Published online Jun 6, 2023. doi: 10.12998/wjcc.v11.i16.3921
Peer-review started: March 30, 2023
First decision: April 13, 2023
Revised: April 20, 2023
Accepted: April 27, 2023
Article in press: April 27, 2023
Published online: June 6, 2023
Processing time: 64 Days and 3.8 Hours
Taiwan has a high prevalence of tuberculosis and urothelial carcinoma. However, the simultaneous occurrence of both disorders in one patient is uncommon. Tuberculosis and urothelial carcinoma share some common risk factors and could demonstrate overlapping clinical manifestations.
Herein, we report the case of a patient who presented with fever, persistent hematuria, and pyuria. Chest computed tomography scans revealed a bilateral upper lobes cavitary lesion with fibrosis. Severe hydronephrosis of the right kidney and renal stones and cysts in the left kidney were observed. Initial microbiological testing was negative; however, a polymerase chain reaction assay of the urine confirmed a urinary tuberculosis infection. The patient was started on an anti-tuberculosis regimen. Ureteroscopy performed to resolve obstructive nephropathy revealed the incidental finding of a left middle-third ureteral tumor. Examination after biopsy and transurethral resection of the bladder tumor indicated urothelial carcinoma. The patient underwent laparoscopic nephroureterectomy, with bladder cuff excision for the right kidney and ureter, and holmium laser ablation of the ureteral lesion to preserve the left kidney and ureter. He has remained stable after the procedures.
Although establishing a causal relationship between tuberculosis and cancer is difficult, medical personnel should consider their correlation.
Core Tip: We have reported the case of a patient who presented with fever, persistent hematuria, and pyuria and was diagnosed with both urinary tuberculosis and urothelial carcinoma. Tuberculosis and urothelial carcinoma have some common risk factors such as smoking, alcohol consumption, chronic diseases, and malnutrition. Although it is difficult to establish a causal relationship between these two diseases, clinical medical personnel should consider the correlation between the two diseases.
- Citation: Tsai YC, Li CC, Chen BT, Wang CY. Coexistence of urinary tuberculosis and urothelial carcinoma: A case report. World J Clin Cases 2023; 11(16): 3921-3928
- URL: https://www.wjgnet.com/2307-8960/full/v11/i16/3921.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i16.3921
Taiwan has a high prevalence of tuberculosis and urothelial carcinoma. However, the simultaneous occurrence of these disorders is uncommon in the same patient. The concurrent development of both of these diseases in the same patient could be explained by the following mechanisms: Local long-term inflammatory response, leading to the destruction of the host protective barrier, resulting in the invasion of microorganisms or cancer cells[1]. In addition, the inflammatory response damages immune cells and weakens the host’s immunity.
Tuberculosis and urothelial carcinoma have some common risk factors, such as smoking, alcohol consumption, chronic diseases, and malnutrition. Although it is difficult to establish a causal relationship between these two diseases, clinical medical personnel should consider the correlation between the two. Herein, we report the case of a patient who presented with fever, persistent hematuria, and pyuria and was diagnosed with both urinary tuberculosis and urothelial carcinoma.
A 70-year-old man was transferred to our hospital for further management of intermittent fever, chills, and fatigue lasting for 10 d.
The patient experienced intermittent symptoms accompanied by severe night sweats. His body temperature was 38.5 °C, and his fever temporarily subsided after taking oral acetaminophen. He denied respiratory symptoms, such as cough, sore throat, or runny nose, and did not experience any gastrointestinal symptoms such as vomiting or diarrhea. Although the patient did not report urinary symptoms such as blood in urine, urgency, difficulty or pain during urination, or frequent voiding, he did report persistent bilateral lower back pain in the past 10 d. The patient had previously been diagnosed with lumbar herniated intervertebral disc disorder and had been undergoing rehabilitation therapy. As he believed that his current back pain was not significantly different from his previous pain, he did not pay particular attention to it.
The patient had developed anorexia, with a weight loss of 5.6 kg during the past year. Moreover, he had third–fourth lumbar herniated intervertebral disc disorder which had been treated with physical rehabilitation therapy since the age of 63. He did not have any systemic disorders, such as diabetes mellitus or liver cirrhosis.
The patient disclosed a smoking history of 31 pack-years and reported infrequent alcohol consumption. He worked as a bus driver and had been experiencing fatigue prior to this visit. The patient denied any recent travel history or engagement in unsafe sexual activities or drug abuse. Furthermore, he had no history of prescription medication use or underlying medical conditions.
During the physical examination, dull percussions were noted bilaterally in the upper back, along with the presence of rhonchi in the same region. Despite these findings, the patient did not exhibit any signs of respiratory distress. His vital signs were within normal limits, with a temperature of 36.5 °C, blood pressure of 144/87 mmHg, heart rate of 102 beats per minute, and respiratory rate of 18 breaths per minute. The patient did not display any pain or discomfort upon percussion of the bilateral kidney areas. No discharge was observed around the urethra, and there were no suspicious lumps, rashes, or wounds in the perineal or other areas of the body.
The patient’s hemogram revealed leukocytosis with neutrophil predominance. The blood electrolytes, glucose levels, and hepatic function were all within normal ranges. His serum urea nitrogen and creatinine levels were elevated. The urinal analysis found microscopic hematuria and pyuria. Detailed laboratory test results are presented in Table 1.
| Variable | Reference range | Illness day 10, on presentation | Illness day 30, on discharge |
| Hemoglobin (g/dL) | 12–15.5 | 14.9 | 12.4 |
| Hematocrit (%) | 34.9–44.5 | 43.3 | 37.2 |
| White-cell count (per mm3) | 4000–11000 | 12710 | 14960 |
| Differential count (%) | |||
| Neutrophils | 40–70 | 83.0 | 86.1 |
| Lymphocytes | 22–44 | 7.9 | 6.8 |
| Monocytes | 4–11 | 8.4 | 5.8 |
| Basophils | 0–3 | 0.4 | 0.5 |
| Eosinophils | 0–8 | 0.3 | 0.8 |
| Platelet count (×103 per mm3) | 135–400 | 253 | 304 |
| Red-cell count (×106 per mm3) | 3.90–5.03 | 4.71 | 4.12 |
| Mean corpuscular volume (fL) | 80.0–100.0 | 91.9 | 90.3 |
| C-reactive protein (mg/dL) | < 1 | 16.11 | 7.69 |
| Sodium (mmol/L) | 136–145 | 132 | 135 |
| Potassium (mmol/L) | 3.5–5.2 | 3.9 | 4.0 |
| Urea nitrogen (mg/dL) | 8–25 | 20 | 20 |
| Creatinine (mg/dL) | 0.60–1.50 | 2.1 | 1.4 |
| Estimated glomerular filtration rate (ml/min/1.73 m2) | > 60 | 33.4 | 53.3 |
| Glucose (mg/dL) | 70–110 | ||
| Aspartate aminotransferase (U/L) | ≤ 33 | 26 | 15 |
| Alanine aminotransferase (U/L) | 10–49 | 24 | 32 |
| Total bilirubin (mg/dL) | 0.3–1.2 | 0.79 | 0.68 |
| Glycated Hemoglobin (%) | < 5.8 | 5.7 | |
| Specimen | |||
| Anti-human immunodeficiency virus test | Negative | Negative | |
| Aspergillus antigen (serum) | Negative | Negative | |
| Cryptococcus antigen (serum) | Negative | Negative | |
| Pneumococcus antigen (urine) | Negative | Negative | |
| Blood culture | Negative | Negative | |
| Sputum culture | Negative | Negative | |
| Sputum acid fasting smear | Negative | Negative | |
| Urine acid fasting smear | Negative | Positive | Negative |
| Urine polymerase chain reaction assay | Negative | Positive; Mycobacterium tuberculosis |
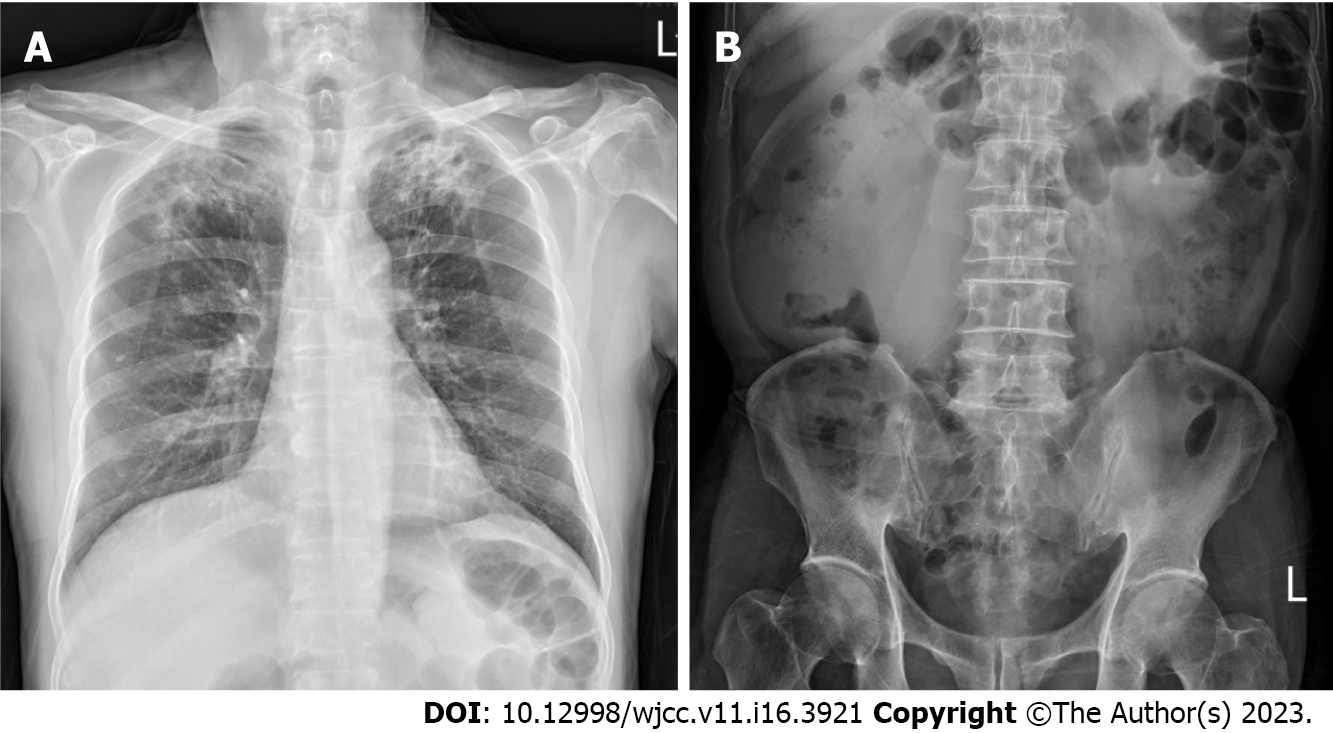
Chest radiography revealed patchy consolidation with fibrosis in both upper lungs (Figure 1).
The patient was admitted to our ward for further management of the bilateral upper lung lesions observed on chest radiography.
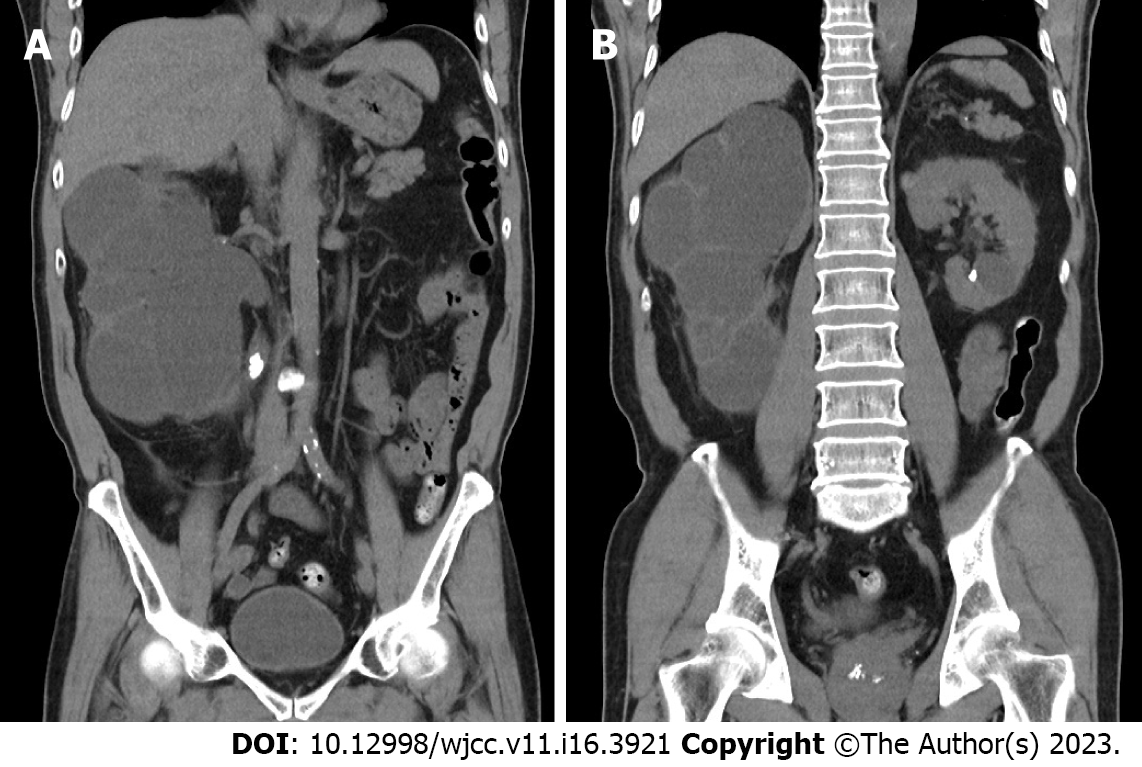
Initially, the patient was prescribed intravenous ceftriaxone 2 g per day after blood and sputum cultures had been obtained. On day 3 of admission, the empiric antibiotics were escalated to intravenous piperacillin/tazobactam 2.25 g every 6 h for broad-spectrum coverage as the patient was experiencing persistent fever and worsening conditions. Additionally, testing for lung lesions was performed. Chest computed tomography (CT) scans revealed fibrotic lesions surrounded by pa
Owing to the lack of an initial adequate microbiological clue, repeated sputum cultures and rapid serology tests, including pneumococcus urine antigen and Cryptococcus antigen serum test, were performed. However, they all demonstrated negative results. Four acid-fast stain sputum smear sets and the final tuberculosis culture results were also all negative. Sputum fungus culture yielded negative results as well.
A urologist was consulted for renal function deterioration and persistent turbid urine passage by the patient. On admission day 5, a decompressive percutaneous nephrostomy (PCN) was performed on the right kidney to correct the worsening infection and collect adequate microbiological evidence. Urine culture and cytology samples from the right kidney were collected, and acid-fast staining of the urine demonstrated positive results.
A polymerase chain reaction assay of the urine confirmed a urinary tuberculosis infection on admission day 12. The patient was initiated on empirical anti-tuberculosis regimen (isoniazid, rifampin, pyrazinamide, and ethambutol).
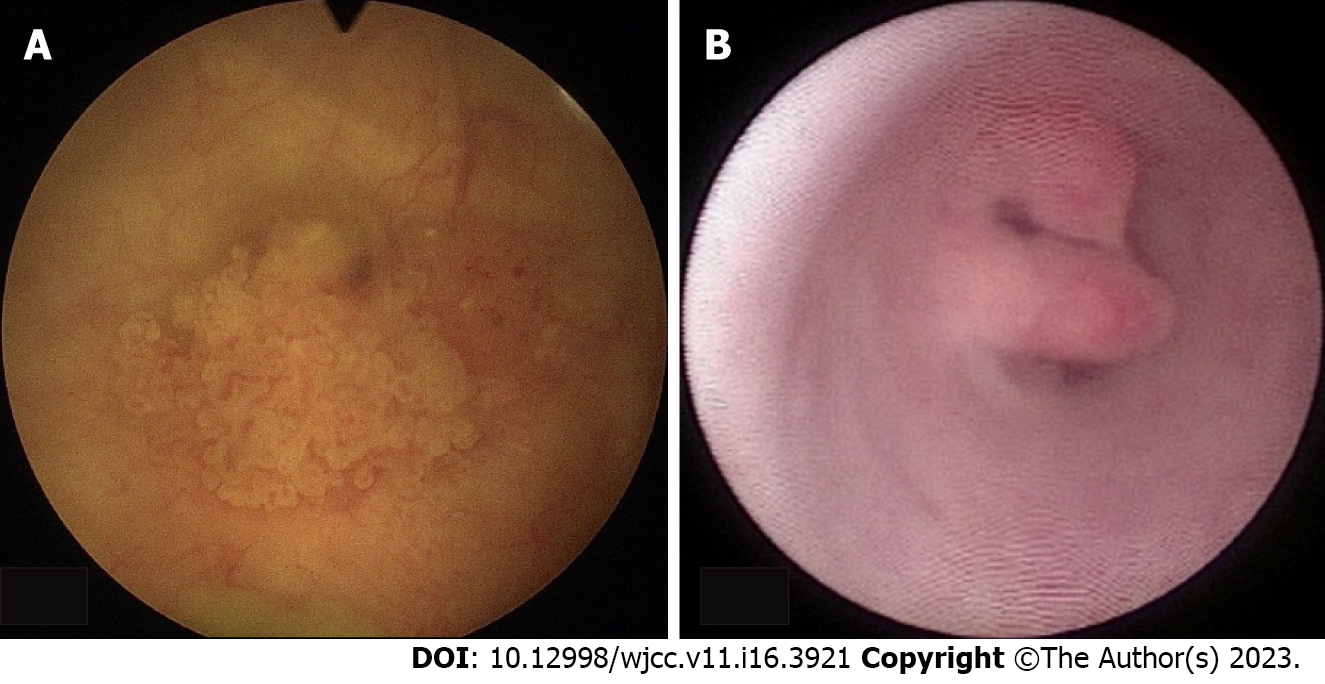
Ureteroscopy was performed to resolve obstructive nephropathy on admission day 15. However, during the procedure, a cauliflower-like lesion was incidentally observed over the right posterior and right lateral wall junction of the urinary bladder. A left middle-third ureter tumor was also incidentally diagnosed during the same procedure (Figure 3). After discussion with the family, a biopsy of the left ureteral tumor with double-J catheterization was performed.
Histological examination of the specimen obtained from the left middle third ureter revealed high-grade papillary pattern urothelial carcinoma with lamina propria invasion (cT1). Immunohistochemical staining was positive for CK7, CK20, and Ki67. Urothelial carcinoma of the urinary bladder was identified based on pathological evidence after transurethral resection of the bladder tumor.
An integrated tumor study was conducted for comprehensive disease staging. Through a dynamic renal function scan, it was discovered that the renal function of the left kidney was intact. Meanwhile, the right kidney was non-functioning.
Based on clinical manifestations, laboratory examinations, imaging examination, and pathological biopsy, the patient was finally diagnosed with coexisting urinary tuberculosis and urothelial carcinoma.
After discussion, the surgeon decided to perform laparoscopic nephroureterectomy, with bladder cuff excision for the right kidney and ureter, and to preserve the left kidney and ureter with holmium laser ablation of the ureteral lesion on admission day 25. There were no surgery-related complications. The patient was discharged on admission day 30.
The initial anti-tuberculosis regimen consisted of four drugs (isoniazid, rifampin, pyrazinamide, and ethambutol) for 2 mo, followed by isoniazid and rifampin for 4 mo owing to susceptibility to the first-line therapy. One month after antibiotic treatment commencement, the patient’s urine culture demonstrated no growth of the tuberculosis species. Although the patient’s renal function did not fully recover, it also did not deteriorate to the extent of requiring dialysis. The patient continued regular follow-ups at our clinic.
In Taiwan, the incidence of genitourinary tuberculosis is approximately 0.19 cases per 100000 indi
The simultaneous occurrence of both diseases in the same patient may be explained by the fact that tuberculosis may cause a long-term local inflammatory response, which may in turn induce cellular carcinogenesis. This could be further explained by the relationship between pulmonary tuberculosis and lung cancer[4], and ulcerative lesions of intestinal tuberculosis acting as a precursor to intestinal mucosal cancer[5]. Mycobacterium tuberculosis may also promote the development of cancer by inducing a chronic inflammatory state and compromising T cell-mediated immunity[6]. However, tumor cells may also weaken the host’s ability to resist the invasion of microorganisms such as mycobacteria by destroying the local infection barrier[7]. These tumor cells can also affect pathogen resistance through systemic immunosuppression[8,9].
Chen et al[6] have conducted a retrospective study of 45455 cancer patients and reported that tuberculosis is an independent risk factor for the development of all cancers. Hence, tuberculosis could increase the risk of specific cancers in patients[6]. A retrospective study by Lien et al[10] in Taiwan that analyzed national data also reported that urinary tract tuberculosis was associated with the development of urothelial carcinoma[10].
However, cancer has been recognized as a risk factor for active Mycobacterium tuberculosis infection since the 1970s. All types of cancers increase the risk of developing active tuberculosis disease, albeit to varying degrees. This could be due to intrinsic immunosuppression caused by the cancer itself, immunosuppressive effects of chemotherapy, or factors related to the patients themselves. Tuberculosis shares several disease risk factors with cancer, such as smoking[11], alcohol consumption, chronic diseases such as diabetes[3], malnutrition, and low socioeconomic status[12]. A previous study conducted in Taiwan on a large population reported that cancer was an independent risk factor for tuberculosis, with the highest risk observed 1 year before and 1 year after cancer diagnosis[13].
Although the temporal association between cancer and tuberculosis and a potential causal relationship between the two have not been established in the literature, as this may be challenging in clinical practice, clinicians should nonetheless be aware of this relationship[6,9,13].
The diagnosis and identification of a suitable treatment strategy in the present case were challenging, primarily because the patient had persistent pyuria and fever, and microbiological pathogenic evidence was lacking. To improve his condition and obtain relevant samples, we arranged decompressive PCN of the right kidney. Although the urine collected via PCN confirmed the tuberculosis infection, this patient already exhibited signs suggestive of urinary tuberculosis, including chest radiography resembling old pulmonary tuberculosis and persistent pyuria despite antibiotic treatment with sterile routine microbial cultures[14]. After several consecutive sets of negative urine cultures, clinicians should perhaps consider urinary tuberculosis as a differential diagnosis.
Surgery is the treatment of choice for early localized urothelial carcinoma. The mainstay of treatment for genitourinary tuberculosis is anti-tuberculosis drugs[15]. Indications for nephrectomy in case of genitourinary tuberculosis combination urothelial carcinoma include a nonfunctioning kidney, extensive disease involving the entire kidney along with hypertension and ureteropelvic junction obstruction, and renal carcinoma[16]. Considering that this patient only had unilateral residual renal function and was worried about the coexistence of potential tumors in the right kidney, he eventually chose the surgical option to remove the non-functional right kidney and is undergoing regular follow-ups to closely monitor the left urinary system that still has renal function.
The association between tuberculosis and cancer could be multifaceted. Although the causal relationship between the two has not yet been established, clinicians should be aware of the link between cancer and tuberculosis.
Yu-Chi Tsai would like to thank his wife Meng-Huang Chang for being considerate and helpful.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Oley MH, Indonesia; Yarmahmoodi F, Iran S-Editor: Li L L-Editor: A P-Editor: Li L
| 1. | Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10123] [Cited by in RCA: 11283] [Article Influence: 490.6] [Reference Citation Analysis (2)] |
| 2. | Huang TY, Hung CH, Hsu WH, Peng KT, Hung MS, Lai LJ, Chuang HJ, Tai WL, Ku YP, Wu TS. Genitourinary tuberculosis in Taiwan: A 15-year experience at a teaching hospital. J Microbiol Immunol Infect. 2019;52:312-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Lin MY, Niu SW, Li WM, Lee HL, Chen LT, Wu WJ, Hwang SJ. Incidence and survival variations of upper tract urothelial cancer in Taiwan (2001-2010). Int J Urol. 2022;29:121-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Molina-Romero C, Arrieta O, Hernández-Pando R. Tuberculosis and lung cancer. Salud Publica Mex. 2019;61:286-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 5. | Chin SN, Foster T, Char G, Garrison A. Concomitant urothelial cancer and renal tuberculosis. Case Rep Urol. 2014;2014:625153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 6. | Chen GL, Guo L, Yang S, Ji DM. Cancer risk in tuberculosis patients in a high endemic area. BMC Cancer. 2021;21:679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Simonsen DF, Farkas DK, Horsburgh CR, Thomsen RW, Sørensen HT. Increased risk of active tuberculosis after cancer diagnosis. J Infect. 2017;74:590-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Suzuki Y, Imokawa S, Sato J, Uto T, Suda T. Cumulative incidence of tuberculosis in lung cancer patients in Japan: A 6-year observational study. Respir Investig. 2016;54:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Kumar DS, Ronald LA, Romanowski K, Rose C, Shulha HP, Cook VJ, Johnston JC. Risk of active tuberculosis in migrants diagnosed with cancer: a retrospective cohort study in British Columbia, Canada. BMJ Open. 2021;11:e037827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Lien YC, Wang JY, Lee MC, Shu CC, Chen HY, Hsieh CH, Lee CH, Lee LN, Chao KM. Urinary tuberculosis is associated with the development of urothelial carcinoma but not renal cell carcinoma: a nationwide cohort study in Taiwan. Br J Cancer. 2013;109:2933-2940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Lin HH, Ezzati M, Chang HY, Murray M. Association between tobacco smoking and active tuberculosis in Taiwan: prospective cohort study. Am J Respir Crit Care Med. 2009;180:475-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Cheng MP, Abou Chakra CN, Yansouni CP, Cnossen S, Shrier I, Menzies D, Greenaway C. Risk of Active Tuberculosis in Patients with Cancer: A Systematic Review and Meta-Analysis. Clin Infect Dis. 2017;64:635-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Shen BJ, Lin HH. Time-dependent association between cancer and risk of tuberculosis: A population-based cohort study. Int J Infect Dis. 2021;108:340-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Figueiredo AA, Lucon AM, Srougi M. Urogenital Tuberculosis. Microbiol Spectr. 2017;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 15. | Abbara A, Davidson RN; Medscape. Etiology and management of genitourinary tuberculosis. Nat Rev Urol. 2011;8:678-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Cek M, Lenk S, Naber KG, Bishop MC, Johansen TE, Botto H, Grabe M, Lobel B, Redorta JP, Tenke P; Members of the Urinary Tract Infection (UTI) Working Group of the European Association of Urology (EAU) Guidelines Office. EAU guidelines for the management of genitourinary tuberculosis. Eur Urol. 2005;48:353-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |