Published online Jun 16, 2023. doi: 10.12998/wjcc.v11.i17.4168
Peer-review started: March 23, 2023
First decision: April 26, 2023
Revised: May 1, 2023
Accepted: May 15, 2023
Article in press: May 15, 2023
Published online: June 16, 2023
Processing time: 81 Days and 1.5 Hours
The use of advanced platelet-rich fibrin (A-PRF) membranes for guided bone and tissue regeneration in through-and-through defects after endodontic surgery was explored in three cases.
Herein, three patients presented to the endodontic clinic suffering from apical periodontitis, associated with large bone resorption and related to previously endodontically treated teeth. Periapical surgery was indicated in these cases and the osteotomy site was covered by A-PRF membrane. Cone-beam computed tomography (CBCT) was used to assess the cases before and after the surgery.
Four months post-surgery, the recall CBCT scan showed complete obliteration of the osteotomy with newly formed bone. A-PRF membrane showed promising results and was an advantageous addition to surgical endodontic treatment.
Core Tip: The use of advanced platelet-rich fibrin (A-PRF) membrane in endodontic surgery in conjunction with cone-beam computed tomography helped establish bone healing after short-term follow-up. The A-PRF membrane’s low expense, low risk of post-surgical infection, and foreign body rejection makes it a useful adjunct to surgical endodontic treatment.
- Citation: Algahtani FN, Almohareb R, Aljamie M, Alkhunaini N, ALHarthi SS, Barakat R. Application of advanced platelet-rich fibrin for through-and-through bony defect during endodontic surgery: Three case reports and review of the literature. World J Clin Cases 2023; 11(17): 4168-4178
- URL: https://www.wjgnet.com/2307-8960/full/v11/i17/4168.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i17.4168
The major reason for failed periapical healing after primary endodontic therapy is the presence of bacteria and diseased tissue[1]. Orthograde retreatment is the first treatment of choice for such cases. However, there are situations where it is not feasible, too complicated or unsuccessful. In such circumstances, apical surgery has proved to be a sound treatment option to save the tooth[2].
Modern technology, materials, and techniques have improved the prognosis of endodontic surgery[2]. Cone-beam computed tomography (CBCT) enabled more accurate determination of the root anatomy and its proximity to critical anatomical structures such as the inferior alveolar nerve which led to better planning of the surgical approach[3]. When the root-end resections and retrograde fillings are performed using microsurgical instruments with current materials and techniques, the success rates was 85% to 97%[4].
In certain clinical situations where lesions have eroded the lingual/palatal cortex (with or without erosion of buccal cortex), resulting in a through-and-through (tunnel) defect, the success of endodontic surgery is less predictable[5-8]. Since the healing usually results in the development of scar tissue in the post-operative area[5-7].
Mesenchymal stem cells are pluripotent cells that have the ability to differentiate into osteoblasts thereby promoting bone healing. This often depends on an adequate stimulator of stem cell gene expression, as well as the presence of platelet derivatives which include within them various pro-inflammatory markers that direct the cells’ maturation. Local administration of hormones, growth factors, ascorbic acid. Plasma derivatives has been advised to promote bone regeneration and soft tissue repair after oral surgery[9,10]. Moreover, bone morphogenic proteins, parathyroid hormone, and enamel matrix proteins have been administered locally to increase the healing capacity of the surgical site[9,11]. However, their usefulness in endodontic surgery is still debatable, and the benefits they bring to both surgeon and patient are modest and debatable[12-14].
Due to the local and continuous release of growth factors and proteins, platelet-rich fibrin (PRF) is frequently utilized to stimulate and accelerate soft tissue and bone healing. This mimics the natural wound healing and reparative tissue processes[15]. Because it is entirely composed of the patient’s blood, PRF acts as a reservoir for growth factors that could be used without exposing the patient to immunogenicity or infection risks[16].
The use of such a specialized biomaterial in endodontic surgery has been discussed in case reports and a randomized clinical trial[17,18]. However, many of these studies excluded through-and-through bony defects[17,18]. Furthermore, many of these cases were assessed and evaluated solely through the use of periapical radiographs[19,20]. As a result, the current study examined three clinical cases in which CBCT was used to assess the success of advanced platelet-rich fibrin (A-PRF) membrane for bone regeneration in through-and-through bony defects following periapical endodontic surgery.
First case: A 32-years-old female, medically fit and unaware of any allergies, was referred to the endodontic clinic for the assessment and treatment of the maxillary right second premolar. The patient complained of persistent pain while chewing, localized to the area of that tooth. The pain intensity was six out of ten according to the verbal numerical rating scale (VNRS).
Second case: A 24-year-old female patient was referred to the endodontic clinic for the assessment and treatment of the maxillary left second premolar. She was medically fit and unaware of any allergies.
Third case: A medically fit, 55 years-old female patient was referred to the endodontic clinic for assessment and treatment of the maxillary right lateral incisor.
First case: Root canal treatment (RCT) had been performed on that tooth several weeks ago, but it did not reduce the symptoms. She was prescribed 500 mg amoxicillin (500 mg) to be taken orally every eight hours with analgesics for five days. However, this measure did not help reduce the symptoms.
Second case: The patient received an RCT on her left maxillary second premolar a year ago, but was continuously complaining of mild pain while chewing, related to that tooth. The pain intensity was four out of ten according to the VNRS.
Third case: The patient had all ceramic dental crowns on the maxillary anterior teeth, placed three to five years ago.
First case: Upon clinical examination, the tooth had a permanent composite core restoration. It did not respond to the cold test, but was tender to the palpation and percussion tests. Periodontal probing was within the normal range.
Second case: Upon clinical examination, the tooth was restored with a porcelain fused to metal crown. Periodontal probing was within the normal range. It did not respond to the cold test, but was tender to percussion.
Third case: The tooth responded negatively to cold, percussion, and palpation. The radiographic examination revealed a localized radiolucency surrounding the apex of the maxillary right lateral incisor (Table 1). The tooth had an adequate RCT, a metallic post, and a ceramic crown. The radiographic quality of the recently performed RCT and crown were adequate
| Tooth No. | Endodontic diagnosis | Coronal coverage | Periapical index using CBCT 47 (before surgery) | Periapical index using CBCT 47 (four months recall) |
| #15 | Previously treated, symptomatic apical periodontitis | Fiber post and composite core build-up | 5 + D: Cortical bone destruction | 0: Intact periapical bone structure |
| #25 | Previously treated, symptomatic apical periodontitis | PFM crown | 4 + E: Cortical bone expansion | 0: Intact periapical bone structure |
| #12 | Previously treated, asymptomatic apical periodontitis | Metallic post and ceramic crown | 5 + D: Cortical bone destruction | 0: Intact periapical bone structure |
First case: The periapical radiograph showed a localized radiolucency surrounding the apex of the tooth, an overextended root canal filling in the buccal canal, and a fiber post-supported core restoration (Table 1).
Second case: The periapical radiograph showed a localized radiolucency surrounding the apex and the tooth had two canals (Table 1).
Third case: The radiographic examination revealed a localized radiolucency surrounding the apex of the maxillary right lateral incisor (Table 1). The tooth had an adequate RCT, a metallic post, and a ceramic crown. The radiographic quality of the recently performed RCT and crown were adequate.
The endodontic diagnosis was previously treated with symptomatic apical periodontitis.
The endodontic diagnosis was previously treated with symptomatic apical periodontitis.
The endodontic diagnosis was previously treated with asymptomatic apical periodontitis.
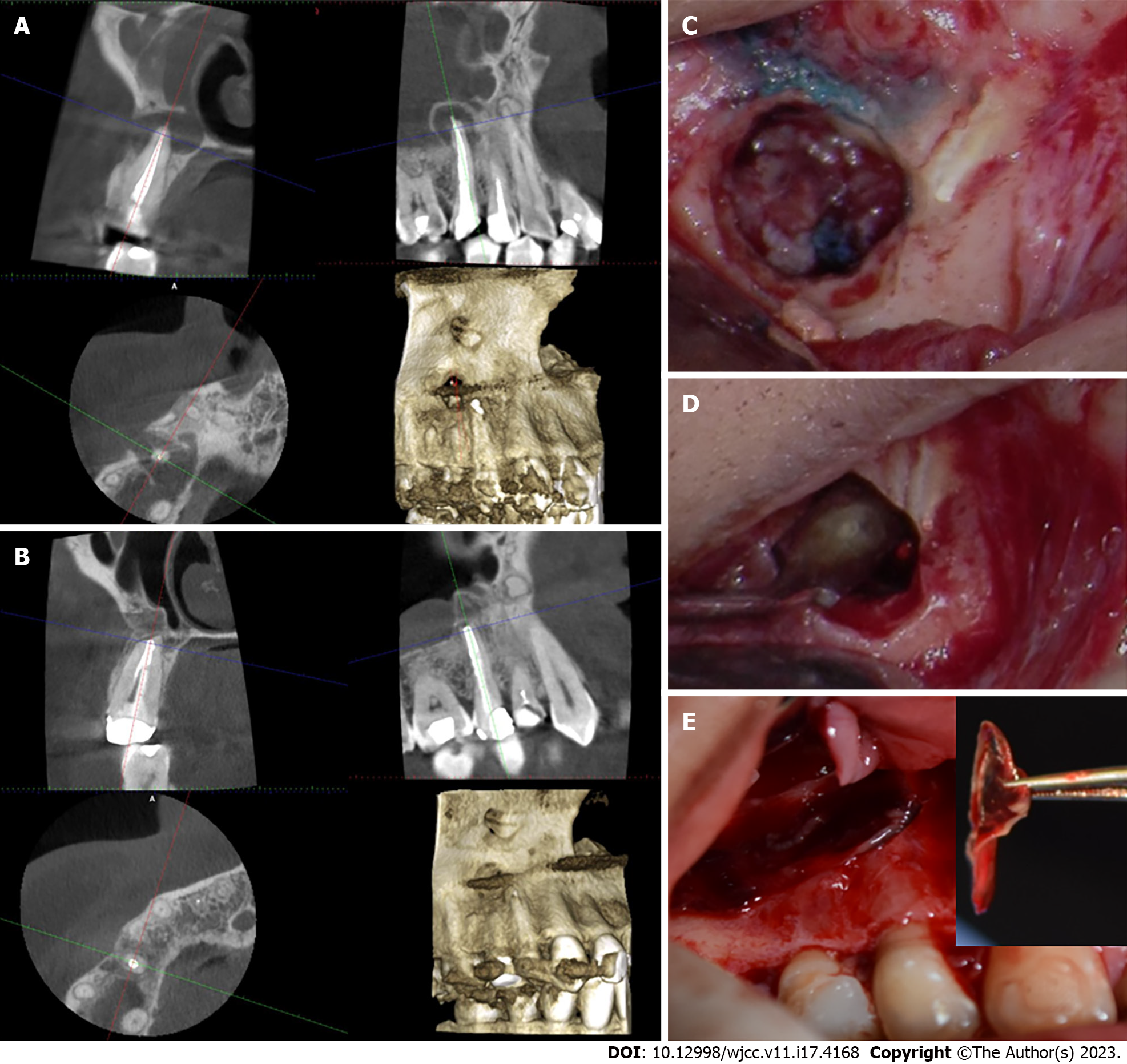
Treatment options were discussed with the patient, and they included orthograde retreatment, periapical surgery, extraction, and no treatment. After the risks and benefits of each option were considered, the treatment of choice was periapical surgery. Small field of view (FOV) CBCT imaging was taken for the area and endodontic microsurgical protocol was followed (Table 2, Figure 1). The osteotomy site was covered by A-PRF membrane (Figure 1). The histological examination revealed granulation tissue. The patient responded negatively to the percussion and palpation tests two weeks and six months after the surgery.
| Treatment phase | Steps performed |
| Before the surgery | The tooth is examined clinically and radiographically to assess the source of the pain and to diagnose the diseased tooth. Following the decision to perform surgical periapical treatment a CBCT scan was performed under the following standardized setting for a small field of view (90 kV, 6 mA, 5.0 cm × 5.5 cm, 160 μm, and 14 s). Using Planmeca device (ProMax 3D Mid, Helsinki, Finland) |
| The treatment risks and benefits, and prognosis were discussed with the patient. The patient was asked to sign the surgical endodontic treatment consent form | |
| A clinical photograph of the surgical area is taken for surgical planning | |
| The CBCT scan is analyzed for the following details and measurements: (1) The quality of root canal obturation and the canal location; (2) the size of the lesion and its proximity to adjacent vital structures such as the maxillary sinus, nerves, and blood vessels; (3) the length of the dental root and the area of resection; (4) the thickness of the buccal plate; and (5) the condition of the dental supporting structure | |
| Surgical planning involved: The type and amount of local anesthesia, the design of the flap, the location and depth of osteotomy, the angle and length of root resection, the retro-filling material, the need for guided tissue regeneration, and the sutures type and technique | |
| During the surgery | Based on the medical history and patient weight, the patient received local anesthesia to achieve analgesia and vasoconstriction |
| The use of a microsurgical blade for the intrasulcular/submarginal incision and a 15c blade for the vertical incision. The vertical incision line is made over the healthy bone to include or exclude the papilla and frenum | |
| The use of a mucoperiosteal elevator to detach the dental flap from the underlying cortical bone while using a technique that minimizes trauma to the reflected flap | |
| The use of rear venting contra-angle handpiece and round bur to prepare the osteotomy while using a copious amount of water to prevent bone necrosis and trauma | |
| The use of the dental operating microscope and methylene blue to differentiate between the root and dental supporting structures and to detect any cracks | |
| The use of a long shank straight diamond or carbide bur to resect the apical 3 mm root tip in a perpendicular direction to the long access of the tooth | |
| The use of the back of a dental surgical curette to scrap and detach the lesion from the bone as a whole or in pieces | |
| The detached lesion is placed inside a 10% buffered formalin container to be sent for histological examination | |
| The area is filled with a hemostatic agent; cotton pellets filled with a high concentration of epinephrine or ferric sulphate. The hemostatic agent is properly removed before the end of the surgery | |
| The retro space is prepared using retrograde piezo ultrasonic tips to prepare a 3 mm retro space inside the canal | |
| The retro space is filled with a bioceramic material using a material carrier and a micro-plugger | |
| A dental radiograph of the surgical site is taken to determine the quality of root resection and retro filling | |
| The PRF membrane is prepared by withdrawing 10 mL of the patient blood within 15 s and placing the blood inside the A-PRF tube. The tube is inserted into the PRF centrifuge (PRF DUO Quatro Centrifuge, Biomedent, New South Wales, and Australia) in the setting recommended for A-PRF (1300 RPM for 14 min). After the centrifugation, the tube is placed in the tube holder for ten minutes. Then A-PRF clot is removed from the tube and placed inside the PRF box to be compressed by the tray for one minute to take the shape of a membrane. The membrane is then used to cover the osteotomy before suturing the flap. In the few cases where a bone graft is needed to support the membrane, Bio-gen cancellous granules xenograft (Biotech company, Italy) is used and mixed with the collected concentrated A-PRF blood clot. Subsequently, the xenograft is placed inside the osteotomy before covering the osteotomy with the membrane | |
| The flap is positioned back into its original position and is hydrated using sterile wet gauze. A non-resorbable monofilament suture is used to secure the flap into the original place using a reverse cutting needle that is 4-0 to 5-0 in diameter | |
| The sutured flap is gently compressed for few minutes using sterile wet gauze to reduce the bleeding and swelling | |
| After the surgery | The patient is instructed to use an ice pack in the first eight hours for twenty minutes on and twenty minutes off. Then the patient is asked to apply wet warm towel intermittently for the next three days |
| The patient is asked to minimize talking, avoid drinking hot drinks and food, and limit exercising in the first week. The dental cleaning has to be gentle and limited to the coronal area | |
| Analgesics are prescribed to manage pain in the first week after the surgery | |
| The patient is scheduled to be seen after one week for suture removal and to examine the surgical site. The patient first recall is four months after the surgery and annually |
After discussing the endodontic treatment options with the patient, which included: Orthograde retreatment, periapical surgery, extraction, and no treatment. The treatment of choice was periapical surgery, since the radiographic quality of the recently performed RCT was adequate, in addition, the tooth had been recently restored with an adequate crown. Small FOV CBCT imaging was taken for the area and endodontic microsurgical protocol was followed (Table 2, Figure 2). Upon accessing the root tip, the osteotomy site was void and filled with a small amount of blood. The osteotomy was proximal to the maxillary Schneider membrane. After the placement of MTA retro-filling material, the osteotomy site was covered by plasma and A-PRF membrane (Figure 2). A histological examination revealed a traumatic bone cyst. The patient responded negatively to the percussion and palpation tests two weeks after the surgery and at the four months follow up. The recall CBCT scan four months after the surgery showed obliteration of the osteotomy with newly formed bone. The patient was content that the procedure went well, and the prognosis was favorable.
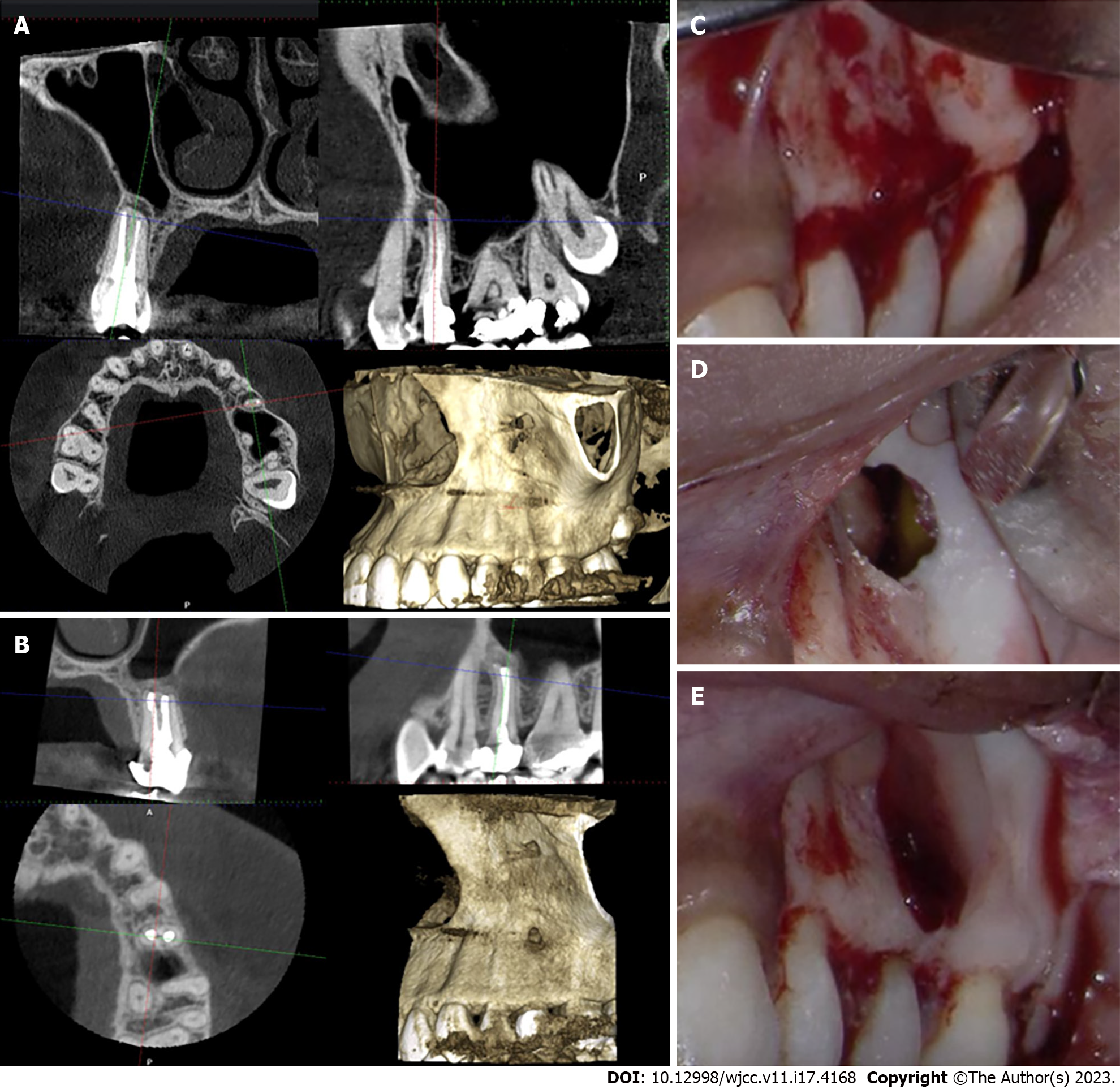
The patient was presented with the risks and benefits of the treatment options: Orthograde retreatment, periapical surgery, extraction, and no treatment. The patient opted for periapical surgery. Small FOV CBCT imaging was taken for the area and endodontic microsurgical protocol was followed (Table 2, Figure 3). The osteotomy was closed with a bone graft and A-PRF membrane. The histological examination revealed a radicular cyst. The patient responded negatively to the percussion and palpation tests two weeks and six months post- surgery. The recall CBCT scan four months after the surgery showed obliteration of the osteotomy with bone (Figure 3). The patient was content that the procedure went well, and the prognosis was favorable.
The recall CBCT scan four months after the surgery showed obliteration of the osteotomy with newly formed bone (Figure 1). The patient was advised to have a permanent crown placed immediately. The patient was content that the procedure went well, and the tooth has favorable prognosis.
The recall CBCT scan four months after the surgery showed obliteration of the osteotomy with newly formed bone. The patient was content that the procedure went well, and the prognosis was favorable.
The recall CBCT scan four months after the surgery showed obliteration of the osteotomy with bone (Figure 3). The patient was content that the procedure went well, and the prognosis was favorable.
Non-surgical endodontic treatment was found to be successful in the management of dental periapical lesions[21,22]. This is related to the ability to eliminate microorganism within the root canal system and the absence of extra-radicular infection. However, a few cases cannot be managed by a non-surgical approach alone and require surgical intervention[1,4].
The modern approach to endodontic surgery has improved the prognosis of peri-radicular surgery[4]. This modern endodontic surgery incorporates the use of CBCT, a microscope, ultrasonic tips, and bio-ceramic materials[4]. Additionally, evidence-based methods were adapted for surgical flap design, root resection, and root end preparation[4]. However, the prognosis of modern endodontic surgery is less predictable in cases with periodontal involvement or through-and-through (Tunnel) defects[5-7]. A through-and-through defect in endodontic surgery is defined as a bony defect that has eroded the buccal and lingual plate caused by the inflammatory lesions, or as a result of buccal access during the osteotomy procedure, in the presence of eroded lingual bone[8]. The first and third cases had inflammatory lesions that caused erosion of the buccal and palatal plates. However, in the second case, the buccal access during the osteotomy resulted in a through-and-through defect after the surgery, as the opposing palatal side was the maxillary sinus. In these configurations, three walls are present (mesial, distal, and caudal) and two walls are missing (buccal and lingual/palatal)[8]. The healing of bone defects after peri-radicular surgery can be summarized in two main events: The first event is the healing of the surgical flap, which takes place during a few days, up to two weeks after surgery[23]. The following event is the healing of the osseous defect, which takes place over several months[24,25]. Usually, the complete healing of an osseous defect can take up to one year; however, the sign of progressive bone formation could become radiographically visible after six months[25]. The use of CBCT for the evaluation of bone healing after periapical surgery is recommended[26].
The usefulness of guided tissue regeneration (GTR) for healing bony defects after peri-radicular surgery was debatable[27]. However, techniques for GTR were considered in through-and-through defects to occupy the space in the bony defect and prevent marginal migration of epithelial cells[27]. Since the epithelium turnover rate is faster than bony stem cells, scar tissue healing was seen in the absence of cortical bone in through-and-through lesions[27].
However, the disadvantage of external bone graft material and membranes is the possibility of infection and foreign body rejection[28]. This can be seriously considered when dealing with infectious lesions that could hold possible residual bacteria[29]. Moreover, the addition of bony grafts and membranes will add to the expense of apical procedures, which could hinder access to care for a few patients.
The centrifuging machine used in this study produces three types of PRF: A-PRF, S-PRF, and I-PRF. The A-PRF is a smart blood concentrate that employs a higher amount of white blood cells in addition to platelet and fibrin to uniquely obtain the A-PRF membrane or plug[30]. The white blood cells in the membrane or plug are meant to become active in stimulating the transformation of monocytes into macrophages[30]. This rapid transformation will speed up the inflammatory cascade and stimulate bone healing[30]. When the A-PRF to the PRF were compared in the regenerative endodontic procedure, it was found that the A-PRF increased the root thickness and length within 13 mo[31]. This can be explained by the amount of growth factors released by the A-PRF which was greater than PRF[30]. Moreover, the use of bone graft in comparison to A-PRF clot alone in periapical surgery yielded remarkable size reduction of the bony defect in the A-PRF group within 6 mo[32]. The significant role of PRF in bony healing is the release of growth factors trapped in platelets during the centrifugation[33]. These growth factors are essential for the stimulation of cellular migration and proliferation[33]. They also assist in guiding tissue maturation and remodeling which promotes healing in injured tissues[32,33].
The main objective of using the bone graft in the third case was to provide structural support and to prevent the A-PRF membrane from collapsing in the deep bony defect[34]. The bone graft in this case was mixed with collected concentrated A-PRF blood clot to benefit from the growth factors embedded there to further promote bone healing. The presence of a blood clot is essential to promote the osteoconductive properties of bone graft[35]. The histological studies confirmed that mixing the PRF with bone accelerates bone healing, as new bone formation was evident within 30 d to 60 d in animals and up to four months in humans[36-38]. However, one of the disadvantages of the bone graft is that it has the same radiopacity as the bone, but it looks like bony chips with voids in the radiograph[39]. In the present case report, the combination of the radiographic appearance in the CBCT together with the absence of clinical signs and symptoms was sufficient to confirm the postsurgical healing.
Generally, the advantages of PRF membranes are their low expense and low risk of body rejection and infection[40]. Not to mention that the use of PRF is recommended in the patients treated with bisphosphonates with periapical lesions to improve bone repair and prevent osteonecrosis[41]. The PRF membrane was used to cover the bony defect in several case reports[17,18]. In accordance with the present case report series, they found that PRF promoted accelerated bone healing evident after 6 mo[15]. However, in many of these reports, the through-and-through defect was excluded[17,18]. Moreover, this study incorporated CBCT to evaluate bone healing after using PRF in periapical surgery. The CBCT proved to be superior to periapical radiographs in sensitivity and specificity[42,43]. The evidence of bone healing was seen in all cases at four months post-surgery, thus reducing the period needed to evaluate the short-term success of surgical endodontic treatment. The CBCT imaging was better than the periapical radiograph in identifying the volumetric changes in the size of the lesion after periapical surgery[44]. Moreover, postsurgical cases that were identified as uncertain or incompletely healed in periapical radiographs can be classified in CBCT imaging[44]. Especially in the third case, the CBCT was better at detecting bone healing after periapical surgery compared to the periapical radiograph.
While the short-term follow up could be considered a limitation of this study, the long-term prognosis of endodontic treatment is multifactorial. Factors such as coronal leakage and occlusal overload could contribute to the final outcome of the procedure, and it will be difficult to attribute the success of the procedure to the use of PRF membrane in periapical surgery[45]. Moreover, short term recall and the use of CBCT allowed accurate assessment of the healing while reducing the incidence of patient dropout that usually happens in longer periods. Recently, a randomized clinical trial used CBCT to evaluate the healing of postsurgical endodontic procedures after six months of the surgery only[32]. The main reason for patient dropout in dental clinics was the improvement of symptoms[46]. In all of the three presented clinical cases, patients were asymptomatic two weeks after the surgery and the patients responded negatively to percussion and palpation. Further clinical studies that explore the benefits of using A-PRF in other complex clinical scenarios, such as apico-marginal bone defects, are recommended.
Within the limitations of this study, the use of A-PRF membrane for GTR in the management of through and through osseous defects was successful. In three cases, the use of A-PRF membrane resulted in the complete healing of peri-radicular tissue and the preservation of root canal-treated teeth that would otherwise be extracted. The advantages of A-PRF membrane in endodontic surgery were low expense, low risk of post-surgical infection, and foreign body rejection.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: Saudi Dental Society, No. 1736.
Specialty type: Dentistry, oral surgery and medicine
Country/Territory of origin: Saudi Arabia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gianfreda F, Italy; Odabasi O, Turkey S-Editor: Chen YL L-Editor: A P-Editor: Yu HG
| 1. | Nair PN. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit Rev Oral Biol Med. 2004;15:348-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 650] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 2. | von Arx T, Peñarrocha M, Jensen S. Prognostic factors in apical surgery with root-end filling: a meta-analysis. J Endod. 2010;36:957-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 3. | Reda R, Zanza A, Bhandi S, Biase A, Testarelli L, Miccoli G. Surgical-anatomical evaluation of mandibular premolars by CBCT among the Italian population. Dent Med Probl. 2022;59:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Kim S, Kratchman S. Modern endodontic surgery concepts and practice: a review. J Endod. 2006;32:601-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 444] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 5. | Hirsch JM, Ahlström U, Henrikson PA, Heyden G, Peterson LE. Periapical surgery. Int J Oral Surg. 1979;8:173-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 156] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Andreasen JO, Rud J. Modes of healing histologically after endodontic surgery in 70 cases. Int J Oral Surg. 1972;1:148-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 76] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Kreisler M, Gockel R, Aubell-Falkenberg S, Kreisler T, Weihe C, Filippi A, Kühl S, Schütz S, d’Hoedt B. Clinical outcome in periradicular surgery: effect of patient- and tooth-related factors--a multicenter study. Quintessence Int. 2013;44:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 8. | von Arx T, Alsaeed M. The use of regenerative techniques in apical surgery: A literature review. Saudi Dent J. 2011;23:113-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Bashutski JD, Wang HL. Periodontal and endodontic regeneration. J Endod. 2009;35:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Bhandi S, Alkahtani A, Mashyakhy M, Abumelha AS, Albar NHM, Renugalakshmi A, Alkahtany MF, Robaian A, Almeslet AS, Patil VR, Varadarajan S, Balaji TM, Reda R, Testarelli L, Patil S. Effect of Ascorbic Acid on Differentiation, Secretome and Stemness of Stem Cells from Human Exfoliated Deciduous Tooth (SHEDs). J Pers Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Bhandi S, Alkahtani A, Reda R, Mashyakhy M, Boreak N, Maganur PC, Vishwanathaiah S, Mehta D, Vyas N, Patil V, Raj AT, Testarelli L, Patil S. Parathyroid Hormone Secretion and Receptor Expression Determine the Age-Related Degree of Osteogenic Differentiation in Dental Pulp Stem Cells. J Pers Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Heijl L, Heden G, Svärdström G, Ostgren A. Enamel matrix derivative (EMDOGAIN) in the treatment of intrabony periodontal defects. J Clin Periodontol. 1997;24:705-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 333] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 13. | Jung RE, Glauser R, Schärer P, Hämmerle CH, Sailer HF, Weber FE. Effect of rhBMP-2 on guided bone regeneration in humans. Clin Oral Implants Res. 2003;14:556-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 212] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 14. | Nevins M, Giannobile WV, McGuire MK, Kao RT, Mellonig JT, Hinrichs JE, McAllister BS, Murphy KS, McClain PK, Nevins ML, Paquette DW, Han TJ, Reddy MS, Lavin PT, Genco RJ, Lynch SE. Platelet-derived growth factor stimulates bone fill and rate of attachment level gain: results of a large multicenter randomized controlled trial. J Periodontol. 2005;76:2205-2215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 313] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 15. | Singh S, Singh A, Singh S, Singh R. Application of PRF in surgical management of periapical lesions. Natl J Maxillofac Surg. 2013;4:94-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Jung MH, Lee JH, Wadhwa P, Jiang HB, Jang HS, Lee ES. Bone regeneration in peri-implant defect using autogenous tooth biomaterial enriched with platelet-rich fibrin in animal model. Appl Sci. 2020;10:1939. [DOI] [Full Text] |
| 17. | Dhiman M, Kumar S, Duhan J, Sangwan P, Tewari S. Effect of Platelet-rich Fibrin on Healing of Apicomarginal Defects: A Randomized Controlled Trial. J Endod. 2015;41:985-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Jung RE, Schmoekel HG, Zwahlen R, Kokovic V, Hammerle CH, Weber FE. Platelet-rich plasma and fibrin as delivery systems for recombinant human bone morphogenetic protein-2. Clin Oral Implants Res. 2005;16:676-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Angerame D, De Biasi M, Kastrioti I, Franco V, Castaldo A, Maglione M. Application of platelet-rich fibrin in endodontic surgery: a pilot study. G Ital Endod. 2015;29:51-57. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Shivashankar VY, Johns DA, Vidyanath S, Sam G. Combination of platelet rich fibrin, hydroxyapatite and PRF membrane in the management of large inflammatory periapical lesion. J Conserv Dent. 2013;16:261-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Maddalone M, Gagliani M. Periapical endodontic surgery: a 3-year follow-up study. Int Endod J. 2003;36:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | von Arx T. Failed root canals: the case for apicoectomy (periradicular surgery). J Oral Maxillofac Surg. 2005;63:832-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Harrison JW, Jurosky KA. Wound healing in the tissues of the periodontium following periradicular surgery. I. The incisional wound. J Endod. 1991;17:425-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Harrison JW, Jurosky KA. Wound healing in the tissues of the periodontium following periradicular surgery. III. The osseous excisional wound. J Endod. 1992;18:76-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Song M, Nam T, Shin SJ, Kim E. Comparison of clinical outcomes of endodontic microsurgery: 1 year vs long-term follow-up. J Endod. 2014;40:490-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Kang S, Ha SW, Kim U, Kim S, Kim E. A One-Year Radiographic Healing Assessment after Endodontic Microsurgery Using Cone-Beam Computed Tomographic Scans. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Corbella S, Taschieri S, Elkabbany A, Del Fabbro M, von Arx T. Guided Tissue Regeneration Using a Barrier Membrane in Endodontic Surgery. Swiss Dent J. 2016;126:13-25. [PubMed] |
| 28. | Caldwell S. Bone graft complications. Misch’s Avoid Complicat Oral Implantol. January 1, 2018. [cited 3 March 2023]. Available from: https://monib-health.com/en/post/111-bone-graft-complications. |
| 29. | Bronzato JD, Davidian MES, de Castro M, de-Jesus-Soares A, Ferraz CCR, Almeida JFA, Marciano MA, Gomes BPFA. Bacteria and virulence factors in periapical lesions associated with teeth following primary and secondary root canal treatment. Int Endod J. 2021;54:660-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Kobayashi E, Flückiger L, Fujioka-Kobayashi M, Sawada K, Sculean A, Schaller B, Miron RJ. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Investig. 2016;20:2353-2360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 435] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 31. | Jayadevan V, Gehlot PM, Manjunath V, Madhunapantula SV, Lakshmikanth JS. A comparative evaluation of Advanced Platelet-Rich Fibrin (A-PRF) and Platelet-Rich Fibrin (PRF) as a Scaffold in Regenerative Endodontic Treatment of Traumatized Immature Non-vital permanent anterior teeth: A Prospective clinical study. J Clin Exp Dent. 2021;13:e463-e472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Kirilova J, Kirov D, Yovchev D, Topalova-Pirinska S, Deliverska E. Endodontic and surgical treatment of chronic apical periodontitis: a randomized clinical study. Biotech & Biotech Equip. 2022;36:737-744. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 33. | Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e37-e44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 1016] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 34. | Kay SA, Wisner-Lynch L, Marxer M, Lynch SE. Guided bone regeneration: integration of a resorbable membrane and a bone graft material. Pract Periodontics Aesthet Dent. 1997;9:185-94; quiz 196. [PubMed] |
| 35. | Milillo L, Cinone F, Lo Presti F, Lauritano D, Petruzzi M. The Role of Blood Clot in Guided Bone Regeneration: Biological Considerations and Clinical Applications with Titanium Foil. Materials (Basel). 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 36. | Karayürek F, Kadiroğlu ET, Nergiz Y, Coşkun Akçay N, Tunik S, Ersöz Kanay B, Uysal E. Combining platelet rich fibrin with different bone graft materials: An experimental study on the histopathological and immunohistochemical aspects of bone healing. J Craniomaxillofac Surg. 2019;47:815-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 37. | Kökdere NN, Baykul T, Findik Y. The use of platelet-rich fibrin (PRF) and PRF-mixed particulated autogenous bone graft in the treatment of bone defects: An experimental and histomorphometrical study. Dent Res J (Isfahan). 2015;12:418-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, Dohan AJ, Mouhyi J, Dohan DM. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part V: histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:299-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 410] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 39. | Kursun-Çakmak ES, Akbulut N, Öztas DD. Comparative Evaluation of the Radiopacity of Bone Graft Materials used in Dentistry. J Contem Dent. 2017;7. [DOI] [Full Text] |
| 40. | Xu Z, Myia W, Kayla F, Vidhi P, Alyson M, Liron S, Gisele WK, Allison M, Christian N, Amgad M, Roman Z, Leanne T, Tanya S, Renee P. Erratum: Home Telemonitoring of Patients With Type 2 Diabetes: A Meta-Analysis and Systematic Review. Diabetes Spectr. 2022;35:384. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 41. | Miranda M, Gianfreda F, Raffone C, Antonacci D, Pistilli V, Bollero P. The Role of Platelet-Rich Fibrin (PRF) in the Prevention of Medication-Related Osteonecrosis of the Jaw (MRONJ). Biomed Res Int. 2021;2021:4948139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 42. | Song D, Shujaat S, de Faria Vasconcelos K, Huang Y, Politis C, Lambrichts I, Jacobs R. Diagnostic accuracy of CBCT vs intraoral imaging for assessment of peri-implant bone defects. BMC Med Imaging. 2021;21:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 43. | Christiansen R, Kirkevang LL, Gotfredsen E, Wenzel A. Periapical radiography and cone beam computed tomography for assessment of the periapical bone defect 1 wk and 12 mo after root-end resection. Dentomaxillofac Radiol. 2009;38:531-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 44. | Schloss T, Sonntag D, Kohli MR, Setzer FC. A Comparison of 2- and 3-dimensional Healing Assessment after Endodontic Surgery Using Cone-beam Computed Tomographic Volumes or Periapical Radiographs. J Endod. 2017;43:1072-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 45. | Haapasalo M, Shen Y, Ricucci DM, Ricucci D. Reasons for persistent and emerging post-treatment endodontic disease. Endod Top. 2008;18:31-50. [RCA] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 46. | Kato T, Mizutani S, Umezaki Y, Sugiyama S, Naito T. Relationship between Type D personality and dropout from dental treatment in middle-aged adults. J Oral Sci. 2019;61:264-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |