Published online Jun 6, 2023. doi: 10.12998/wjcc.v11.i16.3765
Peer-review started: November 30, 2022
First decision: January 17, 2023
Revised: January 17, 2023
Accepted: April 18, 2023
Article in press: April 18, 2023
Published online: June 6, 2023
Processing time: 183 Days and 17.5 Hours
As per the latest Surviving Sepsis Campaign guidelines, fluid resuscitation should be guided by repeated measurements of blood lactate levels until normalization. Nevertheless, raised lactate levels should be interpreted in the clinical context, as there may be other causes of elevated lactate levels. Thus, it may not be the best tool for real-time assessment of the effect of hemodynamic resuscitation, and exploring alternative resuscitation targets should be an essential research priority in sepsis.
To compare the 28-d mortality in two clinical patterns of septic shock: hyperlactatemic patients with hypoperfusion context and hyperlactatemic patients without hypoperfusion context.
This prospective comparative observational study carried out on 135 adult patients with septic shock that met Sepsis-3 definitions compared patients with hyperlactatemia in a hypoperfusion context (Group 1, n = 95) and patients with hyperlactatemia in a non-hypoperfusion context (Group 2, n = 40). Hypoperfusion context was defined by a central venous saturation less than 70%, central venous-arterial PCO2 gradient [P(cv-a)CO2] ≥ 6 mmHg, and capillary refilling time (CRT) ≥ 4 s. The patients were observed for various macro and micro hemodynamic parameters at regular intervals of 0 h, 3 h, and 6 h. All-cause 28-d mortality and all other secondary objective parameters were observed at specified intervals. Nominal categorical data were compared using the χ2 or Fisher’s exact test. Non-normally distributed continuous variables were compared using the Mann-Whitney U test. Receiver operating characteristic curve analysis with the Youden index determined the cutoff values of lactate, CRT, and metabolic perfusion parameters to predict the 28-d all-cause mortality. A P value of < 0.05 was considered significant.
Patient demographics, comorbidities, baseline laboratory, vital parameters, source of infection, baseline lactate levels, and lactate clearance at 3 h and 6 h, Sequential Organ Failure scores, need for invasive mechanical ventilation, days on mechanical ventilation, and renal replacement therapy-free days within 28 d, duration of intensive care unit stay, and hospital stay were comparable between the two groups. The stratification of patients into hypoperfusion and non-hypoperfusion context did not result in a significantly different 28-d mortality (24% vs 15%, respectively; P = 0.234). However, the patients within the hypoperfusion context with high P(cv-a)CO2 and CRT (P = 0.022) at baseline had significantly higher mortality than Group 2. The norepinephrine dose was higher in Group 1 but did not achieve statistical significance with a P > 0.05 at all measured intervals. Group 1 had a higher proportion of patients requiring vasopressin and the mean vasopressor-free days out of the total 28 d were lower in patients with hypoperfusion (18.88 ± 9.04 vs 21.08 ± 8.76; P = 0.011). The mean lactate levels and lactate clearance at 3 h and 6 h, CRT, P(cv-a)CO2 at 0 h, 3 h, and 6 h were found to be associated with 28-d mortality in patients with septic shock, with lactate levels at 6 h having the best predictive value (area under the curve lactate at 6 h: 0.845).
Septic shock patients fulfilling the hypoperfusion and non-hypoperfusion context exhibited similar 28-d all-cause hospital mortality, although patients with hypoperfusion displayed a more severe circulatory dysfunction. Lactate levels at 6 h had a better predictive value in predicting 28-d mortality than other parameters. Persistently high P(cv-a)CO2 (> 6 mmHg) or increased CRT (> 4 s) at 3 h and 6 h during early resuscitation can be a valuable additional aid for prognostication of septic shock patients.
Core Tip: Two different clinical patterns among hyperlactatemic septic shock patients can be effectively differentiated when utilizing three easily employable perfusion parameters. Lactate levels are still the best available tool, but persistence of high central venous-arterial PCO2 gradient (> 6 mmHg) or raised capillary refill time (> 4 s) at 3 h and 6 h along with lactate metrics during early resuscitation can be valuable for guiding resuscitation of septic shock patients.
- Citation: Kataria S, Singh O, Juneja D, Goel A, Bhide M, Yadav D. Hypoperfusion context as a predictor of 28-d all-cause mortality in septic shock patients: A comparative observational study. World J Clin Cases 2023; 11(16): 3765-3779
- URL: https://www.wjgnet.com/2307-8960/full/v11/i16/3765.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i16.3765
Septic shock remains the most frequent cause of mortality in patients admitted to the intensive care unit (ICU), contributing to 33%-50% to the total inpatient hospital deaths[1-3]. Early recognition and adequate resuscitation of patients with sepsis-associated circulatory dysfunction is a fundamental challenge for an intensivist. Undertreatment may lead to persistently impaired tissue oxygenation, whereas overtreatment may lead to a positive fluid balance that can result in pulmonary edema, prolonged mechanical ventilation (MV), and death[4-8].
Viewing the strong relationship between hyperlactatemia, lactate kinetics, and mortality[9] and following the study results by Jansen et al[10], Surviving Sepsis Campaign (SSC) guidelines 2012 suggested fluid resuscitation guided by repeated measurement of blood lactate levels until normalization[11]. However, as per SSC guidelines 2021, lactate level interpretation should be based on the clinical context, and other causes of elevated lactate levels, such as adrenergic-driven aerobic lactate production and impaired hepatic lactate clearance, should be considered[12]. Thus, lactate levels might not be the best tool for real-time assessment of the effect of hemodynamic resuscitation[13,14]. Therefore, exploring alternative resuscitation targets is an important research priority in sepsis.
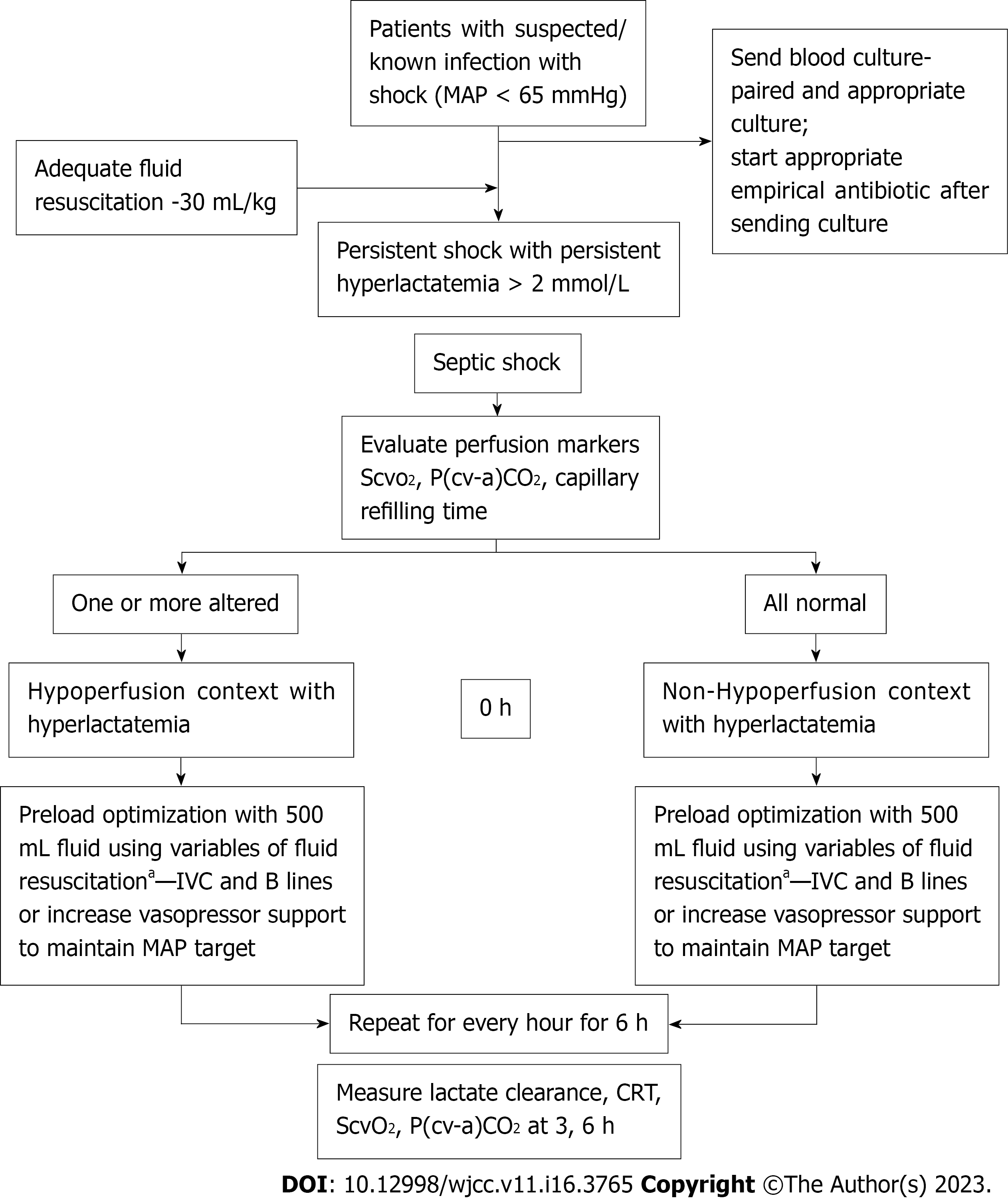
Variables such as central venous saturation (ScvO2), central venous-arterial PCO2 gradient [P(cv-a)CO2], and peripheral (skin) perfusion markers exhibit a very fast normalization rate concerning systemic flow optimization[14]. A concomitant low ScvO2, high P(cv-a)CO2, or abnormal peripheral perfusion define a “hypoperfusion context” in which increasing systemic blood flow may reduce blood lactate levels. Thus, multimodal perfusion monitoring could aid in identifying a hypoperfusion context.
This study aimed to analyze septic shock patients and compare the outcome in two clinical patterns: Hyperlactatemic patients with hypoperfusion and hyperlactatemic patients without hypoperfusion. The hypoperfusion context in the present study was defined similarly to the study by Alegría et al[15]: ScvO2 less than 70%; P(cv-a)CO2 greater than or equal to 6 mmHg; capillary refilling time (CRT) greater than or equal to 4 s; and hyperlactatemia after initial fluid resuscitation in septic shock patients admitted in the ICU.
The present study was a prospective comparative observational study conducted in the medical ICU, Institute of Critical Care Medicine, Max Super Specialty Hospital, Saket, New Delhi from March 2021 to November 2021. Institutional Human Ethics Committee approval was obtained before the commen
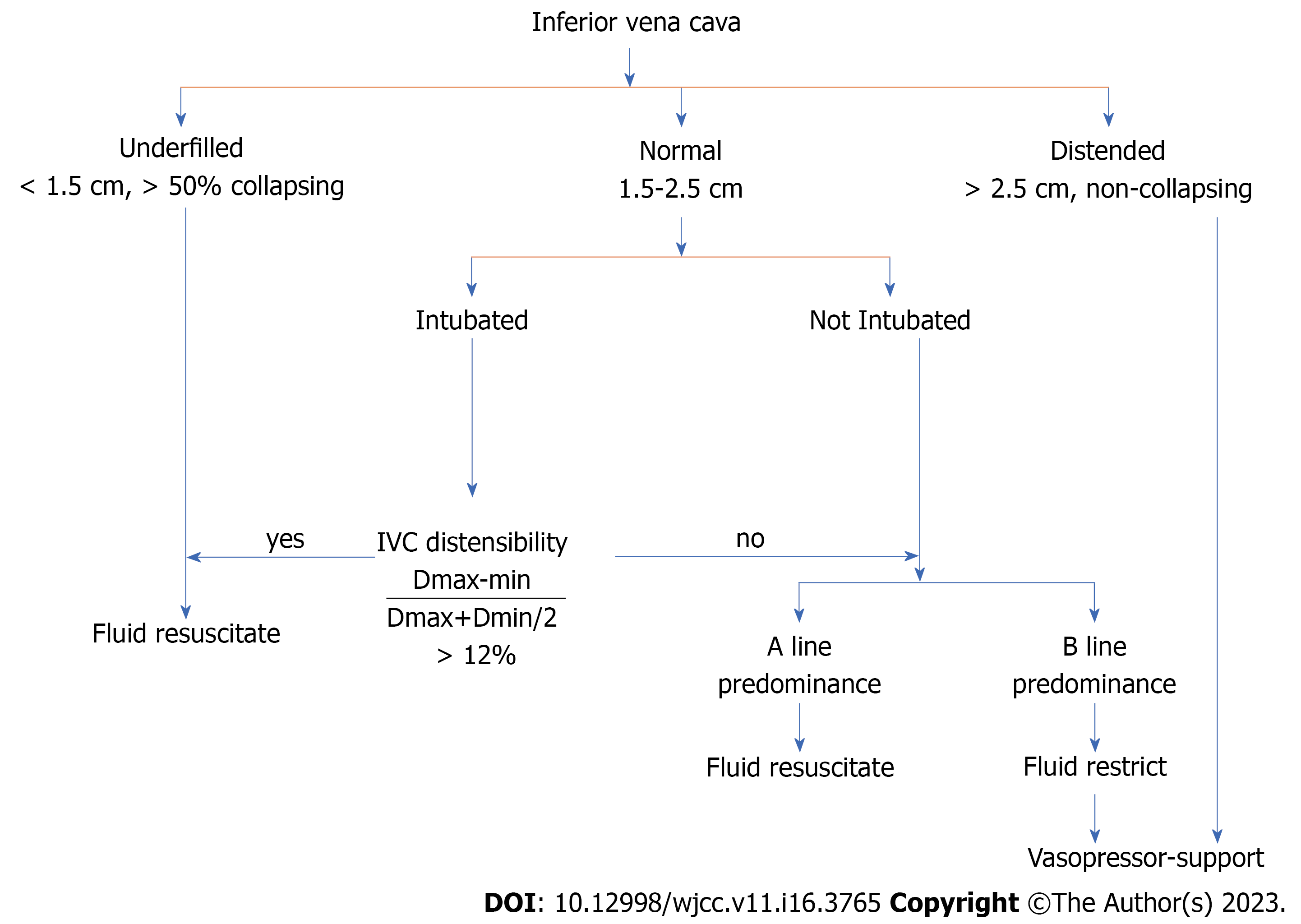
Preload optimization was guided by an algorithm (Figures 1 and 2) that included early fluid loading, followed by vasopressor infusion as needed to maintain a mean arterial pressure > 65 mmHg. SSC guidelines 2016 were followed to guide the treatment of septic shock[1]. All patients were followed for 28 d. The following primary and secondary outcomes were measured as part of the multimodal perfusion assessment.
Primary outcome: all-cause mortality at the 28th d (asked by telephone if patient discharged earlier).
Secondary outcomes: (1) Macro hemodynamic variables measured at baseline including systolic blood pressure, diastolic blood pressure, mean arterial pressure, heart rate, norepinephrine (NE), or vasoactive drug doses; (2) Metabolic-related perfusion variables measured at 0 h (baseline), 3 h, and 6 h including ScvO2 and P(cv-a)CO2; (3) Lactate measurement and percentage of lactate clearance at 0 h (baseline), 3 h, and 6 h. The normal level was defined as less than 2 mmol/L. Lactate was assessed using an arterial sample and processed by a point of care common gas analyzer. The percentage of lactate clearance was defined as: Lactate clearance = (Lactate initial-Lactate time) × 100/Lactate initial; (4) CRT measured at 0 h (baseline), 3 h, and 6 h: Normal values were considered to be ≤ 4.0 s. It was measured by applying firm pressure to the ventral surface of the distal phalanx of the right index finger with a glass microscope slide. The pressure was increased until the skin blanched, was maintained for 10 s, and then released. The time for the return of the normal skin color was recorded using a chronometer, and a refill time greater than 3 s was defined as abnormal; (5) Amount of fluid administered measured at 0 h, 6 h, and 24 h; (6) Vasopressor dose measured at 0 h, 3 h, 6 h, 12 h, and 24 h; (7) Duration of vasopressor use in days; (8) Need of invasive MV, duration on invasive MV in days, and MV-free days within 28 d; (9) Need for renal replacement therapy and renal replacement therapy-free days within 28 d; and (10) ICU and hospital length of stay.
Continuous variables were presented as mean ± standard deviation for normally distributed data and median ± interquartile range for non-normally distributed data. Categorical variables were expressed as frequencies and percentages. The comparison of normally distributed continuous variables between the groups was performed using Student’s t-test. Nominal categorical data between the groups were compared using the χ2 test or Fisher’s exact test. Mann Whitney U test was performed to compare two group means. Receiver operating characteristic curve (ROC) analysis with the Youden index was performed to determine each the cutoff value of each parameter to predict the outcome. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were calculated based on those cutoff values. For all statistical tests, a P < 0.05 indicated a significant difference.
A total of 148 patients met the inclusion criteria in the present study, out of which 7 patients had severe left ventricular systolic dysfunction, 1 patient was pregnant, and 5 patients refused to consent to participate. Therefore, 135 patients were included in the study; 95 patients were in the hypoperfusion context (Group 1), and 40 patients were in the non-hypoperfusion context (Group 2). Patient demographics, comorbidities, baseline laboratory and vital parameters, source of infection, and Sequential Organ Failure scores were comparable between the two groups (Tables 1 and 2). The Acute Physiology and Chronic Health Evaluation (APACHE) II score was higher in Group 2 (23.78 ± 5.414 vs 23.78 ± 5.414; P < 0.002). The baseline lactate levels were 4.84 ± 1.7 mmol/L and were comparable in both the groups at baseline (4.87 ± 1.69 vs 4.76 ± 1.75 mmol/L; P = 0.594) and all measured intervals. The primary and secondary outcomes of Group 2 were compared with Group 1 and with the subgroups of Group 1 (Supplementary Tables 1 and 2).
| Variable | Group 1 | Group 2 | P value | ||||
| mean ± SD | Min-Max | Median (Q1-Q3) | mean ± SD | Min-Max | Median (Q1-Q3) | ||
| Age | 62.34 ± 14.32 | 20-92 | 64 (56-73) | 61.03 ± 15.10 | 25-82 | 66.5 (52-72) | 0.843 |
| APACHE II | 20.82 ± 5.47 | 9-32 | 20 (17-25) | 23.78 ± 5.41 | 8-33 | 24 (21-28) | 0.002a |
| SOFA score | 9.26 ± 4.22 | 2-18 | 8 (6-13) | 8.88 ± 3.44 | 4-16 | 8 (6-11.75) | 0.751 |
| Hemoglobin | 11.24 ± 6.30 | 7.1-16.70 | 10.2 (9.10-12.20) | 10.26 ± 1.93 | 7.1-16 | 10.25 (8.75-11.7) | 0.299 |
| TLC | 12.37 ± 7.92 | 0.1-46.50 | 11.9 (6.70-16.70) | 12.56 ± 9.34 | 1.4-42.80 | 9.65 (6.5-16.65) | 0.668 |
| Platelet count | 2.17 ± 1.01 | 0.1-5.26 | 1.98 (1.63-2.78) | 2.08 ± 0.72 | 0.25-3.18 | 2.02 (1.61-2.75) | 0.904 |
| Serum bilirubin | 1.84 ± 2.78 | 0.16-18.57 | 0.8 (0.55-1.6) | 1.68 ± 2.24 | 0.28-10.93 | 0.81 (0.41-1.84) | 0.507 |
| Serum albumin | 3.0 ± 0.60 | 1.5-4.4 | 2.9 (2.60-3.50) | 2.9 ± 0.60 | 1.7-4 | 2.9 (2.30.-3.40) | 0.584 |
| INR | 1.35 ± 0.36 | 0.95-3.83 | 1.28 (1.12-1.49) | 1.31 ± 0.30 | 1.01-2.52 | 1.26 (1.08-1.44) | 0.323 |
| Creatinine | 1.59 ± 1.31 | 0.2-10.50 | 1.3 (0.70-1.90) | 1.34 ± 0.91 | 0.20-4.20 | 1.15 (0.6-1.85) | 0.265 |
| Urea | 51.68 ± 34.75 | 7.8-229 | 41.7 (29.70-64.60) | 54.12 ± 35.30 | 3.3-157 | 44 (33.1-69.88) | 0.565 |
| Heart rate | 104.15 ± 15.44 | 64-156 | 107 (94-114) | 106.73 ± 15.65 | 70-137 | 112 (98-118.75) | 0.216 |
| SBP | 101.6 ± 22.16 | 50-166 | 102 (90-117) | 99.5 ± 21.53 | 60-133 | 102 (81-117.75) | 0.629 |
| DBP | 56.92 ± 15.26 | 24-97 | 58 (45-66) | 58.58 ± 15.65 | 36-90 | 59 (44-68.75) | 0.685 |
| MAP | 71.62 ± 16.08 | 31-106 | 70 (64-82) | 72.5 ± 16.47 | 44-103 | 70.5 (61.75-87) | 0.808 |
| Lactate at 0 h | 4.87 ± 1.69 | 2.1-9.70 | 4.6 (3.50-5.90) | 4.76 ± 1.75 | 2.3-9.8 | 4.15 (3.5-5.65) | 0.594 |
| Lactate at 3 h | 4.22 ± 2.0 | 1.3-12.00 | 3.6 (2.80-5.20) | 3.82 ± 2.06 | 1.9-12.40 | 3.4 (2.45-4.10) | 0.16 |
| Lactate at 6 h | 3.91 ± 3.01 | 0.6-14.10 | 2.8 (2.0-4.60) | 3.88 ± 3.48 | 0.9-16.70 | 2.7 (2.0-4.20) | 0.7 |
| CRT at 0 h | 5.14 ± 2.16 | 2-12 | 5 (3-7) | 2.25 ± 0.67 | 1-3 | 2 (2-3) | < 0.001a |
| CRT at 3 h | 4.72 ± 2.68 | 1.0-13.00 | 4 (3-7) | 3.55 ± 2 | 2-9 | 3 (2-4) | 0.011a |
| CRT at 6 h | 4.50 ± 3.30 | 1.0-13.00 | 3 (2-7) | 3.88 ± 3.22 | 1-13 | 3 (2-4) | 0.255 |
| ScvO2 at 0 h | 64.5 ± 9.10 | 42.8-74.20 | 70.4 (56.40-71.80) | 71.8 ± 1.30 | 68.1-74.0 | 71.8 (70.9-72.70) | < 0.001a |
| ScvO2 at 3 h | 60.1 ± 7.0 | 36.8-73.70 | 60.8 (56.70-64.70) | 63.8 ± 4.70 | 52.8-73.50 | 63.6 (61.7-67.3) | 0.002a |
| ScvO2 at 6 h | 58.6 ± 9.0 | 36.7-89.20 | 60.2 (52.30-64.90) | 62.4 ± 6.80 | 42.3-71.0 | 63.7 (58.7-68.3) | 0.010a |
| P(cv-a)CO2 at 0 h | 6.63 ± 1.78 | 2.9-9.80 | 7.2 (5-7.9) | 4.24 ± 0.84 | 2.8-5.80 | 4.2 (3.6-4.86) | < 0.001a |
| P(cv-a)CO2 at 3 h | 5.81 ± 2.03 | 2.6-12.30 | 5.5 (4.10-7) | 5.05 ± 1.51 | 2.7-10 | 4.4 (4-5.98) | 0.035a |
| P(cv-a)CO2 at 6 h | 5.70 ± 2.30 | 2.6-12.40 | 5.1 (4-60.5) | 5.88 ± 6.88 | 2.6-47 | 4.3 (3.6-6.375) | 0.059 |
| Lactate clearance at 3 h | 10.2 ± 38.5 | (-183.3-57.7) | 22.7 (-7.30-36.0) | 18.3 ± 29.8 | (-103.3-58.2) | 26.7 (5.4-36.4) | 0.332 |
| Lactate clearance at 6 h | 15.3 ± 76.0 | (-433.3-100.0) | 39.6 (5.1-57.1) | 20.3 ± 54.8 | (-173.8-76.9) | 39.1 (2.4-57.9) | 0.973 |
| Characteristics | Group 1 | Group 2 | Total | P value | |||
| Frequency | % | Frequency | % | Frequency | % | ||
| Sex | |||||||
| Female | 43 | 45% | 20 | 50% | 63 | 95% | 0.614 |
| Male | 52 | 55% | 20 | 50% | 72 | 105% | |
| Comorbidities | |||||||
| DM | 30 | 32% | 12 | 30% | 42 | 31% | 0.856 |
| HTN | 39 | 41% | 15 | 38% | 54 | 40% | 0.700 |
| COPD | 6 | 6% | 5 | 13% | 11 | 8% | 0.393 |
| CLD | 7 | 7% | 4 | 10% | 11 | 8% | 0.868 |
| CKD | 12 | 13% | 8 | 20% | 20 | 15% | 0.271 |
| Malignancy | 7 | 7% | 4 | 10% | 11 | 8% | 0.868 |
| CAD | 8 | 8% | 6 | 15% | 14 | 10% | 0.403 |
| Others IMM | 5 | 5% | 2 | 5% | 7 | 5% | 1.000 |
| Source of infection | |||||||
| Intra-abdominal infection | 27 | 28% | 12 | 30% | 39 | 29% | 0.853 |
| Bacteremia | 11 | 12% | 3 | 8% | 14 | 10% | 0.689 |
| Pneumonia | 30 | 32% | 11 | 28% | 41 | 30% | 0.638 |
| UTI | 12 | 13% | 8 | 20% | 20 | 15% | 0.271 |
| Others | 10 | 11% | 2 | 5% | 12 | 9% | 0.484 |
| Unknown | 5 | 5% | 4 | 10% | 9 | 7% | 0.529 |
The overall 28-d mortality was 21% in 135 patients, 24% in the hypoperfusion context group vs 15% in the non-hypoperfusion context (P = 0.234). However, the patients within the hypoperfusion context with high P(cv-a)CO2 and CRT (P-value 0.022) at baseline had significantly higher mortality as compared to Group 2 (Supplementary Tables 1 and 2). The mean dose of noradrenaline at baseline in all the study patients was 0.19 ± 0.14 µg/kg/min. Although the NE requirement was higher in Group 1, it did not attain statistical significance at any specified interval (P > 0.05). Group 1 had a higher proportion of patients requiring vasopressin, with lower mean vasopressor-free days out of the total 28 d (18.88 ± 9.04 vs 21.08 ± 8.76; P = 0.011). Similarly, Group 1 had a higher fluid requirement than Group 2 at 0 h and 6 h (P = 0.045 and 0.008, respectively). The need for invasive MV, days on MV, renal replacement therapy-free days within 28 d, and ICU and hospital stay duration were comparable between the groups (Supplementary Table 2).
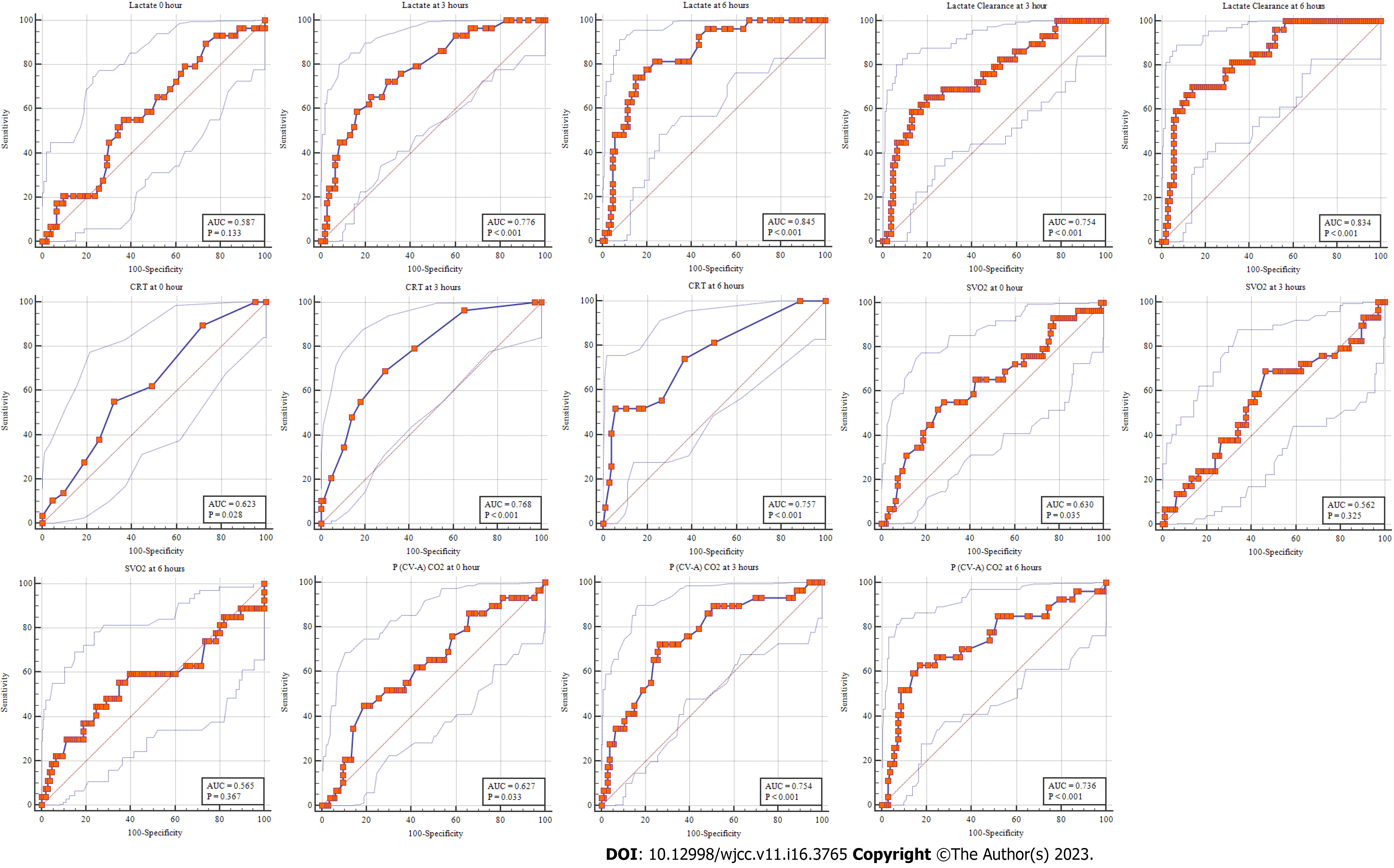
Univariate analysis of baseline variables and primary and secondary outcomes was also performed between the survivors and non-survivors (Table 3). We also analyzed the prognostic value of mean lactate levels, lactate clearance, ScvO2, CRT, and P(cv-a)CO2 at 0 h, 3 h, and 6 h for 28-d all-cause mortality. In the current study, although the lactate levels at baseline were higher in non-survivors than the survivors, they were statistically insignificant (5.2 ± 1.72 vs 4.74 ± 1.69; P = 0.151). Nevertheless, a significant association between lactate levels at 3 h and 6 h and lactate clearance at 3 h and 6 h was observed with the 28-d mortality, with lactate levels at 6 h having a better predictive value than lactate clearance at 6 h [area under the ROC (AUROC) for lactate at 3 h and 6 h: 0.776 and 0.845, respectively; AUROC for lactate clearance at 3 h and 6 h 0.754 and 0.834, respectively] (Figure 3). The optimal cutoff value for lactate values at 3 h in predicting 28-d mortality was ≥ 4.2 mmol/L, with a sensitivity of 55.2%, a specificity of 63.2%, a PPV of 29.1%, and an NPV of 83.8%. Similarly, the cutoff for the 6 h lactate levels was ≥ 4.1 mmol/L with a sensitivity of 74.2%, a specificity of 84.9%, a PPV of 55.6%, and an NPV of 92.8% (Tables 4 and 5).
| Variable | Non-Survivors | Survivors | P value | ||||
| mean ± SD | Min-Max | Median (Q1-Q3) | mean ± SD | Min-max | Median (Q1-Q3) | ||
| Age | 62.41 ± 14.61 | 28-82 | 66 (55.5-74.0) | 61.82 ± 14.55 | 20-92 | 64 (53.75-71.25) | 0.653 |
| APACHE II | 23.93 ± 4.91 | 14-32 | 24 (20.5-28.0) | 21.08 ± 5.64 | 8-33 | 21 (17.00-25.25) | 0.012a |
| SOFA score | 10.38 ± 4.71 | 2-17 | 11 (6-15) | 8.81 ± 3.73 | 2-18 | 8 (6-11) | 0.094 |
| HB | 12.2 ± 11.0 | 7.2-16.7 | 10 (8.8-12.0) | 10.6 ± 2.0 | 7.1-16.9 | 10.3 (9.1-12.0) | 0.493 |
| TLC | 10.0 ± 6.9 | 0.2-24.3 | 8.5 (5.4-13.8) | 13.1 ± 8.6 | 0.1-46.5 | 11.9 (6.8-16.7) | 0.101 |
| PLT | 1.99 ± 0.89 | 0.25-4.08 | 1.83 (1.58-2.01) | 2.18 ± 0.95 | 0.10-5.26 | 2.10 (1.65-2.78) | 0.188 |
| SBIL | 1.02 ± 0.84 | 0.28-4.70 | 0.78 (0.57-1.27) | 2.00 ± 2.90 | 0.16-18.57 | 0.8 (0.5-2.0) | 0.489 |
| SALB | 2.4 ± 0.4 | 1.6-3.1 | 2.3 (2.1-2.7) | 3.1 ± 0.6 | 1.5-4.4 | 3.1 (2.7-3.5) | < 0.001a |
| INR | 1.28 ± 0.29 | 0.97-2.14 | 1.18 (1.09-1.31) | 1.36 ± 0.36 | 0.95-3.83 | 1.30 (1.12-1.48) | 0.099 |
| CREAT | 2.08 ± 0.95 | 0.50-4.90 | 1.80 (1.50-2.40) | 1.36 ± 1.23 | 0.20-10.50 | 1.10 (0.60-1.80) | < 0.001a |
| Urea | 61.24 ± 44.91 | 9.90-229.00 | 45.60 (30.50-86.70) | 49.99 ± 31.30 | 3.30-168.90 | 41.83 (30.10-61.60) | 0.280 |
| HR | 107.00 ± 15.22 | 70-128 | 112 (98-118) | 104.34 ± 15.58 | 64-156 | 106.50 (94.00-116.00) | 0.228 |
| SBP | 94.66 ± 22.81 | 60-138 | 90 (74-116) | 102.71 ± 21.45 | 50-166 | 102 (90-118) | 0.065 |
| DBP | 52.41 ± 14.21 | 26-87 | 50 (41-60) | 58.77 ± 15.41 | 24-97 | 60.00 (46.00-69.25) | 0.060 |
| MAP | 66.24 ± 16.61 | 31-101 | 67.0 (53.0-76.5) | 73.42 ± 15.74 | 33-106 | 71 (64-85) | 0.057 |
| Lactate at 0 h | 5.20 ± 1.72 | 2.10-9.40 | 5.20 (4.0-5.70) | 4.74 ± 1.69 | 2.30-9.80 | 4.40 (3.40-5.90) | 0.151 |
| Lactate at 3 h | 5.54 ± 2.14 | 2.40-10.70 | 5.20 (3.80-6.70) | 3.71 ± 1.80 | 1.30-12.40 | 3.40 (2.50-4.20) | < 0.001a |
| Lactate at 6 h | 6.61 ± 3.35 | 2.10-14.60 | 6.30 (3.90-8.60) | 3.21 ± 2.70 | 0.60-16.70 | 2.45 (1.90-3.30) | < 0.001a |
| CRT at 0 h | 5.07 ± 2.46 | 2-12 | 5 (3-7) | 4.07 ± 2.18 | 1-9 | 3 (2-6) | 0.040a |
| CRT at 3 h | 6.38 ± 2.95 | 2-13 | 6 (4-8) | 3.82 ± 2.14 | 1-10 | 3 (2-5) | < 0.001a |
| CRT at 6 h | 7.10 ± 4.10 | 2-13 | 9 (3-11) | 3.63 ± 2.64 | 1-13 | 2.5 (2.0-5.0) | < 0.001a |
| ScvO2 at 0 h | 68.30 ± 7.80 | 43.20-73.90 | 71.9 (70.2-72.8) | 66.20 ± 8.50 | 42.80-74.20 | 70.80 (58.90-71.90) | 0.033a |
| ScvO2 at 3 h | 61.90 ± 7.10 | 45.80-73.50 | 62.8 (58.9-66.2) | 61.00 ± 6.50 | 36.80-73.70 | 61.70 (57.80-64.90) | 0.304 |
| ScvO2 at 6 h | 58.10 ± 11.80 | 36.70-89.20 | 58.9 (49.7-66.2) | 60.2 ± 7.60 | 37.90-71.0 | 61.90 (56.10-65.90) | 0.299 |
| P(cv-a)CO2 at 0 h | 6.50 ± 1.90 | 2.80-9.40 | 7.3 (4.9-7.9) | 5.70 ± 1.90 | 2.80-9.80 | 5.50 (4.20-7.40) | 0.036a |
| P(cv-a)CO2 at 3 h | 7.00 ± 2.10 | 3.40-12.30 | 6.5 (5.6-8.6) | 5.20 ± 1.60 | 2.7-11.0 | 4.8 (4.0-6.2) | < 0.001a |
| P(cv-a)CO2 at 6 h | 7.10 ± 2.50 | 2.60-10.80 | 7.4 (4.9-9.2) | 5.40 ± 4.50 | 2.60-8.70 | 4.70 (3.60-5.80) | < 0.001a |
| Lactate clearance at 3 h | (-10.20 ± 35.10) | (-88.10-40.40) | (-8.6) (-37.1-24.7) | 18.80 ± 34.20 | (-183.30-58.20) | 26.80 (7.30-37.20) | < 0.001a |
| Lactate clearance at 6 h | (-28.70 ± 72.80) | (-187.0-100.0) | (-46.4) (-61.5-29.9) | 29.20 ± 64.40 | (-433.80-81.30) | 44.90 (23.40-58.70) | < 0.001a |
| Test result variable | Area | Std. Error | P value | Asymptomatic 95%CI | |
| Lower bound | Upper bound | ||||
| Lactate at 0 h | 0.587 | 0.058 | 0.133 | 0.499 | 0.671 |
| Lactate at 3 h | 0.776 | 0.048 | < 0.001a | 0.696 | 0.843 |
| Lactate at 6 h | 0.845 | 0.04 | < 0.001a | 0.772 | 0.902 |
| Lactate clearance at 3 h | 0.754 | 0.053 | < 0.001a | 0.672 | 0.824 |
| Lactate clearance at 6 h | 0.834 | 0.041 | < 0.001a | 0.76 | 0.893 |
| CRT at 0 h | 0.623 | 0.056 | 0.028a | 0.536 | 0.705 |
| CRT at 3 h | 0.768 | 0.047 | < 0.001a | 0.688 | 0.837 |
| CRT at 6 h | 0.757 | 0.054 | < 0.001a | 0.675 | 0.827 |
| ScvO2 at 0 h | 0.630 | 0.062 | 0.035a | 0.542 | 0.711 |
| ScvO2 at 3 h | 0.562 | 0.064 | 0.325 | 0.474 | 0.648 |
| ScvO2 at 6 h | 0.565 | 0.072 | 0.367 | 0.476 | 0.651 |
| P(cv-a)CO2 at 0 h | 0.627 | 0.060 | 0.033a | 0.540 | 0.709 |
| P(cv-a)CO2 at 3 h | 0.754 | 0.052 | < 0.001a | 0.673 | 0.824 |
| P(cv-a)CO2 at 6 h | 0.736 | 0.060 | < 0.001a | 0.652 | 0.808 |
| Marker | Cutoff value | Sensitivity | Specificity | PPV | NPV | Accuracy | P value |
| Lactate at 0 h | > 4.9 | 55.2% | 63.2% | 29.1% | 83.8% | 61.5% | 0.074 |
| Lactate at 3 h | > 4.2 | 65.5% | 77.4% | 44.2% | 89.1% | 74.8% | < 0.001a |
| Lactate at 6 h | > 4.1 | 74.1% | 84.9% | 55.6% | 92.8% | 82.7% | < 0.001a |
| Lactate clearance at 3 h | ≤ -20.6 (≥ 20.6% from baseline) | 44.8% | 93.4% | 65.0% | 86.1% | 83.0% | < 0.001a |
| Lactate clearance at 6 h | ≤ -46.4 (≥ 46.4% from baseline) | 55.6% | 94.3% | 71.4% | 89.3% | 86.5% | < 0.001a |
| CRT at 0 h | > 4 | 55.2% | 67.9% | 32.0% | 84.7% | 65.2% | 0.022a |
| CRT at 3 h | > 4 | 69.0% | 70.8% | 39.2% | 89.3% | 70.4% | < 0.001a |
| CRT at 6 h | > 8 | 51.9% | 94.3% | 70.0% | 88.5% | 85.7% | < 0.001a |
| ScvO2 at 0 h | > 71.7 | 55.2% | 71.7% | 34.8% | 85.4% | 68.2% | 0.007a |
| ScvO2 at 3 h | > 61.7 | 69.0% | 53.8% | 29.0% | 86.4% | 57.0% | 0.03a |
| ScvO2 at 6 h | ≤ 58.9 | 55.6% | 65.1% | 28.9% | 85.2% | 63.2% | 0.05 |
| P(cv-a)CO2 at 0 h | > 7.6 | 44.8% | 81.1% | 39.4% | 84.3% | 73.3% | 0.004a |
| P(cv-a)CO2 at 3 h | > 5.9 | 72.4% | 73.6% | 42.9% | 90.7% | 73.3% | < 0.001a |
| P(cv-a)CO2 at 6 h | > 6.4 | 63.0% | 83.0% | 48.6% | 89.8% | 79.0% | < 0.001a |
A statistical significant (P = 0.033) difference in ScvO2 at baseline between non-survivors and survivors was observed in the present study. However, mean ScvO2 at 3 h and 6 h was comparable between non-survivors and survivors (P = 0.304 and 0.299, respectively) (Table 5).
In the current study, P(cv-a)CO2 ≥ 6 mmHg at baseline was used as one of the criteria of hypoperfusion and was measured at baseline, 3 h, and 6 h. At baseline, the mean P(cv-a)CO2 was 5.92 ± 1.91 mmHg. P(cv-a)CO2 was higher in survivors than non-survivors at baseline, 3 h, and 6 h, which achieved statistical significance with a P value of 0.036, < 0.001, and < 0.001, respectively. In the current study, the cutoff value of P(cv-a)CO2 in predicting 28-d mortality at baseline was ≥ 7.6 mmHg (AUROC: 0.627; sensitivity: 44.8%; specificity: 81.1%; PPV: 39.4%; NPV: 84.3%; accuracy: 73.3%; P = 0.004). Similarly, cutoff values for P(cv-a)CO2 at 3 h and 6 h were ≥ 5.9 and 6.45 mmHg, respectively (Tables 4 and 5).
Similarly, a statistically significant association was found between the 28-d mortality and CRT levels at baseline, 3 h, and 6 h. (P = 0.004, < 0.001, and < 0.001, respectively). The AUROC to estimate mortality for CRT at baseline was 0.623 [95% confidence interval (CI): 0.536-0.705) and at 3 h and 6 h was 0.768 (95%CI: 0.688-0.837) and 0.705 (95%CI: 0.675-0.827), respectively, with the asymptotic significance of < 0.001 and < 0.001, respectively. In the present study, the cutoff point to predict 28-d mortality for CRT at baseline was 4 s, with a sensitivity of 55.2% and specificity of 67.9%, while the cutoff point for CRT at 6 h was 7 s, with a sensitivity of 51.9% and a specificity of 94.3% (P < 0.001) (Tables 4 and 5).
We also performed a multivariate logistic regression analysis to predict variables associated with 28-d mortality. Only lactate levels at 6 h (odds ratio = 1.344; 95%CI: 1.168-1.546; P < 0.001) and baseline serum creatinine (odds ratio = 1.515; 95%CI: 1.036-2.216; P < 0.001) were identified as independent risk factors of 28-d mortality (Table 6).
Although serum lactate has been established as an objective surrogate marker for tissue hypoxia and disease severity in septic shock, an absolute dependence on serial lactate levels to guide fluid resuscitation may lead to over-resuscitation in some cases. Hence, alternative measures for assessing perfusion, such as CRT, ScvO2, and P(cv-a)CO2, might be more pragmatic. A recent study by Algeria et al[15] used CRT, P(cv-a)CO2, and ScvO2 to define hypoperfusion context and demonstrated that patients with hyperlactatemia plus hypoperfusion context exhibited a severe circulatory dysfunction with increased morbidity. However, this study was retrospective and did not examine the superiority of serial measurements of CRT, P(cv-a)CO2, and ScvO2 over serial lactate measurements in predicting poor outcome in patients with septic shock.
In the present prospective observational study involving 135 patients with septic shock, the outcome in two different clinical patterns of septic shock was analyzed: hypoperfusion context vs non-hypoperfusion context. Similar to the results by Algeria et al[15], the stratification of patients in the present study into hypoperfusion and non-hypoperfusion contexts did not result in a significant difference in 28-d mortality. However, in the present study, the subgroup of patients within the hypoperfusion context with a high P(cv-a)CO2 and CRT exhibited significantly higher mortality than those in the non-hypoperfusion context.
Baseline characteristics were comparable between the groups, apart from the APACHE II score, which was higher in Group 2. As the APACHE II score calculation involves chronic comorbidities, a higher APACHE II score in the non-hypoperfusion context could be attributed to more patients with cirrhosis and dialysis dependence.
Although the dose requirement of NE was higher in patients with hypoperfusion at all intervals compared to Group 2, it did not achieve statistical significance. These results differ from Algeria et al[15], who reported significantly higher NE requirements (P < 0.005) in the hypoperfusion context group. This difference could be due to a higher proportion of patients requiring vasopressin in the hypoperfusion context group in the present study. The present study also observed a higher fluid requirement in Group 1 at 0 h and 6 h. Consequently, this signifies the presence of more severe circulatory dysfunction in Group 1 than in Group 2. The rest of the secondary outcomes were comparable between Group 1 and Group 2.
Serum lactate has been established to be of prognostic value in patients with septic shock. Marty et al[16] showed a significant difference between the lactate values at baseline, 6 h, 12 h, or 24 h between the survivors and non-survivors group (P < 0.05 for each time interval). Analysis of the AUROC for lactate levels at baseline, 3 h, and 6 h to predict the 28-d mortality revealed that initial lactate levels had a poor predictive value compared to those at 3 h and 6 h. These results were similar to the study by Lee et al[17], conducted in 2021, in which the lactate levels at 6 h had a better prognostic performance. In the present study, the optimal cutoff value for lactate values in predicting 28-d mortality was ≥ 4.2 mmol/L, with a sensitivity of 55.2%, specificity of 63.2%, PPV of 29.1%, and NPV of 83.8%. Similarly, the cutoff value for the lactate levels at 6 h was ≥ 4.1 mmol/L with a sensitivity of 74.2%, specificity of 84.9%, PPV of 55.6%, and NPV of 92.8%. These findings differ from the study mentioned above by Lee et al[17], where the optimal cutoff of 6 h lactate levels was ≥ 2 mmol/L, with the highest sensitivity [89.2% (95%CI: 83.0%-93.7%)], but the specificity was relatively lower [35.3% (95%CI: 29.0%-42.1%)].
Lactate clearance is defined as the rate of decline in lactate concentration. It has been extensively studied and is a strong independent predictor of survival in patients with septic shock, with lactate non-clearance consistently linked to increased mortality[16]. In our study, lactate clearance remained higher in survivors than non-survivors at all time intervals in the study period. Although the prognostic value of lactate clearance at 6 h was better than at 3 h, the metrics were inferior to the static lactate levels at the corresponding time intervals. Similar results were observed in a study by Ryoo et al[18] in which lactate and lactate clearance at 6 h was associated with higher mortality; lactate levels had significantly higher prognostic value than lactate clearance. On multivariate analysis to evaluate mortality, among all variables assessed, only lactate at 6 h and baseline serum creatinine were independently associated with 28-d mortality (Table 6).
ScvO2 trends correlate well with mixed central venous oxygen saturation and have been independently associated with mortality in septic shock[19,20], with threshold values supporting those published in the SSC guidelines 2012[11]. Normalization of ScvO2 does not rule out persistent tissue hypoperfusion, and the latter can still occur due to severe microcirculatory disorders and mitochondrial dysfunction[21,22]. Moreover, if ScvO2 < 70% is associated with mortality[23], it does not mean that SvcO2 ≥ 70% is associated with survival[24]. Thus, in some circumstances, the use of ScvO2 might mistakenly drive an intensivist to conclude that the patient’s physiologic state has improved when, in fact it has not. According to the results of the current study, ScvO2 appeared to be a valuable tool for initial resuscitation but cannot distinguish between survivors and non-survivors after initial resuscitation.
The P(cv-a)CO2 gap represents an excellent surrogate indicator of the adequacy of cardiac output and tissue perfusion under a given condition of CO2 production. Recently, Ospina-Tascón et al[25] showed that the persistence of high P(v-a)CO2 (≥ 6 mmHg) during the first 6 h of resuscitation of septic shock patients is associated with severe multiple organ dysfunction and increased mortality rate (relative risk = 2.23; P = 0.01). There is a strong agreement between P(v-a)CO2 and P(cv-a)CO2, though it should not be interchanged. In the present study, it was observed that P(cv-a)CO2 was higher in survivors than non-survivors at all time intervals, and persistence of the PCO2 gap > 6.5 mmHg at 3 h and 6 h during early resuscitation of septic shock patients was associated with higher mortality rates. The cutoff values of P(cv-a)CO2 in predicting 28-d mortality at baseline was ≥ 7.6 mmHg (AUROC: 0.627; sensitivity: 44.8%; specificity: 81.1%; PPV: 39.4%; NPV: 84.3%; accuracy: 73.3%; P = 0.004). Similarly, the cutoff value at 6 h was ≥ 6.45 mmHg (AUROC: 0.685; sensitivity: 58.6%; specificity: 83.0%; PPV: 48.6%; NPV: 88.0%; accuracy: 77.8%; P < 0.001). A study by Helmy et al[26] observed a P(cv-a)CO2 cutoff of ≥ 8.4 mmHg at 0 h and ≥ 7.8 mmHg at 6 h as a predictor of all-cause hospital mortality. The difference in cutoff values may be because of the increased specificity. Consequently, high P(cv-a)CO2 > 6 mmHg at 6 h could identify patients with septic shock at high mortality risk in apparently resuscitated patients.
CRT has emerged as a reasonable alternative to guide septic shock resuscitation. The skin territory lacks autoregulatory flow control; therefore, sympathetic activation can impair skin perfusion during circulatory dysfunction, a phenomenon that can be assessed by measuring CRT[27]. CRT can be easily measured at the bedside with no additional equipment required beyond a chronometer (i.e. a clock or the stopwatch on your phone). Measurement of CRT upon admission assesses the alteration in microcirculation at 3 h and 6 h; it also evaluates the response to resuscitation. The present study found a statistically significant association between the 28-d mortality and CRT at baseline, 3 h, and 6 h. Similar results were described by Morocho et al[28], who concluded that the measurement of CRT at baseline, 3 h, and 6 h was a strong predictor of mortality in septic shock, even above the widely studied markers such as lactate.
Castro et al[29] demonstrated that CRT-targeted fluid resuscitation was associated with higher and faster achievement of resuscitation targets and exhibited similar improvement in hypoxia surrogates and regional blood flow to those observed with lactate-targeted fluid resuscitation. These results were in contradiction with that of the ANDROMEDA-SHOCK trial[30]. It may be due to the difference in the duration of intervention periods of both studies and the different kinetics of CRT and lactate. In accordance with the current literature and the results of the present study, CRT is a reliable marker for assessing the severity of clinical perfusion. Its frequent bedside assessment alone can improve resuscitation in septic shock, especially in low-resource settings.
In the present study, it was observed that the cutoff point to predict 28-d mortality for CRT at baseline was 4 s, with a sensitivity of 55.2% and specificity of 67.9%, while the cutoff point for CRT at 6 h was 7 s, with a sensitivity of 51.9%, and a specificity of 94.3%. The corresponding CRT cutoffs by Morocho et al[28] at admission and 6 h were 4.5 s at admission and 3.5 s at 6 h post-resuscitation. This cutoff at 6 h was different from the present study, which may be because of temperature-associated variation, inter-rater variability, and high melanin concentration in our population[31,32]. In dark-skinned people (phototypes V and VI), the high concentration of melanin in the epidermis absorbs much of the light, so the reflected light contribution comes mainly from the melanin contribution and not from the perfusion change caused in the dermis during compression, causing an error in the CRT measurement[33]. This can be overcome by newly developed optical devices to objectively assess CRT. Recently the role of melanin pigment in controlling the immune response has been increasingly recognized. Melanocytes containing little melanin produce more cytokines, such as TNF, IL-1β, IL-6, and IL-10, and can cause fluctuation in the immune response levels[34].
The current study had a few limitations. This non-experimental observational study could only demonstrate an association between hypoperfusion context and 28-d mortality but could not establish the cause-and-effect relationship. We used all-cause in-hospital mortality as our primary outcome; patients might have died from non-sepsis-related causes. Given the various etiologies of hyperlactatemia, drugs or comorbidities causing hyperlactatemia of any clinical significance could not be accounted for, making interpretation of hyperlactatemia challenging. Although the personnel were thoroughly trained to assess CRT using a standardized technique, we did not consider the inter-rater variability and skin temperature, which could alter CRT values. Lastly, this was a single-center study with a small sample size. Future multicenter prospective studies with larger sample sizes must conclusively establish the endpoints of early resuscitation in septic shock to reduce patient mortality.
Septic shock patients fulfilling the hypoperfusion and non-hypoperfusion context exhibit similar 28-d all-cause hospital mortality, although patients with hypoperfusion displayed a more severe circulatory dysfunction. Targeting ScvO2 may not be desirable as normalization of ScvO2 does not rule out persistent tissue hypoperfusion. Lactate levels at 6 h had a better prognostic value in predicting 28-d mortality than other parameters. Persistently high P(cv-a)CO2 (> 6 mmHg) or increased CRT (> 4 s) at 3 h and 6 h during early resuscitation can be a valuable additional tool for prognostication of septic shock patients.
As per the latest Surviving Sepsis Campaign guidelines, fluid resuscitation should be guided by repeated measurements of blood lactate levels until normalization.
Serum lactate is a non-specific biomarker that may be increased by a myriad of clinical conditions. Thus, it may not be the best tool for real-time assessment of the effect of hemodynamic resuscitation, and exploring alternative resuscitation targets should be an essential research priority in sepsis.
To compare the 28-d mortality in two clinical patterns of septic shock: hyperlactatemic patients in the hypoperfusion context and hyperlactatemic patients in the non-hypoperfusion context.
This prospective comparative observational study carried out on 135 adult patients with septic shock that met Sepsis-3 definitions compared patients of hyperlactatemia with hypoperfusion (Group 1, n = 95) and hyperlactatemia without hypoperfusion (Group 2, n = 40). The patients were observed for various macro and micro hemodynamic parameters at regular intervals of 0 h, 3 h, and 6 h. All-cause 28-d mortality and all other secondary objective parameters were observed at specified intervals.
The stratification of patients into hypoperfusion and non-hypoperfusion did not result in a significantly different 28-d mortality (24% vs 15%, respectively; P = 0.234). However, the patients within the hypoperfusion context with high P(cv-a)CO2 and CRT (P = 0.022) at baseline had significantly higher mortality than Group 2. Group 1 had a higher proportion of patients requiring vasopressin and the mean vasopressor-free days out of the total 28 d were lower in patients with hypoperfusion (18.88 ± 9.04 vs 21.08 ± 8.76; P = 0.011). The mean lactate levels and lactate clearance at 3 h and 6 h, CRT, and P(cv-a)CO2 at 0 h, 3 h, and 6 h were found to be associated with 28-d mortality in patients with septic shock, with lactate levels at 6 h having the best predictive value (area under the receiver operating characteristic: 0.845).
Septic shock patients fulfilling the hypoperfusion and non-hypoperfusion context exhibit similar 28-d all-cause hospital mortality, although patients with hypoperfusion displayed a more severe circulatory dysfunction. Lactate levels at 6 h had a better predictive value in predicting 28-d mortality. Persistently high P(cv-a)CO2 (> 6 mmHg) or increased CRT (> 4 s) at 3 h and 6 h during the early resuscitation can be a valuable additional aid for prognostication of septic shock patients.
Multicenter large scale trials should be conducted to further evaluate the role of CRT and PCO2 gap as markers for resuscitation in patients with septic shock.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cai J, China; Cure E, Turkey; Hakimi T, Afghanistan S-Editor: Chang KL L-Editor: Filipodia P-Editor: Chang KL
| 1. | Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15803] [Cited by in RCA: 16968] [Article Influence: 1885.3] [Reference Citation Analysis (2)] |
| 2. | Liu V, Escobar GJ, Greene JD, Soule J, Whippy A, Angus DC, Iwashyna TJ. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA. 2014;312:90-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 679] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 3. | Rhee C, Murphy MV, Li L, Platt R, Klompas M; Centers for Disease Control and Prevention Epicenters Program. Comparison of trends in sepsis incidence and coding using administrative claims vs objective clinical data. Clin Infect Dis. 2015;60:88-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 4. | Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA; Emergency Medicine Shock Research Network (EMShockNet) Investigators. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303:739-746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 804] [Cited by in RCA: 717] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 5. | Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med. 2004;32:1825-1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 830] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 6. | Russell JA, Walley KR, Singer J, Gordon AC, Hébert PC, Cooper DJ, Holmes CL, Mehta S, Granton JT, Storms MM, Cook DJ, Presneill JJ, Ayers D; VASST Investigators. Vasopressin vs norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358:877-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1191] [Cited by in RCA: 1177] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 7. | Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, Nyeko R, Mtove G, Reyburn H, Lang T, Brent B, Evans JA, Tibenderana JK, Crawley J, Russell EC, Levin M, Babiker AG, Gibb DM; FEAST Trial Group. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364:2483-2495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1259] [Cited by in RCA: 1080] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 8. | Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39:259-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 944] [Cited by in RCA: 1008] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 9. | Vincent JL, Quintairos E Silva A, Couto L Jr, Taccone FS. The value of blood lactate kinetics in critically ill patients: a systematic review. Crit Care. 2016;20:257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 327] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 10. | Jansen TC, van Bommel J, Schoonderbeek FJ, Sleeswijk Visser SJ, van der Klooster JM, Lima AP, Willemsen SP, Bakker J; LACTATE study group. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182:752-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 606] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 11. | Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R; Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3146] [Cited by in RCA: 3162] [Article Influence: 263.5] [Reference Citation Analysis (0)] |
| 12. | Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, Mcintyre L, Ostermann M, Prescott HC, Schorr C, Simpson S, Wiersinga WJ, Alshamsi F, Angus DC, Arabi Y, Azevedo L, Beale R, Beilman G, Belley-Cote E, Burry L, Cecconi M, Centofanti J, Coz Yataco A, De Waele J, Dellinger RP, Doi K, Du B, Estenssoro E, Ferrer R, Gomersall C, Hodgson C, Møller MH, Iwashyna T, Jacob S, Kleinpell R, Klompas M, Koh Y, Kumar A, Kwizera A, Lobo S, Masur H, McGloughlin S, Mehta S, Mehta Y, Mer M, Nunnally M, Oczkowski S, Osborn T, Papathanassoglou E, Perner A, Puskarich M, Roberts J, Schweickert W, Seckel M, Sevransky J, Sprung CL, Welte T, Zimmerman J, Levy M. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47:1181-1247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 2194] [Article Influence: 548.5] [Reference Citation Analysis (0)] |
| 13. | Hernandez G, Bruhn A, Castro R, Regueira T. The holistic view on perfusion monitoring in septic shock. Curr Opin Crit Care. 2012;18:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Hernandez G, Luengo C, Bruhn A, Kattan E, Friedman G, Ospina-Tascon GA, Fuentealba A, Castro R, Regueira T, Romero C, Ince C, Bakker J. When to stop septic shock resuscitation: clues from a dynamic perfusion monitoring. Ann Intensive Care. 2014;4:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 15. | Alegría L, Vera M, Dreyse J, Castro R, Carpio D, Henriquez C, Gajardo D, Bravo S, Araneda F, Kattan E, Torres P, Ospina-Tascón G, Teboul JL, Bakker J, Hernández G. A hypoperfusion context may aid to interpret hyperlactatemia in sepsis-3 septic shock patients: a proof-of-concept study. Ann Intensive Care. 2017;7:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Marty P, Roquilly A, Vallée F, Luzi A, Ferré F, Fourcade O, Asehnoune K, Minville V. Lactate clearance for death prediction in severe sepsis or septic shock patients during the first 24 hours in Intensive Care Unit: an observational study. Ann Intensive Care. 2013;3:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 17. | Lee SG, Song J, Park DW, Moon S, Cho HJ, Kim JY, Park J, Cha JH. Prognostic value of lactate levels and lactate clearance in sepsis and septic shock with initial hyperlactatemia: A retrospective cohort study according to the Sepsis-3 definitions. Medicine (Baltimore). 2021;100:e24835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Ryoo SM, Lee J, Lee YS, Lee JH, Lim KS, Huh JW, Hong SB, Lim CM, Koh Y, Kim WY. Lactate Level Versus Lactate Clearance for Predicting Mortality in Patients With Septic Shock Defined by Sepsis-3. Crit Care Med. 2018;46:e489-e495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 19. | Reinhart K, Kuhn HJ, Hartog C, Bredle DL. Continuous central venous and pulmonary artery oxygen saturation monitoring in the critically ill. Intensive Care Med. 2004;30:1572-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 248] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 20. | Dueck MH, Klimek M, Appenrodt S, Weigand C, Boerner U. Trends but not individual values of central venous oxygen saturation agree with mixed venous oxygen saturation during varying hemodynamic conditions. Anesthesiology. 2005;103:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Puskarich MA, Trzeciak S, Shapiro NI, Albers AB, Heffner AC, Kline JA, Jones AE. Whole blood lactate kinetics in patients undergoing quantitative resuscitation for severe sepsis and septic shock. Chest. 2013;143:1548-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 22. | Mallat J, Lemyze M, Tronchon L, Vallet B, Thevenin D. Use of venous-to-arterial carbon dioxide tension difference to guide resuscitation therapy in septic shock. World J Crit Care Med. 2016;5:47-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 95] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (1)] |
| 23. | Boulain T, Garot D, Vignon P, Lascarrou JB, Desachy A, Botoc V, Follin A, Frat JP, Bellec F, Quenot JP, Mathonnet A, Dequin PF; Clinical Research in Intensive Care and Sepsis Group. Prevalence of low central venous oxygen saturation in the first hours of intensive care unit admission and associated mortality in septic shock patients: a prospective multicentre study. Crit Care. 2014;18:609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Textoris J, Fouché L, Wiramus S, Antonini F, Tho S, Martin C, Leone M. High central venous oxygen saturation in the latter stages of septic shock is associated with increased mortality. Crit Care. 2011;15:R176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Ospina-Tascón GA, Bautista-Rincón DF, Umaña M, Tafur JD, Gutiérrez A, García AF, Bermúdez W, Granados M, Arango-Dávila C, Hernández G. Persistently high venous-to-arterial carbon dioxide differences during early resuscitation are associated with poor outcomes in septic shock. Crit Care. 2013;17:R294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 26. | Helmy TA, El-Reweny EM, Ghazy FG. Prognostic Value of Venous to Arterial Carbon Dioxide Difference during Early Resuscitation in Critically Ill Patients with Septic Shock. Indian J Crit Care Med. 2017;21:589-593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Hernández G, Castro R, Bakker J. Capillary refill time: the missing link between macrocirculation and microcirculation in septic shock? J Thorac Dis. 2020;12:1127-1129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Morocho JP, Martínez AF, Cevallos MM, Vasconez-Gonzalez J, Ortiz-Prado E, Barreto-Grimaldos A, Vélez-Páez JL. Prolonged Capillary Refilling as a Predictor of Mortality in Patients With Septic Shock. J Intensive Care Med. 2022;37:423-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 29. | Castro R, Kattan E, Ferri G, Pairumani R, Valenzuela ED, Alegría L, Oviedo V, Pavez N, Soto D, Vera M, Santis C, Astudillo B, Cid MA, Bravo S, Ospina-Tascón G, Bakker J, Hernández G. Effects of capillary refill time-vs. lactate-targeted fluid resuscitation on regional, microcirculatory and hypoxia-related perfusion parameters in septic shock: a randomized controlled trial. Ann Intensive Care. 2020;10:150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 30. | Hernández G, Ospina-Tascón GA, Damiani LP, Estenssoro E, Dubin A, Hurtado J, Friedman G, Castro R, Alegría L, Teboul JL, Cecconi M, Ferri G, Jibaja M, Pairumani R, Fernández P, Barahona D, Granda-Luna V, Cavalcanti AB, Bakker J; The ANDROMEDA SHOCK Investigators and the Latin America Intensive Care Network (LIVEN), Hernández G, Ospina-Tascón G, Petri Damiani L, Estenssoro E, Dubin A, Hurtado J, Friedman G, Castro R, Alegría L, Teboul JL, Cecconi M, Cecconi M, Ferri G, Jibaja M, Pairumani R, Fernández P, Barahona D, Cavalcanti AB, Bakker J, Hernández G, Alegría L, Ferri G, Rodriguez N, Holger P, Soto N, Pozo M, Bakker J, Cook D, Vincent JL, Rhodes A, Kavanagh BP, Dellinger P, Rietdijk W, Carpio D, Pavéz N, Henriquez E, Bravo S, Valenzuela ED, Vera M, Dreyse J, Oviedo V, Cid MA, Larroulet M, Petruska E, Sarabia C, Gallardo D, Sanchez JE, González H, Arancibia JM, Muñoz A, Ramirez G, Aravena F, Aquevedo A, Zambrano F, Bozinovic M, Valle F, Ramirez M, Rossel V, Muñoz P, Ceballos C, Esveile C, Carmona C, Candia E, Mendoza D, Sanchez A, Ponce D, Ponce D, Lastra J, Nahuelpán B, Fasce F, Luengo C, Medel N, Cortés C, Campassi L, Rubatto P, Horna N, Furche M, Pendino JC, Bettini L, Lovesio C, González MC, Rodruguez J, Canales H, Caminos F, Galletti C, Minoldo E, Aramburu MJ, Olmos D, Nin N, Tenzi J, Quiroga C, Lacuesta P, Gaudín A, Pais R, Silvestre A, Olivera G, Rieppi G, Berrutti D, Ochoa M, Cobos P, Vintimilla F, Ramirez V, Tobar M, García F, Picoita F, Remache N, Granda V, Paredes F, Barzallo E, Garcés P, Guerrero F, Salazar S, Torres G, Tana C, Calahorrano J, Solis F, Torres P, Herrera L, Ornes A, Peréz V, Delgado G, López A, Espinosa E, Moreira J, Salcedo B, Villacres I, Suing J, Lopez M, Gomez L, Toctaquiza G, Cadena Zapata M, Orazabal MA, Pardo Espejo R, Jimenez J, Calderón A, Paredes G, Barberán JL, Moya T, Atehortua H, Sabogal R, Ortiz G, Lara A, Sanchez F, Hernán Portilla A, Dávila H, Mora JA, Calderón LE, Alvarez I, Escobar E, Bejarano A, Bustamante LA, Aldana JL. Effect of a Resuscitation Strategy Targeting Peripheral Perfusion Status vs Serum Lactate Levels on 28-Day Mortality Among Patients With Septic Shock: The ANDROMEDA-SHOCK Randomized Clinical Trial. JAMA. 2019;321:654-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 499] [Article Influence: 83.2] [Reference Citation Analysis (0)] |
| 31. | Anderson B, Kelly AM, Kerr D, Clooney M, Jolley D. Impact of patient and environmental factors on capillary refill time in adults. Am J Emerg Med. 2008;26:62-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Brown LH, Prasad NH, Whitley TW. Adverse lighting condition effects on the assessment of capillary refill. Am J Emerg Med. 1994;12:46-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Bachour RP, Dias EL, Cardoso GC. Skin color independent robust assessment of capillary refill time. 2021 preprint. Available from: arXiv:2102.13611. [DOI] [Full Text] |
| 34. | Koike S, Yamasaki K. Melanogenesis Connection with Innate Immunity and Toll-Like Receptors. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |