Published online Jun 16, 2023. doi: 10.12998/wjcc.v11.i17.3980
Peer-review started: March 21, 2023
First decision: April 27, 2023
Revised: April 30, 2023
Accepted: May 16, 2023
Article in press: May 16, 2023
Published online: June 16, 2023
Processing time: 82 Days and 9 Hours
Kidney disease in patients with liver disease is serious and increases mortality. Up to 50% of patients hospitalized experience an episode of acute kidney injury. In general, men with liver disease are thought to be at increased risk of kidney disease. However, this association should be considered with caution because most studies use creatinine-based inclusion criteria, which is negatively biased against women. In this review, we synthesize data on sex differences in kidney disease in patients with chronic liver disease in the clinical setting and discuss potential physiologic underpinnings.
Core Tip: Kidney disease in patients with chronic liver disease is common and associated with increased morbidity and mortality. Most literature on this topic is based in men. However, there are important sex differences to consider in pathophysiology and outcomes in men and women that are poorly addressed in the literature. Here, we synthesize data on sex differences in kidney disease amongst patients with liver disease.
- Citation: Cooper KM, Colletta A, Moulton K, Ralto KM, Devuni D. Kidney disease in patients with chronic liver disease: Does sex matter? World J Clin Cases 2023; 11(17): 3980-3992
- URL: https://www.wjgnet.com/2307-8960/full/v11/i17/3980.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i17.3980
Kidney disease is a serious comorbidity in patients with chronic liver disease (CLD). Both acute and chronic kidney diseases portend a worse prognosis in patients with CLD[1-3]. Sex differences in markers of kidney function have been a topic of interest in hepatology due to the relationship between serum creatinine and access to liver transplantation in women[4]. However, there are other differences regarding kidney disease in men and women that are infrequently addressed in the literature. We aim to review the existing literature to identify sex differences in the clinical course and outcomes of men and women with CLD complicated by renal impairment. We will discuss the potential pathophysiologic basis of sex differences, including differences in splanchnic vasodilation, autonomic regulation, portal hypertension, sarcopenia, hormones, and inflammation. We will also discuss future directions and alternative markers of kidney function that may reduce gender disparities in renal outcomes.
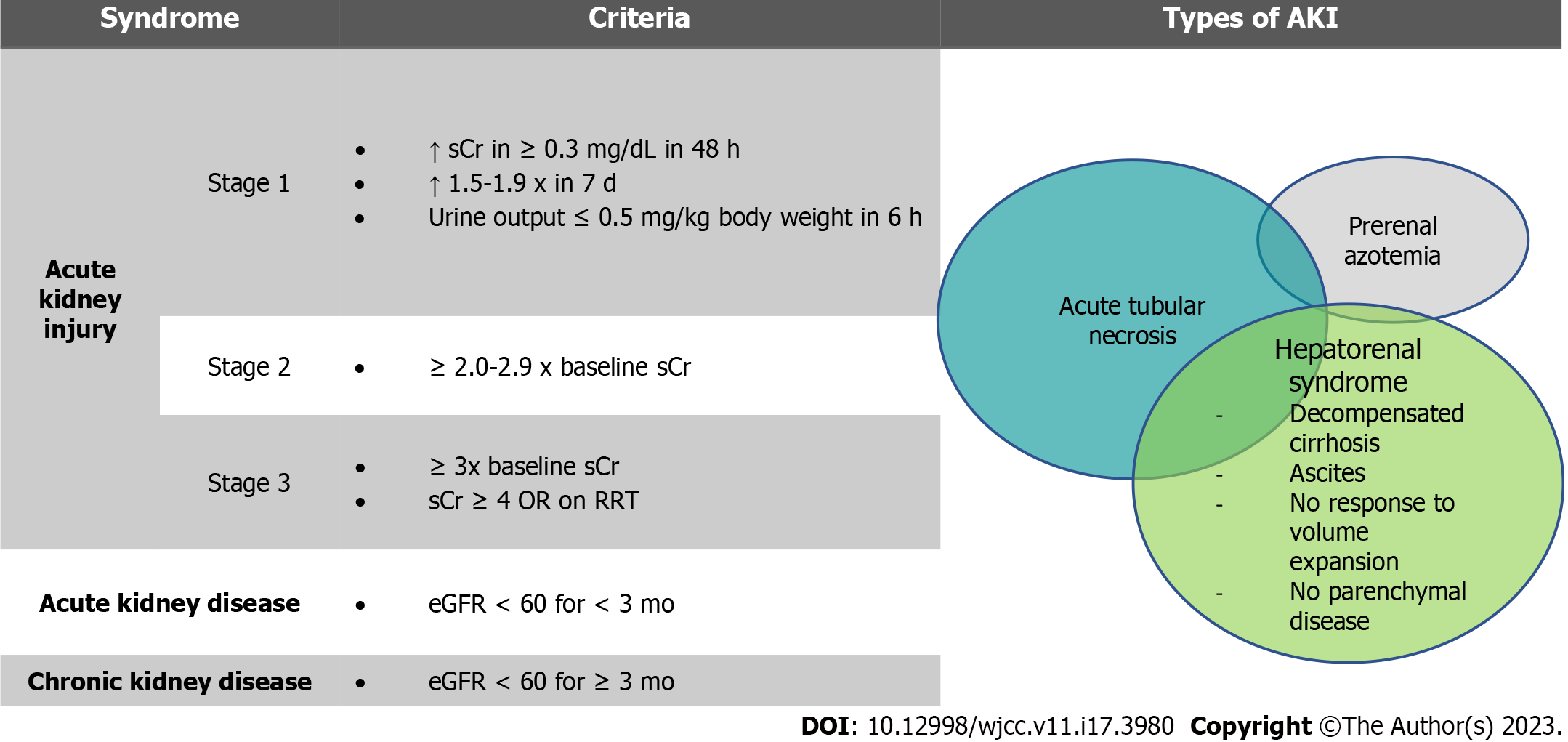
Kidney dysfunction occurs along a spectrum and is common in patients with CLD. Chronic kidney disease (CKD) affects 1 of 5 patients with cirrhosis[5] while acute kidney disease (AKD) and acute kidney injury (AKI) affect between one half and one third of decompensated patients[6]. The most common forms of AKI in patients with cirrhosis are pre-renal azotemia, acute tubular injury/necrosis, and hepatorenal syndrome (HRS)[7]. Other less common causes are IgA nephropathy, cholemic nephropathy, abdominal compartment syndrome, and nephritic syndromes related to viral hepatitis[8].
Of these pathologies, HRS is a unique entity with pathophysiology specific to advanced liver disease[9]. Prior to 2015, the diagnostic criteria for HRS included a final serum creatinine cutoff value of > 2.5 mg/dL[10,11]. However, this definition was thought to delay treatment and negatively affect outcomes[12]. Thus in 2015, the International Club of Ascites (ICA) revised the diagnostic criteria to be similar to those for AKI in other forms of critical illness without an absolute creatinine cutoff[13]. While these changes have reduced underdiagnosis of severe renal dysfunction, they have also: (1) Affected the interpretation and comparison of the literature published before and after this change period; and (2) allowed for more overlap between AKI syndromes. It is now more accepted that hepatorenal physiology occurs along a spectrum and may be present in conjunction with or independent of other causes of kidney injury[8]. The 2015 ICA guidelines also defined AKD and updated the definition of CKD (Figure 1).
There are currently no sex specific diagnostic criteria for AKI, AKD, or CKD in patients with CLD. We would like to highlight that it is unclear whether the change in diagnostic criteria will completely address underdiagnosis in women relative to men. While studies have shown that the required change of 30% serum creatinine occurs at similar rates between men and women with cirrhosis and AKI, this relies on capturing patients at baseline. Given that many patients with cirrhosis remain unrecognized until first decompensation[14], it is possible that women may already have renal impairment despite a normal presenting serum creatinine.
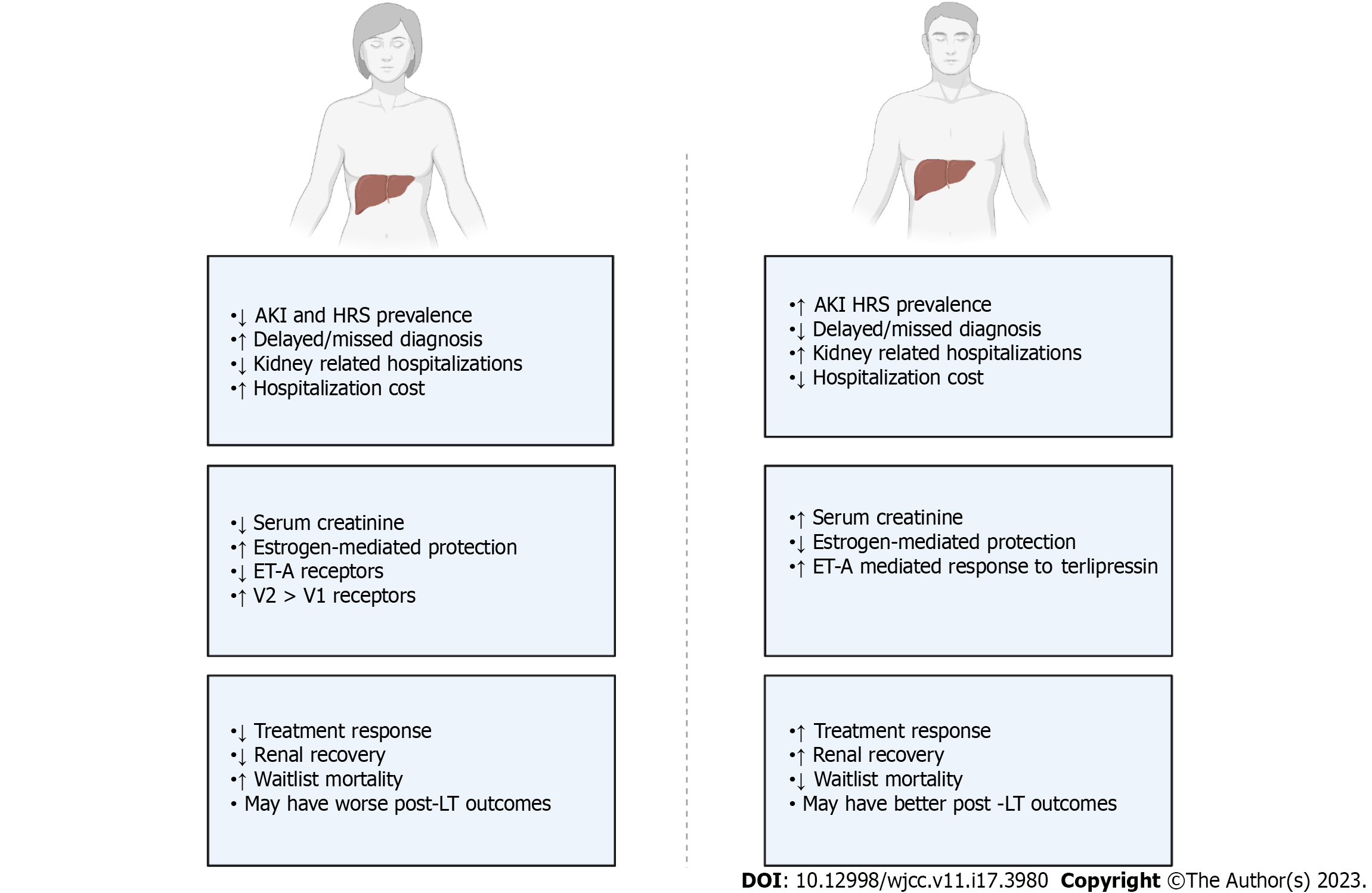
Male sex is associated with increased risk of AKI in the general population, while population-based studies indicate that CKD affects more women than men[15,16]. Similarly, many studies evaluating AKI in patients with cirrhosis show a male predominance[7]. A recent 10-year analysis found that 60%-65% of hospitalizations for cirrhosis with AKI are in male patients. This study also observed a significant increase in the number of hospitalizations for female patients over the study period[17]. The economic burden of these hospitalizations is significant. Hospitalizations for cirrhosis complicated by AKI are almost twice as costly as those without AKI[18]. In 2019, the health care burden of HRS alone in the United States was 4.2 billion dollars[19]. Some data suggest that women may have more costly hospital stays when hospitalized with AKI compared to men[20].
Thus, while most data suggest that males experience more AKI episodes, these should be interpreted with caution. First, the data suggesting a greater incidence of AKI in men is not generalizable to the diagnosis of HRS-AKI, since most studies on decompensated cirrhosis are male predominant. Second, women are at increased risk of underdiagnosis of AKI compared to men due to lower baseline serum creatinine[21]. This disparity was likely exacerbated when diagnosis of HRS required an absolute serum creatinine level > 2.5 mg/dL[22]. Thus, studies conducted prior to 2015 may have failed to capture the true sex differences. Further, the male to female ratio in many HRS studies is similar to that in decompensated cirrhosis, which suggests a similar risk between sexes rather than an increased risk specific to men.
The relationship between sex and persistent kidney disease in patients with cirrhosis is less clear. Female sex has been associated with what was previously considered type 2 HRS, which is now more consistent with HRS-CKD[23]. Conversely, a large consortium study found that there was no difference in the risk of AKD in men and women hospitalized for cirrhosis with AKI[2]. Similarly, an analysis of hospitalized patients with cirrhosis found rates of established CKD to be similar in men and women. This study also found that males had decompensated liver disease significantly more often and did not control for how this affected risk of CKD[5]. However, of those that have received a liver transplant, female sex is a risk factor for post-transplant CKD; this may suggest more significant yet underestimated pre-transplant kidney dysfunction in women[24].
To date, there are limited studies evaluating sex differences in kidney disease in patients with specific etiologies of cirrhosis. A small analysis conducted in patients hospitalized with alcohol-related liver disease found males to make up a larger proportion of the AKI cohort than non-AKI cohort (76% vs 57%, P = 0.006), but did not identify male sex as a predictor of AKI on multivariate regression modeling[25]. Patterns in non-alcoholic fatty liver disease (NAFLD) are unique compared with those in other etiologies: Men appear to be at increased risk of CKD compared to women but they have similar risk of HRS[26]. A study using iothalamate clearance tests to study renal function in a cohort of NAFLD patients found that more women had a glomerular filtration rate (GFR) less than 60 mL/min than men prior to liver transplant (39% vs 27%)[27]. Unfortunately, there is limited data on kidney disease in autoimmune related liver diseases (e.g., autoimmune hepatitis and primary biliary cirrhosis). As these diseases are female predominant, their underrepresentation in the literature further supports the bias surrounding CKD and AKI/AKI-HRS[28]. This concept is bolstered by lack of sex-specific data in cirrhosis from viral hepatitis, despite these being some of the most prevalent worldwide.
For HRS-AKI specifically, many studies have been performed in male predominant populations. However, a recent Cochrane meta-analysis demonstrates variability in sex composition among studies employing medical treatment for HRS. In the 25 studies included, females comprised 6%-61% of study participants with a pooled average of 28%[28]. A nationwide analysis of over 500000 patients hospitalized for cirrhosis noted that a higher proportion of men had a diagnosis of HRS compared to women (3.9% vs 3.3%, P < 0.01)[5]. While this evidence purports a higher risk of HRS in males, the authors did not provide diagnostic criteria used in this determination and thus this data should be interpreted with caution. Ultimately, high quality studies that directly compare the risk of HRS-AKI in men and women are lacking. Interestingly, there is a female predominance amongst pediatric patients who develop HRS as this most often occurs in the setting of biliary atresia, a female predominant congenital malformation[29]. Thus, studies in the pediatric population may provide a unique opportunity to limit the underrepresentation of female subjects and better understand sex differences in HRS.
The negative impact of AKI on survival in this cohort is well recognized. Up to 50% of patients hospitalized with decompensated cirrhosis present with or develop AKI[7,30,31]. A recent meta-analysis of 32 high quality studies found that AKI was associated with significantly higher mortality in-hospital [odds ratio (OR) 5.92], at 30 d (OR 4.78), at 90 d (OR 4.34), and at 1 year follow-up (OR 4.82) compared to patients without AKI[32]. Of note, the authors did not present sex-specific differences in outcomes.
Women hospitalized with AKI may be less likely to recover normal renal function compared to men. Patidar et al[6] found that female sex was a predictor of persistently elevated serum creatinine 3 mo after an AKI event. While we are unaware of studies reporting sex specific data regarding response to treatment with midodrine-octreotide, there are definitive sex differences in response to terlipressin treatment for HRS-AKI. A study by Wong et al[33] demonstrated that male sex is a predictor of improvement of AKI in patients with HRS-AKI, regardless of initial AKI stage. Overall, they found that men were 1.58 times as likely to have a clinically significant reduction in serum creatinine compared to women after 14 d of treatment with terlipressin plus albumin.
A randomized, multicenter, placebo-controlled, double-blind study assessing the efficacy and safety of intravenous terlipressin plus albumin vs placebo plus albumin found that HRS reversal in female patients (15.6%) was lower than that in male patients (23.1%). Furhter, when evaluating for baseline factors predictive of a response to terlipressin and overall survival using multivariate logistic regression, male sex was a significant positive predictor of overall survival (P = 0.02)[34]. Frederick et al[35] analyzed pooled data from three landmark studies on terlipressin use in HRS and found that while both men and women have some improvement in serum creatinine with treatment, men were significantly more likely to achieve HRS-AKI reversal. The lower response in women may reflect more severe renal dysfunction at the time of treatment initiation. This is consistent with data showing worse kidney disease in women compared to men despite lower serum creatinine, due to inherent differences in muscle mass[36]. Despite these differences, there remain no sex specific recommendations to guide treatment[37].
For patients with HRS-AKI and poorly controlled ascites, transjugular intrahepatic shunt (TIPS) has been utilized[38]. Trivedi et al[39] studied outcomes for HRS-AKI after TIPS and found that male patients had better outcomes than female patients. Men with HRS-AKI treated with TIPS experienced a reduction in mortality compared to medical management. Conversely, women with HRS-AKI treated with TIPS had no mortality benefit compared to medical management. Moreover, women were at a striking six-fold increased risk of death after TIPS compared to men. Of note, the use of TIPS in management of HRS-AKI is not well studied and is not recommended by the American Association for the Study of Liver Disease (AASLD) at this time[9].
Patients who do not respond to medical therapy for HRS require renal replacement therapy (RRT), often as a bridge to liver transplant evaluation. While women respond less to treatment, sex differences in utilization of RRT are inconsistent. A 15-center consortium study found that women with AKI received RRT more often than men, despite a similar change in serum creatinine from baseline[31]. In patients not listed for transplant, a larger proportion of women received RRT than men[40]. Conversely, a large study evaluating over 3 million hospitalizations for decompensated cirrhosis over a 6-year period found male sex to be an independent predictor of AKI requiring RRT. Specifically, the study found that AKI requiring RRT occurred in 2.2% of male hospitalizations for decompensated cirrhosis compared to 2.1% of female hospitalizations for decompensated cirrhosis. Interestingly, the study did not comment on sex differences in AKI without RRT. Further, AKI requiring RRT was associated with increased hospital complications and death, but the authors did not comment on sex differences in these events. Most importantly, the study did not discuss access to transplantation and how this affected the relationship between sex and RRT utilization[41]. The heterogenous nature of these findings is not surprising given the complexity of pursuing dialysis in patients with cirrhosis. Specifically, dialysis candidacy is closely associated with access to and candidacy for liver transplantation in patients with irreversible severe AKI associated with hepatic decompensation[42,43]. As such, requiring dialysis is not necessarily associated with receiving dialysis in patients who are deemed not to be candidates for liver transplantation. Interestingly, amongst patients with lower GFR, female transplant candidates have increased mortality relative to male candidates when there are no differences in transplant access[36].
Once initiated on dialysis, there appear to be no differences in renal outcomes or mortality in the acute setting. A study by Wang et al[44] utilized multivariable competing risks analysis to identify predictors of kidney recovery with death and transplant as competing events and found no differences between men and women. This has also been shown among both transplant and non-transplant candidates for whom RRT was initiated for ATN-AKI or HRS-AKI[40]. Similarly, there are no known sex differences in renal recovery or mortality once initiated on chronic maintenance RRT[45]. There appear to be sex differences in post liver transplant outcomes for men and women with pre-liver transplant AKI. A study performed in living donor liver transplant recipients found that men with HRS may be at reduced risk of death after transplant compared to women with HRS (P = 0.07)[46].
The remainder of this review will focus on physiologic processes that may contribute to sex-based differences in kidney disease. Most of this section will focus on HRS given its specificity to advanced liver disease and unique pathophysiology. Specifically, HRS is a functional renal disorder caused by a complex cascade of circulatory and inflammatory changes that result in dysregulated renal vasoconstriction and reduced renal perfusion (Figure 2)[9]. In general, severe portal hypertension in the setting of CLD leads to low systemic vascular resistance (SVR) and splanchnic vasodilatation. Together, splanchnic vasodilation and low SVR result in reduced effective circulating volume which activates central baroreceptors and the renin-angiotensin-aldosterone system (RAAS). The RAAS activation increases over time with the declining ability of the cirrhotic liver to degrade renin. In parallel, systemic inflammation related to translocation of enteric bacteria results in nitric oxide production which exacerbates peripheral vasodilation and central hypovolemia. The interplay of hemodynamic changes with systemic inflammation ultimately predisposes patients to hemodynamic collapse when exposed to physiologic stressors such as gastrointestinal bleeding, dehydration, or infection. The second hit typically leads to a further impairment of circulatory dysfunction and worsening renal perfusion and ultimately development of severe AKI.
Sex differences in splanchnic circulation and portal pressures may contribute to differences in HRS-AKI outcomes. First, sex differences in splanchnic autoregulation have been demonstrated in the literature. Specifically, women have been found to have smaller splanchnic vasoconstrictor reserve than men[47]. For example, women have been shown to have lower tilt table tolerance associated with failure to increase splanchnic blood flow relative to circulation. Though this improves with octreotide administration, sex differences persist despite treatment with somatostatin analogue[48]. The latter finding may suggest that women have a decreased response to midodrine and octreotide based HRS protocols, though no studies have investigated this further.
Conversely, there are established sex differences in treatment response for HRS using TIPS. The goal of a TIPS procedure is to redirect flow in the portal system to minimize complications of portal hypertension, as reflected by a reduction in the hepatic venous pressure gradient (HVPG). The HVPG serves as a diagnostic tool in portal hypertension and has prognostication value for related clinical outcomes (ascites, varices, and HRS)[49]. An HVPG greater than or equal to 12 mmHg has been associated with advanced fibrosis and clinically significant portal hypertension[50]. However, this cutoff is derived from mostly male predominant studies. A recent study by Wortham et al[51] showed that men and women may respond differently to similar portal pressure. The study followed 20 patients (12 female, 8 male) with compensated cirrhosis who were monitored for clinical decompensation over a three-year period. They found that females tended to decompensate more than males (7 females vs 1 male, P = 0.13) and the HPVG at the time of decompensation was similar between groups. More females decompensating at similar HPVG suggest that women may not tolerate higher portal pressures as well as men. Ultimately, differences in tolerance to elevated portal pressures and changes in splanchnic hemodynamics may contribute to lower response to TIPS in women or may suggest different contributions of portal hypertension to the development of HRS across genders. Unfortunately, this is not well explored in the literature and no study has specifically assessed sex differences in HVPG with regards to predisposition for development of HRS.
Current data suggest that women respond differently to terlipressin treatment than men. Pooled data from 535 patients across three randomized control trials found that women were less likely to have improvement in serum creatinine with terlipressin[35]. This discrepancy in response persists when matching males and females by serum creatinine. While there is no clear reason for this incongruence, systemic vasoregulation is likely at the crux of these findings.
First, there are established sex differences in RAAS response as men and women are known to respond differently to both RAAS activation and inhibition[52]. For example, women have enhanced response to angiotensin stimulation at the level of the kidneys[53,54]. Hyperresponsiveness has been shown to accelerate renal disease and decompensation in mice models of congestive heart failure, another state of decreased renal perfusion[55]. Unfortunately, this has yet to be studied within the context of cirrhosis. However, given that RAAS dysregulation is a key component of HRS development, it is difficult to discount this as a potential driving factor in sex differences.
Receptor level differences in vasoregulatory response may contribute to differences in treatment response observed between sexes. Terlipressin acts predominantly trough vasopressin receptor 1 (V1R). Interestingly, pre-clinical models have found that females may have increased V2 receptor expression in the kidney compared to males[56]. Studies in other human organ tissue have also shown that males have increased V1R binding activity than females. It is possible that there is increased V2 stimulation relative to V1 stimulation in women, which may evoke a weaker treatment response.
Endothelin-1 (ET-1) is a vasoconstrictor that acts via endothelin-A receptors at the level of the kidneys and thus has potential implications in the dysregulation of renal microcirculation in HRS[57,58]. While terlipressin is not known to act directly on endothelin receptors, Abdel-Razik et al[59] found that a positive renal response to terlipressin was associated with early reduction of serum ET-1 Levels. Interestingly, women express fewer endothelin A receptors than men systemically. Differences in endothelin-A receptor expression may explain why terlipressin treatment is associated with a greater rate of HRS reversal in men[35]. Similarly, it may account for sex differences in rate of HRS, as women are less prone to ET-1 mediated vasoconstriction of renal microvasculature. Conversely, it may suggest different underlying HRS pathophysiology in men and women.
Differences in sex hormones may portend differing risk profiles for HRS by gender. Estrogen has been shown to have multiple protective effects on the kidneys, including reduction of apoptosis, promoting mesangial cell enhancement, and regulating the renin-angiotensin system[60,61]. Conversely, androgens have been shown to have a negative impact due to promotion of tubular injury and upregulation of the renin-angiotensin system[62,63]. Furthermore, a study evaluating the relationship between renal function and muscle mass reported that loss of testosterone promotes sarcopenia which may exacerbate renal dysfunction independent of other causes.
While no studies have assessed sex hormones in HRS specifically, animal studies have shown estrogen to be protective against toxin driven hepatorenal toxicity[64]. In clinical practice, post-menopausal women have been shown to be at higher risk for kidney injury compared to pre-menopausal women, likely due to loss of estrogen mediated protection[65,66]. However, no studies evaluating AKI risk by menstrual state have been conducted in patients with CLD.
It is important to note that elevated levels of estradiol and progesterone are present at baseline both in men and women with cirrhosis due to impaired metabolism[67]. It is unclear whether this creates a dose dependent protective response in women or counteracts androgen mediated damage in men. In future studies, evaluating sex differences of HRS in the pediatric population could provide further insight to these relationships given presumably less profound sex differences in muscle mass and steroid hormones in pediatrics compared to adults.
Systemic inflammation is common in CLD and increases with disease progression[68]. Two key inflammatory markers are interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) which have been shown to have predictive power in hepatic decompensation, including HRS[69,70]. Patients with HRS-AKI have marked systemic inflammation with altered cytokine profile relative to patients with decompensated cirrhosis without HRS-AKI. Further, inflammatory makers have implications in treatment response and monitoring. Sole et al[71] showed that response to terlipressin is associated with a change in cytokine levels and significant reduction in IL-6 and TNF-α.
While there are studies showing sex specific inflammatory responses in certain subsets of CLD[72], this type of literature in HRS is lacking. Most HRS studies are male predominant and do not comment on sex differences in systemic inflammation. However, there are physiologic differences that may serve as a basis for sex differences in inflammation. As described, females express fewer endothelin-A receptors than males and prolonged ET-1 activity is associated with the release of inflammatory cytokines, including TNF-α and IL-6, in smooth muscle cells in the vasculature and mesangial cells in the kidney[73-75]. This calls to question whether ET-1 promotes local inflammation at the level of the renal vasculature and whether that inflammation contributes to sex differences in HRS incidence and treatment response[76]. Tissue hypoxia is known to increase local inflammation in the kidney[77]. Studies assessing the relationship between anemia and HRS development have found low serum hemoglobin to be associated with HRS type 2[78]. As anemia is more common and is more pronounced in males with CLD[79], it is possible that hypoxia related inflammation predisposes males to the more profound inflammatory response in HRS-AKI.
Pathologic bacterial translocation and infection cause a significant and systemic inflammatory response and are well-known triggers of HRS[80,81]. Unfortunately, there are no studies assessing sex differences in inflammatory response to infection in patients with HRS. Ultimately, given the profound effect of inflammation on the development of HRS, further studies are needed to determine if a relationship between gender and inflammation exists.
Serum creatinine is the standard biochemical marker of renal function in most health care settings, yet it has several limitations in patients with liver disease. Serum creatinine underestimates the severity of kidney dysfunction in patients with CLD due to a reduction in the hepatic synthesis of creatinine, severe malnutrition, sarcopenia, and anasarca[82-84]. This is more pronounced in women due to the inherent differences in muscle mass between sexes. Thus, absolute creatinine values and creatinine based GFR calculations fail to represent kidney function equally across genders as described; reliance on absolute serum creatinine level cutoffs likely results in both underdiagnosing and delaying diagnosis of HRS in women[85,86]. Further, the Model for End-stage Liver Disease-Sodium (MELD-Na) includes serum creatinine as one of its four values. The MELD-Na score not only predicts mortality in patients with end-stage liver disease but also directly determines priority on the liver transplant waitlist. The use of serum creatinine in the MELD-Na score has been shown to perpetuate disparities in access to liver transplantation in women.
Multiple studies that utilized creatinine-based definitions of AKI discuss the differential impact of creatinine by sex. In a study by Mindikoglu et al[36], women on the liver transplant list had not only lower serum creatinine compared to men, but also lower true GFR. Despite more severe renal impairment, women were also less likely to receive dialysis than men due to the use of creatinine based GFR estimations. O’Leary et al[31] found that women required dialysis and had a worse prognosis compared to men despite similar levels of serum creatinine. Similarly, Frederick et al[35] attributed differences in response rate to terlipressin in part to delayed diagnosis and treatment in female patients due to lower overall serum creatine. Underdiagnosis of renal dysfunction in women is also supported by the literature[36,87]. A study by Yoo et al[87] found that creatinine overestimated GFR significantly more often in females than in males (152/192 females and 303/587 males). The authors concluded that serum creatine concentrations were controlled by different mechanisms in men compared to woman[87].
New strategies monitor kidney function in patients with decompensated liver disease are under investigation. Measuring GFR directly (using exogenous substances) may better approximate renal function in women[88]. However, this is time consuming and difficult to complete during acute decompensation and in the hospital setting. Likewise, using urine output-based definitions of kidney injury in theory would have less sex differences because these are based on volume per kilogram. While less expensive than obtaining a measured GFR, monitoring intake and output in the hospital is labor intensive and requires patients’ strict adherence or the insertion of urethral catheters, which: (1) Increases risk of infection, and (2) is not indicated in most patients outside of the intensive care setting. MicroRNAs have garnered attention as innovative markers to evaluate kidney injury in cirrhotic patients due to their ability to differentiate ATN and HRS. However, pre-clinical models have shown that these markers are expressed variably across sexes[89]. Fortunately, there is increasing literature on alternative biomarkers for renal function. Two newer markers shown to be predictive of renal dysfunction in cirrhosis include cystatin C and neutrophil gelatinase-associated lipocalin (NGAL)[90].
NGAL is a small protein produced by the distal nephron in response to kidney injury[91]. Unlike serum creatinine concentrations, its value does not vary widely with age, gender, muscle mass, muscle metabolism, and hydration status[91]. Huelin et al[92] showed that urinary NGAL has high accuracy in differentiating between ATN, HRS, and pre-renal azotemia. Gambino et al[93] identified a urinary NGAL level of 220 ng/mL to be sensitive and specific in differentiating these pathologies, predicting treatment response to terlipressin, and predicting mortality. Unfortunately, this study did not have equal gender representation with 76.5% of participants being male. Interestingly, of the patients who died during the hospitalization, only 70% were male (P = 0.135). Although this did not reach the threshold for significance, the trend raises the question of whether a larger and more balanced study population would lead to different results.
Cystatin C has gained attraction for its superiority in assessing GFR and lack of sex specificity. This is because cystatin C is produced by all nucleated cells and serum levels are independent of muscle mass[94]. Cystatin C is better correlated with GFR than serum creatinine in patients of all Child-Pugh classes and more accurately estimates GFR for patients with a true GFR less than 70 mL/min[95,96]. Further, serum cystatin C level is an independent predictor of HRS[97]. Unlike creatinine, cystatin C does not depend on muscle mass or age and is not influenced by inflammatory disorders or malignancy. Multiple studies have found cystatin C to be a more neutral marker of renal function. First, the sensitivity of cystatin C is significantly higher (P < 0.05) than that of creatinine in women with cirrhosis[95]. Furthermore, Mindikoglu et al[36] found no association between cystatin C and biologic sex after controlling for potential confounding factors[98]. Lastly, Torner et al[99] found that cystatin C was a predictor of mortality in both male and female patients with HRS compared to creatinine which only predicted mortality in males.
Despite kidney disease in patients with cirrhosis being thoroughly studied and well documented in the literature, information regarding sex differences in liver disease is overall lacking (see summary Figure 3). It is known that serum creatinine continues to pose a barrier to care for women with cirrhosis who develop renal dysfunction. Additionally, the use of creatinine in MELD-Na scoring limits and may even preclude access to liver transplant in women with more advanced renal dysfunction, whose creatinine does not accurately represent their true GFR. However, the historical use of serum creatinine as a marker for AKI in cirrhosis complicates interpretation of existing data relative to sex. Newer studies that employ updated AKI definitions demonstrate sex differences in clinical course and outcomes, suggesting that there are likely underlying pathophysiologic differences beyond serum creatinine between men and women. Given that there are known differences in renal physiology and risk of renal dysfunction between men and women, further research is needed to define sex specific diagnostic and treatment recommendations for kidney disease in patients with cirrhosis.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: American Association for the Study of Liver Diseases; American Society of Transplanation; Society of Hospital Medicine.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cabezuelo AS, Spain; Ferrarese A, Italy S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Zhao S
| 1. | Wong F, Reddy KR, O'Leary JG, Tandon P, Biggins SW, Garcia-Tsao G, Maliakkal BJ, Lai JC, Fallon MB, Vargas HE, Subramanian R, Thuluvath PJ, Kamath PS, Thacker L, Bajaj JS. Impact of Chronic Kidney Disease on Outcomes in Cirrhosis. Liver Transpl. 2019;25:870-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 2. | Wong F, Garcia-Tsao G, Reddy KR, O'Leary JG, Kamath PS, Tandon P, Lai JC, Vargas HE, Biggins SW, Fallon MB, Thuluvath PJ, Maliakkal BJ, Subramanian R, Thacker L, Bajaj JS. Prognosis of hospitalized patients with cirrhosis and acute kidney disease. Liver Int. 2022;42:896-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Scott RA, Austin AS, Kolhe NV, McIntyre CW, Selby NM. Acute kidney injury is independently associated with death in patients with cirrhosis. Frontline Gastroenterol. 2013;4:191-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Allen AM, Heimbach JK, Larson JJ, Mara KC, Kim WR, Kamath PS, Therneau TM. Reduced Access to Liver Transplantation in Women: Role of Height, MELD Exception Scores, and Renal Function Underestimation. Transplantation. 2018;102:1710-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 133] [Article Influence: 22.2] [Reference Citation Analysis (1)] |
| 5. | Rubin JB, Sundaram V, Lai JC. Gender Differences Among Patients Hospitalized With Cirrhosis in the United States. J Clin Gastroenterol. 2020;54:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Patidar KR, Naved MA, Grama A, Adibuzzaman M, Aziz Ali A, Slaven JE, Desai AP, Ghabril MS, Nephew L, Chalasani N, Orman ES. Acute kidney disease is common and associated with poor outcomes in patients with cirrhosis and acute kidney injury. J Hepatol. 2022;77:108-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology. 2008;48:2064-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 457] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 8. | Cullaro G, Kanduri SR, Velez JCQ. Acute Kidney Injury in Patients with Liver Disease. Clin J Am Soc Nephrol. 2022;17:1674-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 9. | Biggins SW, Angeli P, Garcia-Tsao G, Ginès P, Ling SC, Nadim MK, Wong F, Kim WR. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 460] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 10. | Arroyo V, Ginès P, Gerbes AL, Dudley FJ, Gentilini P, Laffi G, Reynolds TB, Ring-Larsen H, Schölmerich J. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996;23:164-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1084] [Cited by in RCA: 1020] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 11. | Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 356] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 12. | Boyer TD, Sanyal AJ, Garcia-Tsao G, Blei A, Carl D, Bexon AS, Teuber P; Terlipressin Study Group. Predictors of response to terlipressin plus albumin in hepatorenal syndrome (HRS) type 1: relationship of serum creatinine to hemodynamics. J Hepatol. 2011;55:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 186] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 13. | Angeli P, Gines P, Wong F, Bernardi M, Boyer TD, Gerbes A, Moreau R, Jalan R, Sarin SK, Piano S, Moore K, Lee SS, Durand F, Salerno F, Caraceni P, Kim WR, Arroyo V, Garcia-Tsao G; International Club of Ascites. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut. 2015;64:531-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 387] [Article Influence: 38.7] [Reference Citation Analysis (1)] |
| 14. | Blais P, Husain N, Kramer JR, Kowalkowski M, El-Serag H, Kanwal F. Nonalcoholic fatty liver disease is underrecognized in the primary care setting. Am J Gastroenterol. 2015;110:10-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 15. | Loutradis C, Pickup L, Law JP, Dasgupta I, Townend JN, Cockwell P, Sharif A, Sarafidis P, Ferro CJ. Acute kidney injury is more common in men than women after accounting for socioeconomic status, ethnicity, alcohol intake and smoking history. Biol Sex Differ. 2021;12:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 16. | Carrero JJ, Hecking M, Chesnaye NC, Jager KJ. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol. 2018;14:151-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 553] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 17. | Singh J, Dahiya DS, Kichloo A, Singh G, Khoshbin K, Shaka H. Hepatorenal syndrome: a Nationwide Trend Analysis from 2008 to 2018. Ann Med. 2021;53:2018-2024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Desai AP, Knapp SM, Orman ES, Ghabril MS, Nephew LD, Anderson M, Ginès P, Chalasani NP, Patidar KR. Changing epidemiology and outcomes of acute kidney injury in hospitalized patients with cirrhosis - a US population-based study. J Hepatol. 2020;73:1092-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 19. | Singal AK, Kuo YF, Reddy KR, Bataller R, Kwo P. Healthcare burden and outcomes of hepatorenal syndrome among cirrhosis-related hospitalisations in the US. Aliment Pharmacol Ther. 2022;56:1486-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Jamil K, Huang X, Lovelace B, Pham AT, Lodaya K, Wan G. The burden of illness of hepatorenal syndrome (HRS) in the United States: a retrospective analysis of electronic health records. J Med Econ. 2019;22:421-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Grams ME, Sang Y, Ballew SH, Gansevoort RT, Kimm H, Kovesdy CP, Naimark D, Oien C, Smith DH, Coresh J, Sarnak MJ, Stengel B, Tonelli M; CKD Prognosis Consortium. A Meta-analysis of the Association of Estimated GFR, Albuminuria, Age, Race, and Sex With Acute Kidney Injury. Am J Kidney Dis. 2015;66:591-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 137] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 22. | Flamm SL, Wong F, Ahn J, Kamath PS. AGA Clinical Practice Update on the Evaluation and Management of Acute Kidney Injury in Patients With Cirrhosis: Expert Review. Clin Gastroenterol Hepatol. 2022;20:2707-2716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 23. | Rey RM, Delgado AF, De Zubiria A, Pinto R, De la Hoz-Valle JA, Pérez-Riveros ED, Ardila G, Sierra-Arango F. Prevalence and short-term outcome of hepatorenal syndrome: A 9-year experience in a high-complexity hospital in Colombia. PLoS One. 2020;15:e0239834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Tan HK, Marquez M, Wong F, Renner EL. Pretransplant Type 2 Hepatorenal Syndrome Is Associated With Persistently Impaired Renal Function After Liver Transplantation. Transplantation. 2015;99:1441-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Ravi S, Kaif M, Black T, Singal A. Acute Kidney Injury Among Hospitalized Patients With Alcoholic Liver Disease and Acute or Chronic Liver Failure: 2132. Offic J of ACG. 2015;110:S890-S1. [DOI] [Full Text] |
| 26. | Abu-Freha N, Cohen B, Weissmann S, Hizkiya R, Abu-Hammad R, Taha G, Gordon M. Comorbidities and Outcomes among Females with Non-Alcoholic Fatty Liver Disease Compared to Males. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 27. | Fussner LA, Charlton MR, Heimbach JK, Fan C, Dierkhising R, Coss E, Watt KD. The impact of gender and NASH on chronic kidney disease before and after liver transplantation. Liver Int. 2014;34:1259-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Best LM, Freeman SC, Sutton AJ, Cooper NJ, Tng EL, Csenar M, Hawkins N, Pavlov CS, Davidson BR, Thorburn D, Cowlin M, Milne EJ, Tsochatzis E, Gurusamy KS. Treatment for hepatorenal syndrome in people with decompensated liver cirrhosis: a network meta-analysis. Cochrane Database Syst Rev. 2019;9:CD013103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 29. | Elizabeth Parsons C, Nelson R, Book LS, Kyle Jensen M. Renal replacement therapy in infants and children with hepatorenal syndrome awaiting liver transplantation: a case-control study. Liver Transpl. 2014;20:1468-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Huelin P, Piano S, Solà E, Stanco M, Solé C, Moreira R, Pose E, Fasolato S, Fabrellas N, de Prada G, Pilutti C, Graupera I, Ariza X, Romano A, Elia C, Cárdenas A, Fernández J, Angeli P, Ginès P. Validation of a Staging System for Acute Kidney Injury in Patients With Cirrhosis and Association With Acute-on-Chronic Liver Failure. Clin Gastroenterol Hepatol. 2017;15:438-445.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 130] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 31. | O'Leary JG, Wong F, Reddy KR, Garcia-Tsao G, Kamath PS, Biggins SW, Fallon MB, Subramanian RM, Maliakkal B, Thacker L, Bajaj JS. Gender-Specific Differences in Baseline, Peak, and Delta Serum Creatinine: The NACSELD Experience. Dig Dis Sci. 2017;62:768-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 32. | Ning Y, Zou X, Xu J, Wang X, Ding M, Lu H. Impact of acute kidney injury on the risk of mortality in patients with cirrhosis: a systematic review and meta-analysis. Ren Fail. 2022;44:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 33. | Wong F, Boyer TD, Sanyal AJ, Pappas SC, Escalante S, Jamil K. Reduction in acute kidney injury stage predicts survival in patients with type-1 hepatorenal syndrome. Nephrol Dial Transplant. 2020;35:1554-1561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 34. | Boyer TD, Sanyal AJ, Wong F, Frederick RT, Lake JR, O'Leary JG, Ganger D, Jamil K, Pappas SC; REVERSE Study Investigators. Terlipressin Plus Albumin Is More Effective Than Albumin Alone in Improving Renal Function in Patients With Cirrhosis and Hepatorenal Syndrome Type 1. Gastroenterology. 2016;150:1579-1589.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 223] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 35. | Frederick RT, Pappas C, Jamil K. Gender affects the association between serum creatinine levels and clinical response to terlipressin in patients with hepatorenal syndrome type of acute kidney injury. J Hepatol. 2022;77:S611. [DOI] [Full Text] |
| 36. | Mindikoglu AL, Regev A, Seliger SL, Magder LS. Gender disparity in liver transplant waiting-list mortality: the importance of kidney function. Liver Transpl. 2010;16:1147-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 37. | Flamm SL, Brown K, Wadei HM, Brown RS Jr, Kugelmas M, Samaniego-Picota M, Burra P, Poordad F, Saab S. The Current Management of Hepatorenal Syndrome-Acute Kidney Injury in the United States and the Potential of Terlipressin. Liver Transpl. 2021;27:1191-1202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 38. | Rössle M, Gerbes AL. TIPS for the treatment of refractory ascites, hepatorenal syndrome and hepatic hydrothorax: a critical update. Gut. 2010;59:988-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 165] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 39. | Trivedi PS, Brown MA, Rochon PJ, Ryu RK, Johnson DT. Gender Disparity in Inpatient Mortality After Transjugular Intrahepatic Portosystemic Shunt Creation in Patients Admitted With Hepatorenal Syndrome: A Nationwide Study. J Am Coll Radiol. 2020;17:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Allegretti AS, Parada XV, Eneanya ND, Gilligan H, Xu D, Zhao S, Dienstag JL, Chung RT, Thadhani RI. Prognosis of Patients with Cirrhosis and AKI Who Initiate RRT. Clin J Am Soc Nephrol. 2018;13:16-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 110] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 41. | Nadkarni GN, Simoes PK, Patel A, Patel S, Yacoub R, Konstantinidis I, Kamat S, Annapureddy N, Parikh CR, Coca SG. National trends of acute kidney injury requiring dialysis in decompensated cirrhosis hospitalizations in the United States. Hepatol Int. 2016;10:525-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Francoz C, Durand F, Kahn JA, Genyk YS, Nadim MK. Hepatorenal Syndrome. Clin J Am Soc Nephrol. 2019;14:774-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 43. | Velez JCQ. Patients with Hepatorenal Syndrome Should Be Dialyzed? PRO. Kidney360. 2021;2:406-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | Wang PL, Silver SA, Djerboua M, Thanabalasingam S, Zarnke S, Flemming JA. Recovery From Dialysis-Treated Acute Kidney Injury in Patients With Cirrhosis: A Population-Based Study. Am J Kidney Dis. 2022;80:55-64.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 45. | McAllister S, Lai JC, Copeland TP, Johansen KL, McCulloch CE, Kwong YD, Seth D, Grimes B, Ku E. Renal Recovery and Mortality Risk among Patients with Hepatorenal Syndrome Receiving Chronic Maintenance Dialysis. Kidney360. 2021;2:819-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Park CS, Yoon YI, Kim N, Hwang S, Ha TY, Jung DH, Song GW, Moon DB, Ahn CS, Park GC, Kim KH, Cho YP, Lee SG. Analysis of outcomes and renal recovery after adult living-donor liver transplantation among recipients with hepatorenal syndrome. Am J Transplant. 2022;22:2381-2391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Jarvis SS, Florian JP, Curren MJ, Pawelczyk JA. Sex differences in vasoconstrictor reserve during 70 deg head-up tilt. Exp Physiol. 2010;95:184-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 48. | Jarvis SS, Florian JP, Curren MJ, Pawelczyk JA. A somatostatin analog improves tilt table tolerance by decreasing splanchnic vascular conductance. J Appl Physiol (1985). 2012;112:1504-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 49. | Ripoll C, Groszmann R, Garcia-Tsao G, Grace N, Burroughs A, Planas R, Escorsell A, Garcia-Pagan JC, Makuch R, Patch D, Matloff DS, Bosch J; Portal Hypertension Collaborative Group. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 811] [Article Influence: 45.1] [Reference Citation Analysis (1)] |
| 50. | Suk KT, Kim DJ. Staging of liver fibrosis or cirrhosis: The role of hepatic venous pressure gradient measurement. World J Hepatol. 2015;7:607-615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 51. | Wortham A, Khalifa A, Rockey DC. The natural history of patients with compensated cirrhosis and elevated hepatic venous pressure gradient. Portal Hypertension & Cirrhosis. 2022;1:101-106. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Colafella KMM, Denton KM. Sex-specific differences in hypertension and associated cardiovascular disease. Nat Rev Nephrol. 2018;14:185-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 322] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 53. | Barsha G, Mirabito Colafella KM, Walton SL, Gaspari TA, Spizzo I, Pinar AA, Hilliard Krause LM, Widdop RE, Samuel CS, Denton KM. In Aged Females, the Enhanced Pressor Response to Angiotensin II Is Attenuated By Estrogen Replacement via an Angiotensin Type 2 Receptor-Mediated Mechanism. Hypertension. 2021;78:128-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 54. | Gandhi SK, Gainer J, King D, Brown NJ. Gender affects renal vasoconstrictor response to Ang I and Ang II. Hypertension. 1998;31:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 55. | Krátký V, Kikerlová S, Husková Z, Sadowski J, Kolář F, Červenka L. Enhanced Renal Vascular Responsiveness to Angiotensin II and Norepinephrine: A Unique Feature of Female Rats with Congestive Heart Failure. Kidney Blood Press Res. 2019;44:1128-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 56. | Liu J, Sharma N, Zheng W, Ji H, Tam H, Wu X, Manigrasso MB, Sandberg K, Verbalis JG. Sex differences in vasopressin V₂ receptor expression and vasopressin-induced antidiuresis. Am J Physiol Renal Physiol. 2011;300:F433-F440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 57. | Guan Z, VanBeusecum JP, Inscho EW. Endothelin and the renal microcirculation. Semin Nephrol. 2015;35:145-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 58. | Zanatta CM, Veronese FV, Loreto Mda S, Sortica DA, Carpio VN, Eldeweiss MI, da Silva VD, Lopes TG, Gross JL, Canani LH. Endothelin-1 and endothelin a receptor immunoreactivity is increased in patients with diabetic nephropathy. Ren Fail. 2012;34:308-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 59. | Abdel-Razik A, Mousa N, Abdelsalam M, Abdelwahab A, Tawfik M, Tawfik AM, Hasan AS, Elhelaly R, El-Wakeel N, Eldars W. Endothelin-1/Nitric Oxide Ratio as a Predictive Factor of Response to Therapy With Terlipressin and Albumin in Patients With Type-1 Hepatorenal Syndrome. Front Pharmacol. 2020;11:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 60. | Farahmand M, Ramezani Tehrani F, Khalili D, Cheraghi L, Azizi F. Endogenous estrogen exposure and chronic kidney disease; a 15-year prospective cohort study. BMC Endocr Disord. 2021;21:155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 61. | Neugarten J, Golestaneh L. Gender and the prevalence and progression of renal disease. Adv Chronic Kidney Dis. 2013;20:390-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 62. | Müller V, Szabó A, Viklicky O, Gaul I, Pörtl S, Philipp T, Heemann UW. Sex hormones and gender-related differences: their influence on chronic renal allograft rejection. Kidney Int. 1999;55:2011-2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 90] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 63. | Song J, Kost CK Jr, Martin DS. Androgens augment renal vascular responses to ANG II in New Zealand genetically hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1608-R1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 64. | Koyuncuoğlu T, Yıldırım A, Dertsiz EK, Yüksel M, Ercan F, Yeğen BÇ. Estrogen receptor agonists protect against acetaminophen-induced hepatorenal toxicity in rats. Life Sci. 2020;263:118561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 65. | Frydman S, Freund O, Banai A, Zornitzki L, Banai S, Shacham Y. Relation of Gender to the Occurrence of AKI in STEMI Patients. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 66. | Güzel C, Yeşiltaş S, Daşkaya H, Uysal H, Sümer I, Türkay M. The effect of gender on acute kidney injury developing in the intensive care unit. Hippokratia. 2019;23:126-130. [PubMed] |
| 67. | Van Thiel DH, Gavaler JS, Schade RR. Liver disease and the hypothalamic pituitary gonadal axis. Semin Liver Dis. 1985;5:35-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 68. | Bernardi M, Moreau R, Angeli P, Schnabl B, Arroyo V. Mechanisms of decompensation and organ failure in cirrhosis: From peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015;63:1272-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 432] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 69. | Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 850] [Article Influence: 77.3] [Reference Citation Analysis (1)] |
| 70. | Costa D, Simbrunner B, Jachs M, Hartl L, Bauer D, Paternostro R, Schwabl P, Scheiner B, Stättermayer AF, Pinter M, Trauner M, Mandorfer M, Reiberger T. Systemic inflammation increases across distinct stages of advanced chronic liver disease and correlates with decompensation and mortality. J Hepatol. 2021;74:819-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 126] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 71. | Solé C, Solà E, Huelin P, Carol M, Moreira R, Cereijo U, Mas JM, Graupera I, Pose E, Napoleone L, dePrada G, Juanola A, Fabrellas N, Torres F, Morales-Ruiz M, Farrés J, Jiménez W, Ginès P. Characterization of inflammatory response in hepatorenal syndrome: Relationship with kidney outcome and survival. Liver Int. 2019;39:1246-1255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 72. | Lonardo A, Nascimbeni F, Ballestri S, Fairweather D, Win S, Than TA, Abdelmalek MF, Suzuki A. Sex Differences in Nonalcoholic Fatty Liver Disease: State of the Art and Identification of Research Gaps. Hepatology. 2019;70:1457-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 698] [Article Influence: 116.3] [Reference Citation Analysis (0)] |
| 73. | Browatzki M, Schmidt J, Kübler W, Kranzhöfer R. Endothelin-1 induces interleukin-6 release via activation of the transcription factor NF-kappaB in human vascular smooth muscle cells. Basic Res Cardiol. 2000;95:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 120] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 74. | Bruno CM, D'Amico R, Ierna D, Neri S. Does endothelin-1 play a role in the renal function of cirrhotic patients? Panminerva Med. 1998;40:196-198. [PubMed] |
| 75. | Moore K. Endothelin and vascular function in liver disease. Gut. 2004;53:159-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 76. | Örmeci N. Endothelins and liver cirrhosis. Potal Hypertension & Cirrhosis. 2022;1:66-72. [DOI] [Full Text] |
| 77. | Haase VH. Inflammation and hypoxia in the kidney: friends or foes? Kidney Int. 2015;88:213-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 78. | Bizid S, Yacoub H, Mohamed G, Ben Slimane B, Boughoula K, Ben Abdallah H, Bouali R, Abedelli N. Does Anemia Have a Potential Effect on Type 2 Hepatorenal Syndrome? Can J Gastroenterol Hepatol. 2020;2020:1134744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 79. | Scheiner B, Semmler G, Maurer F, Schwabl P, Bucsics TA, Paternostro R, Bauer D, Simbrunner B, Trauner M, Mandorfer M, Reiberger T. Prevalence of and risk factors for anaemia in patients with advanced chronic liver disease. Liver Int. 2020;40:194-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (4)] |
| 80. | Skinner C, Thompson AJ, Thursz MR, Marchesi JR, Vergis N. Intestinal permeability and bacterial translocation in patients with liver disease, focusing on alcoholic aetiology: methods of assessment and therapeutic intervention. Therap Adv Gastroenterol. 2020;13:1756284820942616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 81. | Salerno F, Monti V. Hepatorenal syndrome type 1 and bacterial infection: a catastrophic association in patients with cirrhosis. Hepatology. 2014;59:1239-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 82. | Skluzacek PA, Szewc RG, Nolan CR 3rd, Riley DJ, Lee S, Pergola PE. Prediction of GFR in liver transplant candidates. Am J Kidney Dis. 2003;42:1169-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 83. | Kumar R, Priyadarshi RN, Anand U. Chronic renal dysfunction in cirrhosis: A new frontier in hepatology. World J Gastroenterol. 2021;27:990-1005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (3)] |
| 84. | Dhaliwal A, Armstrong MJ. Sarcopenia in cirrhosis: A practical overview. Clin Med (Lond). 2020;20:489-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 85. | Davenport A. Difficulties in assessing renal function in patients with cirrhosis: potential impact on patient treatment. Intensive Care Med. 2011;37:930-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 86. | Cholongitas E, Marelli L, Kerry A, Goodier DW, Nair D, Thomas M, Patch D, Burroughs AK. Female liver transplant recipients with the same GFR as male recipients have lower MELD scores--a systematic bias. Am J Transplant. 2007;7:685-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 87. | Yoo JJ, Kim SG, Kim YS, Lee B, Lee MH, Jeong SW, Jang JY, Lee SH, Kim HS, Kim YD, Cheon GJ. Estimation of renal function in patients with liver cirrhosis: Impact of muscle mass and sex. J Hepatol. 2019;70:847-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 88. | Heaf JG, Yahya R, Dahl M. The ratio of measured to estimated glomerular filtration rate may be a marker of early mortality and dialysis requirement. BMC Nephrol. 2021;22:370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 89. | Kwekel JC, Vijay V, Desai VG, Moland CL, Fuscoe JC. Age and sex differences in kidney microRNA expression during the life span of F344 rats. Biol Sex Differ. 2015;6:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 90. | Francoz C, Nadim MK, Durand F. Kidney biomarkers in cirrhosis. J Hepatol. 2016;65:809-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 114] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 91. | Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL): a new marker of kidney disease. Scand J Clin Lab Invest Suppl. 2008;241:89-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 272] [Cited by in RCA: 246] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 92. | Huelin P, Solà E, Elia C, Solé C, Risso A, Moreira R, Carol M, Fabrellas N, Bassegoda O, Juanola A, de Prada G, Albertos S, Piano S, Graupera I, Ariza X, Napoleone L, Pose E, Filella X, Morales-Ruiz M, Rios J, Fernández J, Jiménez W, Poch E, Torres F, Ginès P. Neutrophil Gelatinase-Associated Lipocalin for Assessment of Acute Kidney Injury in Cirrhosis: A Prospective Study. Hepatology. 2019;70:319-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 93. | Gambino C, Piano S, Stenico M, Tonon M, Brocca A, Calvino V, Incicco S, Zeni N, Gagliardi R, Cosma C, Zaninotto M, Burra P, Cillo U, Basso D, Angeli P. Diagnostic and prognostic performance of urinary neutrophil gelatinase-associated lipocalin in patients with cirrhosis and acute kidney injury. Hepatology. 2023;77:1630-1638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 39] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 94. | Randers E, Kristensen JH, Erlandsen EJ, Danielsen H. Serum cystatin C as a marker of the renal function. Scand J Clin Lab Invest. 1998;58:585-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 92] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 95. | Gerbes AL, Gülberg V, Bilzer M, Vogeser M. Evaluation of serum cystatin C concentration as a marker of renal function in patients with cirrhosis of the liver. Gut. 2002;50:106-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 102] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 96. | Ahn HS, Kim YS, Kim SG, Kim HK, Min SK, Jeong SW, Jang JY, Lee SH, Kim HS, Kim BS, Park JM. Cystatin C is a good predictor of hepatorenal syndrome and survival in patients with cirrhosis who have normal serum creatinine levels. Hepatogastroenterology. 2012;59:1168-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 97. | Markwardt D, Holdt L, Steib C, Benesic A, Bendtsen F, Bernardi M, Moreau R, Teupser D, Wendon J, Nevens F, Trebicka J, Garcia E, Pavesi M, Arroyo V, Gerbes AL. Plasma cystatin C is a predictor of renal dysfunction, acute-on-chronic liver failure, and mortality in patients with acutely decompensated liver cirrhosis. Hepatology. 2017;66:1232-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 98. | Mindikoglu AL, Opekun AR, Mitch WE, Magder LS, Christenson RH, Dowling TC, Weir MR, Seliger SL, Howell CD, Raufman JP, Rana A, Goss JA, Khaderi SA, Vierling JM. Cystatin C Is a Gender-Neutral Glomerular Filtration Rate Biomarker in Patients with Cirrhosis. Dig Dis Sci. 2018;63:665-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 99. | Torner M, Mangal A, Scharnagl H, Jansen C, Praktiknjo M, Queck A, Gu W, Schierwagen R, Lehmann J, Uschner FE, Graf C, Strassburg CP, Fernandez J, Stojakovic T, Woitas R, Trebicka J. Sex specificity of kidney markers to assess prognosis in cirrhotic patients with TIPS. Liver Int. 2020;40:186-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |