Published online Jun 16, 2023. doi: 10.12998/wjcc.v11.i17.4152
Peer-review started: March 22, 2023
First decision: April 28, 2023
Revised: April 29, 2023
Accepted: May 16, 2023
Article in press: May 16, 2023
Published online: June 16, 2023
Processing time: 82 Days and 7.3 Hours
Pulmonary fibrosis often occurs as a sequel of coronavirus disease 2019 (COVID-19); however, in some cases, it can rapidly progress, similar to the acute exacerbation of interstitial lung disease. Glucocorticoids are the standard treatment for severe COVID-19 pneumonia requiring oxygen supply; however, the post-COVID-19 efficacy of high-dose steroid therapy remains unclear. Here, we presented a case of an 81-year-old man who developed acute respiratory failure after COVID-19 and was treated with glucocorticoid pulse therapy.
An 81-year-old man with no respiratory symptoms was admitted due to a diabetic foot. He had been previously treated for COVID-19 pneumonia six weeks prior. However, upon admission, he suddenly complained of dyspnea and required a high-flow oxygen supply. Initial simple chest radiography and computed tomography (CT) revealed diffuse ground-glass opacities and consolidation in both lungs. However, repeated sputum tests did not identify any infectious pathogens, and initial broad-spectrum antibiotic therapy did not result in any clinical improvement with the patient having an increasing oxygen demand. The patient was diagnosed with post-COVID-19 organizing pneumonia. Thus, we initiated glucocorticoid pulse therapy of 500 mg for three days followed by a tapered dose on hospital day (HD) 9. After three days of pulse treatment, the patient's oxygen demand decreased. The patient was subsequently discharged on HD 41, and chest radiography and CT scans have almost normalized nine months after discharge.
Glucocorticoid pulse therapy may be considered when the usual glucocorticoid dose is ineffective for patients with COVID-19 sequelae.
Core Tip: In cases wherein standard glucocorticoid therapy is ineffective in patients with coronavirus disease 2019 (COVID-19) sequelae, glucocorticoid pulse therapy may be considered. This treatment approach was effective in the present case of an 81-year-old man with post-COVID-19 organizing pneumonia who developed acute respiratory failure after infection. The patient showed significant improvement in oxygen demand and imaging tests after three days of pulse treatment; he eventually recovered without any symptoms of interstitial lung disease. Therefore, glucocorticoid pulse therapy is a potential treatment option for patients with COVID-19 sequelae who require oxygen therapy and do not respond to standard therapy.
- Citation: Park S, Jang Y, Koo SM, Nam BD, Yoon HY. Glucocorticoid pulse therapy in an elderly patient with post-COVID-19 organizing pneumonia: A case report. World J Clin Cases 2023; 11(17): 4152-4158
- URL: https://www.wjgnet.com/2307-8960/full/v11/i17/4152.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i17.4152
As the coronavirus disease 2019 (COVID-19) pandemic persists into its third year, various manifestations of COVID-19 sequelae have been documented[1]. Pulmonary fibrosis is frequently reported as a COVID-19 sequel, ranging from 10%-84%, depending on the disease severity, demographics, and initial radiological findings[2]. Unlike certain types of interstitial lung disease (ILD) that can progress over time, post-COVID-19 fibrosis generally stabilizes or may even show gradual improvement after the acute phase of the disease. However, irreversible or progressive fibrotic changes have been documented in only a few cases[3,4].
Acute respiratory failure has been reported as an acute exacerbation (AE) of ILD after COVID-19 in patients without a history of ILD, which has shown a poor prognosis. Currently, effective treatment options for AE of ILD are limited. Glucocorticoids are currently recommended, with a weak positive recommendation for the management of AE of idiopathic pulmonary fibrosis, which is one of the most common types of ILD. However, evidence supporting the use of glucocorticoids in this context is limited and mainly based on expert consensus, small uncontrolled studies, or retrospective studies[5]. Corticosteroids have been shown to improve clinical outcomes in patients with COVID-19 pneumonia requiring oxygen therapy in several randomized trials[6]. Here, we presented a case of a patient who developed acute respiratory failure after COVID-19 and was treated with glucocorticoid pulse therapy. This report contributes to the limited evidence for the use of glucocorticoids in the management of post-COVID-19 pneumonia, specifically in cases of AE of ILD.
An 81-year-old man who presented with shortness of breath immediately after admission.
The patient was scheduled for admission to the Department of Endocrinology for glycemic control, as indicated by a hemoglobin A1C (HbA1c) level of 9.5%, and management of diabetic gangrene in both feet. The patient had been taking metformin for one year to control his blood sugar levels. However, his serum glucose levels have not been well controlled for the past month, as measured at home, and he subsequently developed diabetic foot, requiring hospitalization. The patient had no respiratory symptoms at the time of admission. However, shortly after admission, the patient suddenly complained of dyspnea, and his initial peripheral transcutaneous oxygen saturation (SpO2) level was 67% while breathing room air.
The patient had been diagnosed with COVID-19 at another institution six weeks before admission. He was hospitalized for approximately two weeks due to COVID-19 pneumonia and received treatment with remdesivir and steroids. After discharge, the patient did not require additional medication or home oxygen, and he did not complain of any other symptoms, such as respiratory distress, despite being almost inactive due to diabetic gangrene. Six weeks later, the patient was admitted to our institute for further management of uncontrolled diabetes and diabetic foot disease.
The medical history included medications, such as digoxin, diltiazem, and edoxaban for atrial fibrillation and metformin for diabetes mellitus. His baseline HbA1c level was 7.2% during a hospital visit six months prior. No other respiratory illness was observed. The patient had a smoking history of 2.5 pack years.
Upon admission, the patient's vital signs were as follows: blood pressure, 114/77 mmHg; heart rate, 138/min; respiratory rate, 28/min; body temperature, 37.0 °C; SpO2, 67% on room air. The patient presented with coarse breath sounds and rhonchi in both lung fields.
Arterial blood gas analysis (ABGA) revealed hypoxemia [arterial oxygen partial pressure (PaO2): 63.2 mmHg], hypocapnia (arterial carbon dioxide partial pressure: 28.6 mmHg), and respiratory alkalosis (pH7.5) with oxygen supply at 5 L/min via nasal prong. However, his bicarbonate (HCO3, 21.7 mmol/L) and lactic acid (2.0 mmol/L) levels were within normal range. Mild leukocytosis with a white blood cell counts of 12.1 × 109/L, elevated C-reactive protein (17.53 mg/dL), and erythrocyte sedimentation rate (120 mm/h) were found. In addition, the patient's initial blood glucose level was markedly elevated (387 mg/dL). However, procalcitonin level was within the normal range (0.25 ng/mL). No obvious abnormalities were observed in any biochemical indices.
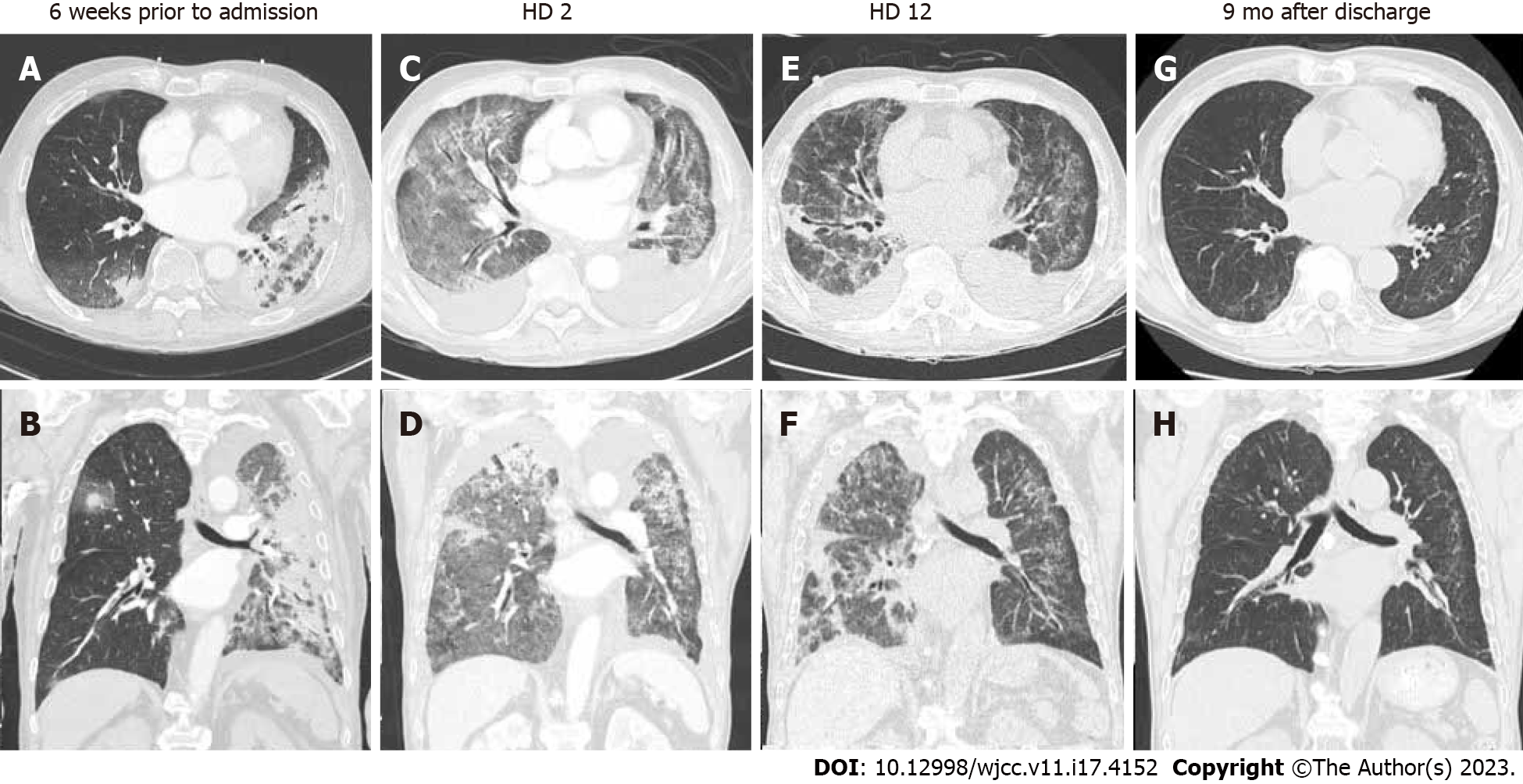
On initial chest radiography, diffuse bilateral consolidation with ground-glass opacities (GGO) were observed in both lungs, and a small amount of bilateral pleural effusion was noted (Figure 1A). Chest computed tomography (CT) performed 6 weeks after the patient was hospitalized for COVID-19 pneumonia revealed peribronchovascular and peripherally distributed consolidations, with greater severity in the left lung (Figure 2A and B). On admission, enhanced chest CT revealed diffuse and multifocal patchy consolidation and GGOs in both lungs, along with a small to moderate amount of bilateral effusion (Figure 2C and D).
To evaluate breathing difficulty, transthoracic echocardiography was performed to assess cardiac function. The results revealed mild diastolic dysfunction, mild pulmonary hypertension with a mean pressure gradient of 38 mmHg, and left ventricular hypertrophy. No abnormalities were observed.
Initially, severe community-acquired pneumonia was suspected, and broad-spectrum antibiotics (piperacillin/tazobactam and levofloxacin) were administered. On hospital day (HD) 2, due to progressing dyspnea and increased oxygen demand, the patient was transferred to the intensive care unit (ICU), and high-flow nasal cannula (HFNC) with a fraction of inspired oxygen (FiO2) of 0.7 was initiated. Bronchoalveolar lavage was performed under bronchoscopy for diagnostic purposes; however, it was not performed because of the high oxygen demand and the patient's refusal to undergo tracheal intubation, as specified in their advance healthcare directives. Repeated sputum tests, including Gram stain/culture, acid-fast bacilli stain/culture, COVID-19, respiratory viruses, atypical pathogens, tuberculosis, and Pneumocystis jiroveci polymerase chain reaction did not reveal any pathogens. Additionally, all autoantibodies confirming underlying ILD, such as rheumatoid factors and antinuclear antibodies, tested negative. Although the patient was not immunocompromised, he was treated for Pneumocystis jiroveci using trimethoprim/sulfamethoxazole in addition to methylprednisolone (62.5 mg) since he did not respond to broad-spectrum antibiotics on HD 5.
Based on the patient's recent history of COVID-19 and steroid responsiveness, the final diagnosis was post-COVID-19 organizing pneumonia.
Despite the use of broad-spectrum empirical antibiotics and usual doses of steroids, the patient's oxygen demand did not decrease, and there was no significant improvement in bilateral lung parenchymal disease on follow-up chest radiography (Figure 1B) until HD 9. While maintaining the HFNC FiO2 at 0.7, the patient's ABGA showed hypoxemia and respiratory alkalosis (PaO2, 72.3; PCO2, 38.2; pH, 7.52; HCO3, 30.4). The patient's PaO2/FiO2 ratio was 103.3, indicating moderate acute respiratory distress syndrome (ARDS) according to the Berlin definition[7]. Since repeated sputum tests did not reveal any pathogens, we initiated high-dose steroid pulse therapy (methylprednisolone at a dose of 500 mg/day for three days) in combination with broad-spectrum antibiotics, considering other undiagnosed ILD or post-COVID ILD. After initiating pulse therapy, the patient's dyspnea slowly improved, and the oxygen demand decreased. On follow-up chest radiography and low-dose CT on HD 12, the extent of bilateral consolidation with GGOs had partially decreased, and there was mild progression of bronchial dilatation (Figures 1C, 2E and F). We concluded that steroid pulse therapy was effective during the patient's clinical course. After reducing the methylprednisolone dose to 60 mg, we slowly tapered the dose while monitoring the patient's condition.
The patient was transferred to the general ward on HD 12 in stable condition. He was weaned off HFNC on HD 17 and was administered low-flow oxygen via nasal cannula at 4 L/min. Although the patient still required oxygen, he recovered sufficiently to withstand general anesthesia. Therefore, on HD 28, the patient underwent below-knee amputation under general anesthesia for diabetic gangrene. The patient recovered safely after surgery and was discharged on HD 41.
Upon discharge, the patient required portable oxygen supplementation because of oxygen demand. However, oxygen supplementation was no longer necessary after six months of pulse therapy. Nine months after pulse therapy, the extensive and heterogeneous bilateral GGOs and consolidations that were previously observed had almost resolved on follow-up chest radiography and CT scans. There was no evidence of ILD in the follow-up images (Figures 1D, 2G and H).
Here, we presented a case of ARDS caused by an AE pattern of ILD after COVID-19 that resolved after steroid pulse treatment. Several reports have indicated that organizing pneumonia (OP) can worsen in the late period after COVID-19[8-10]. Vadász et al[10] reported cases of biopsy-confirmed OP that occurred between two and four weeks after severe COVID-19, followed by intubation. Both cases improved after a 4-week treatment with 1 mg/kg methylprednisolone. Ng et al[9] also reported two cases of post-COVID-19 OP that were successfully treated with corticosteroids. A medium dose (0.75-1 mg/kg) of corticosteroids is typically administered for 4-8 wk as the initial treatment for OP, followed by gradual tapering over several months[11]. Glucocorticoid therapy at low to medium doses is usually sufficient to treat OP; therefore, the use of high doses of steroids for cryptogenic or secondary OP is rare.
In contrast, the World Health Organization recommends the systemic administration of dexa
Although our patient did not undergo invasive diagnostic tests, such as bronchoscopy or transbronchial lung biopsy because of the impaired general condition and high oxygen demand, the dramatic clinical improvement after steroid pulse therapy suggested a high possibility of post-COVID-19 OP clinically. Additionally, while a biopsy is necessary to diagnose OP, it may not be feasible for severely ill patients with high oxygen demand. In cases wherein ARDS with an OP pattern is confirmed after COVID-19, there is no clear evidence of late-phase infection, and steroid pulse therapy should be cautiously considered without biopsy confirmation.
It is important to consider the possibility of secondary OP in patients with prolonged respiratory symptoms and radiological involvement after COVID-19. In cases where the standard dose of glucocorticoid therapy is ineffective, pulse therapy may be considered as the last resort. However, additional clinical trials are necessary to obtain further information on this topic.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Respiratory System
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Al-Ani RM, Iraq; Shen TC, Taiwan S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Carfì A, Bernabei R, Landi F; Gemelli. Against COVID-19 post-acute care study group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2398] [Cited by in RCA: 2812] [Article Influence: 562.4] [Reference Citation Analysis (0)] |
| 2. | Hama Amin BJ, Kakamad FH, Ahmed GS, Ahmed SF, Abdulla BA, Mohammed SH, Mikael TM, Salih RQ, Ali RK, Salh AM, Hussein DA. Post COVID-19 pulmonary fibrosis; a meta-analysis study. Ann Med Surg (Lond). 2022;77:103590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 93] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 3. | Gulati A, Lakhani P. Interstitial lung abnormalities and pulmonary fibrosis in COVID-19 patients: a short-term follow-up case series. Clin Imaging. 2021;77:180-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Rai DK, Kumar S, Sahay N. Post-COVID-19 pulmonary fibrosis: A case series and review of literature. J Family Med Prim Care. 2021;10:2028-2031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Collard HR, Ryerson CJ, Corte TJ, Jenkins G, Kondoh Y, Lederer DJ, Lee JS, Maher TM, Wells AU, Antoniou KM, Behr J, Brown KK, Cottin V, Flaherty KR, Fukuoka J, Hansell DM, Johkoh T, Kaminski N, Kim DS, Kolb M, Lynch DA, Myers JL, Raghu G, Richeldi L, Taniguchi H, Martinez FJ. Acute exacerbation of idiopathic pulmonary fibrosis. An International Working Group Report. Am J Respir Crit Care Med. 2016;194:265-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 996] [Article Influence: 110.7] [Reference Citation Analysis (0)] |
| 6. | Ebrahimi Chaharom F, Pourafkari L, Ebrahimi Chaharom AA, Nader ND. Effects of corticosteroids on Covid-19 patients: A systematic review and meta-analysis on clinical outcomes. Pulm Pharmacol Ther. 2022;72:102107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526-2533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1812] [Cited by in RCA: 4297] [Article Influence: 330.5] [Reference Citation Analysis (0)] |
| 8. | Bieksiene K, Zaveckiene J, Malakauskas K, Vaguliene N, Zemaitis M, Miliauskas S. Post COVID-19 organizing pneumonia: The right time to interfere. Medicina (Kaunas). 2021;57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Ng BH, Ban AY, Nik Abeed NN, Faisal M. Organising pneumonia manifesting as a late-phase complication of COVID-19. BMJ Case Rep. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Vadász I, Husain-Syed F, Dorfmüller P, Roller FC, Tello K, Hecker M, Morty RE, Gattenlöhner S, Walmrath HD, Grimminger F, Herold S, Seeger W. Severe organising pneumonia following COVID-19. Thorax. 2021;76:201-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 11. | King TE Jr, Lee JS. Cryptogenic organizing pneumonia. N Engl J Med. 2022;386:1058-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 75] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 12. | Recovery Collaborative Group; Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6762] [Cited by in RCA: 7389] [Article Influence: 1847.3] [Reference Citation Analysis (1)] |
| 13. | Tan RSJ, Ng KT, Xin CE, Atan R, Yunos NM, Hasan MS. High-dose vs low-dose corticosteroids in COVID-19 patients: A systematic review and meta-analysis. J Cardiothorac Vasc Anesth. 2022;36:3576-3586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Kumar G, Patel D, Hererra M, Jefferies D, Sakhuja A, Meersman M, Dalton D, Nanchal R, Guddati AK. Do high-dose corticosteroids improve outcomes in hospitalized COVID-19 patients? J Med Virol. 2022;94:372-379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Edalatifard M, Akhtari M, Salehi M, Naderi Z, Jamshidi A, Mostafaei S, Najafizadeh SR, Farhadi E, Jalili N, Esfahani M, Rahimi B, Kazemzadeh H, Mahmoodi Aliabadi M, Ghazanfari T, Sattarian M, Ebrahimi Louyeh H, Raeeskarami SR, Jamalimoghadamsiahkali S, Khajavirad N, Mahmoudi M, Rostamian A. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: results from a randomised controlled clinical trial. Eur Respir J. 2020;56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 255] [Article Influence: 51.0] [Reference Citation Analysis (0)] |