Published online Jun 16, 2023. doi: 10.12998/wjcc.v11.i17.4019
Peer-review started: February 3, 2023
First decision: April 10, 2023
Revised: April 23, 2023
Accepted: May 6, 2023
Article in press: May 6, 2023
Published online: June 16, 2023
Processing time: 128 Days and 14.2 Hours
Granuloma annulare (GA) has diverse clinical manifestations, multiple subtypes, and unknown etiology and pathogenesis. Existing studies regarding GA in children are scarce.
To examine the correlation between clinical manifestation and histopathology of pediatric GA.
A total of 39 patients under 18 years of age with both a clinical and pathological diagnosis of GA at Kunming Children's Hospital from 2017 to 2022 were retrieved. Their medical records were consulted, and clinical data of the children were recorded and summarized, including gender, age, disease site, etc. Existing wax blocks of skin lesion specimens of children and pathological films were retrieved for further study and relevant histology, including hematoxylin-eosin, Alcian blue, elastic fiber (Victoria blue-Lichon red method), and antacid staining. Finally, the children’s clinical manifestations, histopathological results, and special staining characteristics were analyzed.
The clinical manifestations of granuloma annulare in children were diverse: 11 cases presented with a single lesion, 25 with multiple lesions, and 3 with generalized lesions. The pathological typing comprised histiocytic infiltration, palisading granuloma, epithelioid nodular, and mixed types in 4, 11, 9, and 15 cases, respectively. Thirty-nine cases were negative for antacid staining. The positive rate of Alcian blue staining was 92.3%, and that of elastic fiber staining was 100%. The degree of elastic fiber dissolution and granuloma annulare histopathological typing were positively correlated (r = 0.432, P < 0.05). No correlation was found between clinical presentation and histopathological typing of the granuloma annulare in children. In the pathological diagnosis of granuloma annulare, the positive elastic fiber staining rate was higher than that of Alcian blue staining. A correlation was found between elastic fiber dissolution degree and histopathological staging. However, the differences in pathological staging may have been related to the pathological manifestation of granuloma annulare at different periods.
Elastic fiber degradation may be a critical step in the pathogenesis of pediatric granuloma annulare. This is also one of the first studies focused on granuloma annulare in children.
Core Tip: This study examined the correlation between clinical manifestation and histopathology of pediatric granuloma annulare. We performed a retrospective analysis of thirty-nine cases of granuloma annulare in children and demonstrated a correlation between histopathological staining and elastic fiber dissolution. Different pathological staging may reflect the pathological manifestation of granuloma annulare at different periods. Our study demonstrates that elastic fiber degradation may be a critical step in the pathogenesis of pediatric granuloma annulare. Moreover, it is one of the first studies on granuloma annulare in children.
- Citation: Zhang DY, Zhang L, Yang QY, Xie YC, Jiang HC, Li JZ, Shu H. Elastic fiber degradation in the development of pediatric granuloma annulare: Report of 39 cases. World J Clin Cases 2023; 11(17): 4019-4025
- URL: https://www.wjgnet.com/2307-8960/full/v11/i17/4019.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i17.4019
Granuloma annulare (GA) manifests primarily as granulomatous inflammation infiltrating the dermis and subcutaneous tissues. Fox first reported GA in 1895, and Crocker officially named GA in 1902. The prevalence of GA is approximately 0.04% according to large cross-sectional studies, with a predilection for patients aged 3–50 years and a male-to-female ratio of approximately 2:1. However, some small single-center studies suggest that GA is common in children[1-3]. GA has diverse clinical manifestations, multiple subtypes, and unknown etiology and pathogenesis. Recent studies have shown that activation of the Th1, Th2, and JAK-STAT pathways is involved in the pathogenesis of GA[4]. In the early stages, the Th1-dominated immune response induces the infiltration of M1 macrophages, which triggers collagen degradation, followed by successive induction of M2 macrophage responses that mediate mucin deposition and tissue regeneration[5]. However, existing studies regarding GA in children are limited. Therefore, in this study, we analyzed and discussed the clinical manifestations and histopathological features of GA in children and investigated the role of elastic fiber degradation and mucin deposition in the pathogenesis of GA in children with elastic fiber and Alcian blue staining.
Thirty-nine cases of clinically and pathologically confirmed GA in children under 18 years of age from Kunming Children's Hospital between 2017 and 2022 were included.
Medical records were consulted and clinical data of the children were recorded and summarized, including gender, age, and disease site. Specimen wax blocks and existing pathological staining sheets were also collected for further study and relevant histological staining, including hematoxylin-eosin (HE), Alcian blue, elastic fiber (Victoria blue-Lichon red method), and antacid staining. The results were determined by two pathologists. The Medical Ethics Committee of Kunming Children's Hospital approved this study (No. 2022-03-325-K01).
HE staining was primarily used to analyze the pattern of histocyte infiltration. Referring to the histopathological typing of GA by Friedman-Birnbaum et al[4], GA was classified into these four types: Palisading granuloma, histiocytic infiltrative, epithelioid nodular, and mixed histologic types.
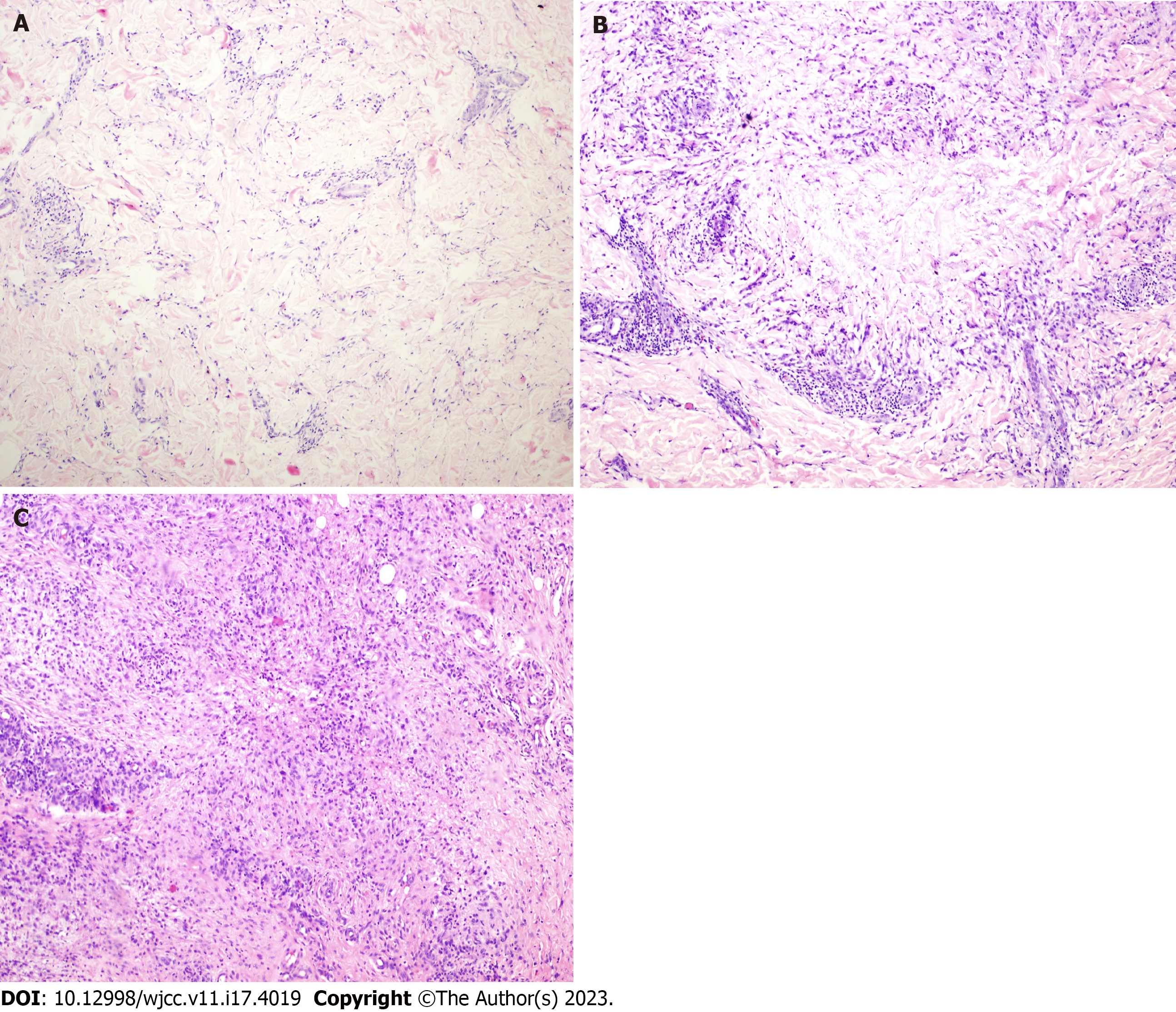
Elastic fiber staining was categorized into four grades according to elastic fibers' dissolution and destruction. Grade I: Disorganized arrangement of elastic fibers in the lesion without obvious disappearance and dissolution (Figure 1A). Grade II: Partial breakage of elastic fibers in the lesion with slight dissolution (Figure 1B). Grade III: Partial dissolution of elastic fibers in the lesion, but the visibility of some discontinuous elastic fibers (Figure 1C). Grade IV: Complete dissolution and disappearance of elastic fibers in the lesion (Figure 1D).
Statistical Package for the Social Sciences (SPSS, v. 20.0) was used to analyze histopathological staging and the degree of elastic fiber dissolution (grades I–IV) using Kendall's tau-b correlation analysis. Statistical significance was set at P < 0.05.
Thirty-nine patients were included, 14 males and 25 females; the age distribution ranged from 2 mo and 13 d to 11 years, with a mean age of onset of 4.2 years. Eleven patients had single lesions, 25 had multiple lesions, and 3 had generalized lesions. The 3 patients with generalized lesions were all infants aged 2–4 mo. The lesions were distributed on the head in 6, face in 2, trunk in 1, extremities in 24, and whole body in 6 cases. Six cases of GA on the head involved subcutaneous nodule-like lesions (single or multiple), and three cases of generalized GA involved papule-like lesions. The lesions were plaques in 20, papules in 4, nodules in 12, and mixed manifestations in 3 cases.
Among the 39 cases, 4 showed histiocytic infiltration (Figure 2A), 11 showed palisading granuloma (Figure 2B), 9 showed epithelioid nodules (Figure 2C), and 15 showed mixed type findings. Of these 15 mixed-type cases, 2 showed a mixture of palisading and histiocytic infiltration, and 13 showed a mixture of palisading and epithelioid nodules (Table 1).
| Clinical and special staining | Clinical manifestation | Special staining | Dissolution degree of elastic fiber | ||||||||
| Pathological type | Plaque | Nodule | Papules | Mixed | Alcian blue staining (positive) | Dissolution and disappearance of elastic fibers | Acid-fast staining (negative) | Grade I | Grade II | Grade III | Grade IV |
| Histiocytic infiltration type | 4 | 0 | 0 | 0 | 4 | 4 | 4 | 1 | 3 | 0 | 0 |
| Palisading granuloma type | 6 | 0 | 2 | 3 | 11 | 11 | 11 | 0 | 3 | 4 | 4 |
| Mixed type | 8 | 7 | 0 | 0 | 15 | 15 | 15 | 0 | 1 | 5 | 9 |
| Epithelioid nodular type | 2 | 5 | 2 | 0 | 6 | 9 | 9 | 0 | 0 | 4 | 5 |
Antacid staining was negative in all 39 cases. Alcian blue staining was positive in 36 cases (positivity rate: 92.3%) and negative in only 3 cases (all epithelioid nodular types). Elastic fiber staining showed different degrees of dissolution and disappearance of elastic fibers, with a positivity rate of 100% (39/39 cases). The cases were classified into grades I–IV according to the degree of elastic fiber dissolution and destruction (Table 1). There was a single grade I and 3 grade II cases with histiocytic infiltration; 3, 4, and 4 grade II, grade III, and grade IV cases with palisading granuloma; 1, 5, and 9 grade II, grade III, and grade IV cases with mixed type, respectively; and 4 and 5 grade III and five grade IV cases with epithelioid nodules, respectively.
A positive correlation was found between the degree of elastolysis and GA histopathological typing (r = 0.432, P < 0.05).
Telephone follow-up was conducted for all 39 children prior to the write-up (2022/10/10). Eleven were lost to follow-up, and 25 had subsided after diagnosis and were now asymptomatic. Only 3 children still had annular papules in the extremities, but no treatment was given because they were asymptomatic.
The etiology and pathogenesis of GA are unknown, and the clinical manifestations are diverse and differ between children and adults. This study included 39 cases of GA in children. The male-to-female ratio was approximately 0.56:1, which contradicts data from other studies, possibly due to the inclusion of only children in this study, although this needs to be validated in larger studies on children with GA. Clinical manifestations were more common as single or multiple annular plaques, mostly skin-colored, with a small proportion being red; however, no clear standard was available for the histopathological types of the different clinical manifestations.
Researchers have suggested that interferon produced by Th1 Lymphocytes in GA lesions activates macrophages to produce tumor necrosis factor and matrix metalloproteases (MMPs, including MMP2 and MMP9), triggering matrix degradation. Meanwhile, MMP-12 expression is also increased in tissues, which can lead to the degradation of elastic fibers and basement membrane bands. In ultrastructural studies, the main lesion of GA has been found to involve elastic fiber degeneration, suggesting that the disease is essentially an elastic fiber damage disease [5]. In this study, the positivity rate of elastic fiber staining (100%) was significantly higher than that of Alcian blue staining (92.3%), suggesting that elastic fiber changes are more common in GA.
In this study, we graded the degree of elastic fiber dissolution and disappearance in 39 cases of GA. The number of histiocytes was relatively low in histiocytic infiltrative GA, and the degree of elastolysis was grade I–II in all cases. Therefore, we speculate that this may be the early stage of the disease. The degree of elastolysis in palisading granuloma type GA and mixed type GA was mainly grade II–IV, and the elastolysis in epithelioid nodular GA was predominantly grade III–IV. The correlation between the degree of GA elastolysis and histopathological staging was verified by Kendall's tau-b correlation analysis, and we believe that these four stages are the pathological manifestations of GA at different periods. Additionally, the four pathological stages may be the gradual evolution and derivation of the course of GA. However, due to this study’s limited data and low correlation, more studies are needed to support our findings.
Recent studies have shown that activation of the Th1, Th2, and JAK-STAT pathways is involved in the pathogenesis of GA[6]. In the early stages, the Th1-dominated immune response induces M1 macrophage infiltration, triggers collagen degradation, and subsequently induces an M2 macrophage response that mediates mucin deposition and tissue regeneration[7]. For the 36 cases with positive Alcian blue staining in this study, mucin was usually distributed in the center of a relatively concentrated site of histiocytes. The remaining three cases with negative Alcian blue staining showed epithelioid nodular GA. We speculate that in the later stages of the disease, histiocytes and macro
For the first time, we established that the GA lesions in all 39 cases had different degrees of elastin fiber change. Moreover, the positivity rate of elastic fiber staining was approximately 100%, significantly higher than that of Alcian blue staining. There is a correlation between the degree of elastolysis and histopathological staging; and different pathological staging may be the pathological manifestation of GA at different periods. However, larger samples and prospective studies are needed to validate our results further and confirm whether elastic fiber degradation is critical for the pathogenesis of pediatric GA.
Granuloma annulare (GA) is more common in children than in adults, and has diverse clinical manifestations, multiple subtypes, and unknown etiology and pathogenesis. However its correlation is rarely studied.
The main criteria for the diagnosis of GA is histopathologic biopsy, and the possible pathogenesis of GA is preliminarily discussed by analyzing the pathological characteristics of GA in children.
We analyzed and discussed the clinical manifestations and histopathological features of GA in children and performed elastic fiber staining and Alcian blue staining to initially investigate the role of elastic fiber degradation and mucin deposition in the pathogenesis of GA in children.
A total of 39 cases with a pathological diagnosis of GA in Kunming Children’s Hospital between 2017 and 2022 were retrieved, and the clinical manifestations, histopathological, and special staining characteristics of the children were analyzed.
There is a correlation between the degree of elastolysis and histopathological staging, and different pathological staging may be the pathological manifestation of GA at different periods.
Elastolysis is the key link of annular granuloma in children.
Larger samples and prospective studies are needed to further validate our results and to confirm whether elastic fiber degradation is critical for the pathogenesis of pediatric GA.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pathology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hegazy AA, Egypt; Viswanathan VK, United States S-Editor: Liu JH L-Editor: A P-Editor: Ji MX
| 1. | Barbieri JS, Rodriguez O, Rosenbach M, Margolis D. Incidence and Prevalence of Granuloma Annulare in the United States. JAMA Dermatol. 2021;157:824-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 2. | Joshi TP, Duvic M. Granuloma Annulare: An Updated Review of Epidemiology, Pathogenesis, and Treatment Options. Am J Clin Dermatol. 2022;23:37-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 3. | Leasure AC, Damsky W, Cohen JM. Prevalence of granuloma annulare in the United States: a cross-sectional study in the All of Us Research Program. Int J Dermatol. 2022;61:e301-e302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Friedman-Birnbaum R, Weltfriend S, Munichor M, Lichtig C. A comparative histopathologic study of generalized and localized granuloma annulare. Am J Dermatopathol. 1989;11:144-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Fayyazi A, Schweyer S, Eichmeyer B, Herms J, Hemmerlein B, Radzun HJ, Berger H. Expression of IFNγ, coexpression of TNFα and matrix metalloproteinases and apoptosis of T lymphocytes and macrophages in granuloma annulare. Arch Dermatol Res. 2000;292:384-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 79] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Min MS, Wu J, He H, Sanz-Cabanillas JL, Del Duca E, Zhang N, Renert-Yuval Y, Pavel AB, Lebwohl M, Guttman-Yassky E. Granuloma annulare skin profile shows activation of T-helper cell type 1, T-helper cell type 2, and Janus kinase pathways. J Am Acad Dermatol. 2020;83:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Wang A, Rahman NT, McGeary MK, Murphy M, McHenry A, Peterson D, Bosenberg M, Flavell RA, King B, Damsky W. Treatment of granuloma annulare and suppression of proinflammatory cytokine activity with tofacitinib. J Allergy Clin Immunol. 2021;147:1795-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |