. For localized MCC, surgery is the preferred treatment option. However, for advanced MCC, radiotherapy and chemotherapy have proven to be effective. In cases where chemotherapy is not effective or in the advanced stages of MCC, immune therapy plays an important role in treatment. As with any rare disease, the management of MCC remains an enormous challenge for clinicians; thus, follow-up should be individualized and future progress needs multidisciplinary collaborative efforts. Furthermore, physicians should include MCC in their list of possible diagnoses when they come across painless, rapidly growing lesions, particularly in patients with chronic HBV infection or diabetes, as these patients are more susce
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Handra-Luca A, France; Hegazy AA, Egypt S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Villani A, Fabbrocini G, Costa C, Carmela Annunziata M, Scalvenzi M. Merkel Cell Carcinoma: Therapeutic Update and Emerging Therapies. Dermatol Ther (Heidelb). 2019;9:209-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 2. | Becker JC, Stang A, Hausen AZ, Fischer N, DeCaprio JA, Tothill RW, Lyngaa R, Hansen UK, Ritter C, Nghiem P, Bichakjian CK, Ugurel S, Schrama D. Epidemiology, biology and therapy of Merkel cell carcinoma: conclusions from the EU project IMMOMEC. Cancer Immunol Immunother. 2018;67:341-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 3. | Hernandez LE, Mohsin N, Yaghi M, Frech FS, Dreyfuss I, Nouri K. Merkel cell carcinoma: An updated review of pathogenesis, diagnosis, and treatment options. Dermatol Ther. 2022;35:e15292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Calder KB, Smoller BR. New insights into merkel cell carcinoma. Adv Anat Pathol. 2010;17:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Tadmor T, Aviv A, Polliack A. Merkel cell carcinoma, chronic lymphocytic leukemia and other lymphoproliferative disorders: an old bond with possible new viral ties. Ann Oncol. 2011;22:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Manganoni MA, Farisoglio C, Tucci G, Venturini M, Marocolo D, Aquilano MC, El-Hamad I, Ferrari VD, Calzavara Pinton PG. Merkel cell carcinoma and HIV infection: a case report and review of the literature. AIDS Patient Care STDS. 2007;21:447-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Heath M, Jaimes N, Lemos B, Mostaghimi A, Wang LC, Peñas PF, Nghiem P. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 728] [Cited by in RCA: 603] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 8. | Ziprin P, Smith S, Salerno G, Rosin RD. Two cases of merkel cell tumour arising in patients with chronic lymphocytic leukaemia. Br J Dermatol. 2000;142:525-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Becker JC, Stang A, DeCaprio JA, Cerroni L, Lebbé C, Veness M, Nghiem P. Merkel cell carcinoma. Nat Rev Dis Primers. 2017;3:17077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 387] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 10. | Sahi H, Sihto H, Artama M, Koljonen V, Böhling T, Pukkala E. History of chronic inflammatory disorders increases the risk of Merkel cell carcinoma, but does not correlate with Merkel cell polyomavirus infection. Br J Cancer. 2017;116:260-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Li Y, Qu T, Sun Q, Mao X, Fang K. Merkel cell carcinoma of the earlobe in a diabetes mellitus patient. Chin Med J (Engl). 2014;127:1596. [PubMed] |
| 12. | Cestaro G, Festa P, Cricrì AM, Antropoli M, Castriconi M. Unexpected histopathologic result of a wide surgical excision of a bleeding lesion of the skin: a case of Merkel cell carcinoma of the leg. G Chir. 2015;36:231-235. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13:871-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 799] [Cited by in RCA: 957] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 14. | El-Zayat SR, Sibaii H, Mannaa FA. Toll-like receptors activation, signaling, and targeting: An overview. Bull Natl Res Centre. 2019;43:187. [DOI] [Full Text] |
| 15. | Samuel Duek O, Ben Naftali Y, Ullmann Y. [Merkel cell carcinoma: a retrospective study]. Harefuah. 2020;159:550-553. [PubMed] |
| 16. | Starup-Linde J, Karlstad O, Eriksen SA, Vestergaard P, Bronsveld HK, de Vries F, Andersen M, Auvinen A, Haukka J, Hjellvik V, Bazelier MT, Boer Ad, Furu K, De Bruin ML. CARING (CAncer Risk and INsulin analoGues): the association of diabetes mellitus and cancer risk with focus on possible determinants - a systematic review and a meta-analysis. Curr Drug Saf. 2013;8:296-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Coggshall K, Tello TL, North JP, Yu SS. Merkel cell carcinoma: An update and review: Pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78:433-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 132] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 18. | Leech SN, Kolar AJ, Barrett PD, Sinclair SA, Leonard N. Merkel cell carcinoma can be distinguished from metastatic small cell carcinoma using antibodies to cytokeratin 20 and thyroid transcription factor 1. J Clin Pathol. 2001;54:727-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 100] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Lango M, Shnayder Y. Surgical Management of Merkel Cell Carcinoma. Otolaryngol Clin North Am. 2021;54:357-368. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Strom T, Naghavi AO, Messina JL, Kim S, Torres-Roca JF, Russell J, Sondak VK, Padhya TA, Trotti AM, Caudell JJ, Harrison LB. Improved local and regional control with radiotherapy for Merkel cell carcinoma of the head and neck. Head Neck. 2017;39:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Stachyra K, Dudzisz-Śledź M, Bylina E, Szumera-Ciećkiewicz A, Spałek MJ, Bartnik E, Rutkowski P, Czarnecka AM. Merkel Cell Carcinoma from Molecular Pathology to Novel Therapies. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Patel P, Hussain K. Merkel cell carcinoma. Clin Exp Dermatol. 2021;46:814-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9936] [Cited by in RCA: 10282] [Article Influence: 790.9] [Reference Citation Analysis (34)] |
| 24. | Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, Berry S, Chartash EK, Daud A, Fling SP, Friedlander PA, Kluger HM, Kohrt HE, Lundgren L, Margolin K, Mitchell A, Olencki T, Pardoll DM, Reddy SA, Shantha EM, Sharfman WH, Sharon E, Shemanski LR, Shinohara MM, Sunshine JC, Taube JM, Thompson JA, Townson SM, Yearley JH, Topalian SL, Cheever MA. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N Engl J Med. 2016;374:2542-2552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 974] [Cited by in RCA: 969] [Article Influence: 107.7] [Reference Citation Analysis (0)] |
| 25. | Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D'Angelo SP, Shih KC, Lebbé C, Linette GP, Milella M, Brownell I, Lewis KD, Lorch JH, Chin K, Mahnke L, von Heydebreck A, Cuillerot JM, Nghiem P. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17:1374-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 830] [Cited by in RCA: 945] [Article Influence: 105.0] [Reference Citation Analysis (0)] |
| 26. | Nishino M, Ramaiya NH, Awad MM, Sholl LM, Maattala JA, Taibi M, Hatabu H, Ott PA, Armand PF, Hodi FS. PD-1 Inhibitor-Related Pneumonitis in Advanced Cancer Patients: Radiographic Patterns and Clinical Course. Clin Cancer Res. 2016;22:6051-6060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 355] [Article Influence: 39.4] [Reference Citation Analysis (1)] |
| 27. | Spallarossa P, Meliota G, Brunelli C, Arboscello E, Ameri P, Dessalvi CC, Grossi F, Deidda M, Mele D, Sarocchi M, Bellodi A, Madonna R, Mercuro G. Potential cardiac risk of immune-checkpoint blockade as anticancer treatment: What we know, what we do not know, and what we can do to prevent adverse effects. Med Res Rev. 2018;38:1447-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |