Published online May 26, 2023. doi: 10.12998/wjcc.v11.i15.3408
Peer-review started: January 10, 2023
First decision: February 8, 2023
Revised: February 26, 2023
Accepted: April 17, 2023
Article in press: April 17, 2023
Published online: May 26, 2023
Processing time: 134 Days and 19.4 Hours
High rates of extrapancreatic malignancies, in particular colorectal cancer (CRC), have been detected in patients with intraductal papillary mucinous neoplasm (IPMN). So far, there is no distinct explanation in the literature for the deve
Core Tip: In this mini-review, we highlighted the genetic alterations that occur in intraductal papillary mucinous neoplasm (IPMN) and colorectal cancer to understand common genetic or epigenetic risk factors that could explain their synchronous manifestation. The process of malignant transformation in both entities is complex, but some distinctive features of IPMN lesions are linked with their genetic heterogeneity. Specific mutations in GNAS and KRAS are mainly expressed in IPMN. A significantly lower frequency of mutations is detected in other cancer-related genes, such as SMAD4, PI3KCA, PTEN, and BRAF.
- Citation: Mirchev MB, Boeva I, Peshevska-Sekulovska M, Stoitsov V, Peruhova M. Synchronous manifestation of colorectal cancer and intraductal papillary mucinous neoplasms. World J Clin Cases 2023; 11(15): 3408-3417
- URL: https://www.wjgnet.com/2307-8960/full/v11/i15/3408.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i15.3408
Intraductal papillary mucinous neoplasm (IPMN) of the pancreas is characterized histologically by a broad spectrum of transformation starting from low-grade dysplasia, moderate dysplasia, high-grade dysplasia, and invasive pancreatic carcinoma (PC)[1]. Depending on the epithelial type, IPMN has a variable prognosis with the unambiguous potential to transform into invasive PC[2].
IPMN is characterized by intraductal papillary growth and mucous secretion, which leads to ductal dilatation of the pancreas. IPMN is classified into main duct IPMN, branch duct IPMN, or mixed type IPMN and is based on anatomical involvement of the pancreatic ductal system[3]. It was estimated that the main duct type IPMN has a higher malignancy potential, with a range of 36%-100%[4]. It is important to note that even malignant IPMN can be resected and has a better prognosis compared with pancreatic ductal adenocarcinoma[5]. In the last few decades, many studies have been published representing an interesting correlation between IPMN and other malignancies, which emerge before or simultaneously with the diagnosis of IPMN. It was estimated that in patients with IPMN, the incidence of additional malignancy is in the range of 10%-52%[6].
Typically, the gastrointestinal (GI) tract is involved, with a prevalence of colon polyps and colorectal cancer (CRC) in Western countries and gastric cancer in Asian countries[7]. The incidence of synchronous CRCs and IPMN is about 3%-12% in Western countries[8]. In some publications, it was pointed out that the frequency of colonic adenomas was uncommonly higher in patients with IPMN than in those with pancreatic ductal adenocarcinoma[9].
In the last two decades, a unique carcinogenesis model for CRC has been revealed with a detailed analysis of underlying genetic and epigenetic alterations[10]. On the contrary, the mechanisms of malignant transformation in IPMN remain poorly understood. It is believed that IPMN is fundamentally characterized by a genetic lesion and that an accumulation of somatic mutations drives the histologic progression, ultimately leading to malignant transformation[11].
So far, there is no distinct explanation in the literature for the development of secondary or synchronous malignancies in patients with IPMN. In the past few years, data were published related to common genetic alterations between IPMN and other affiliated cancers[12,13]. This review elucidates the association between IPMN and CRC. We also discuss the most relevant information concerning the molecular mechanism of genetic alterations leading to the synchronous development of IPMN and CRC.
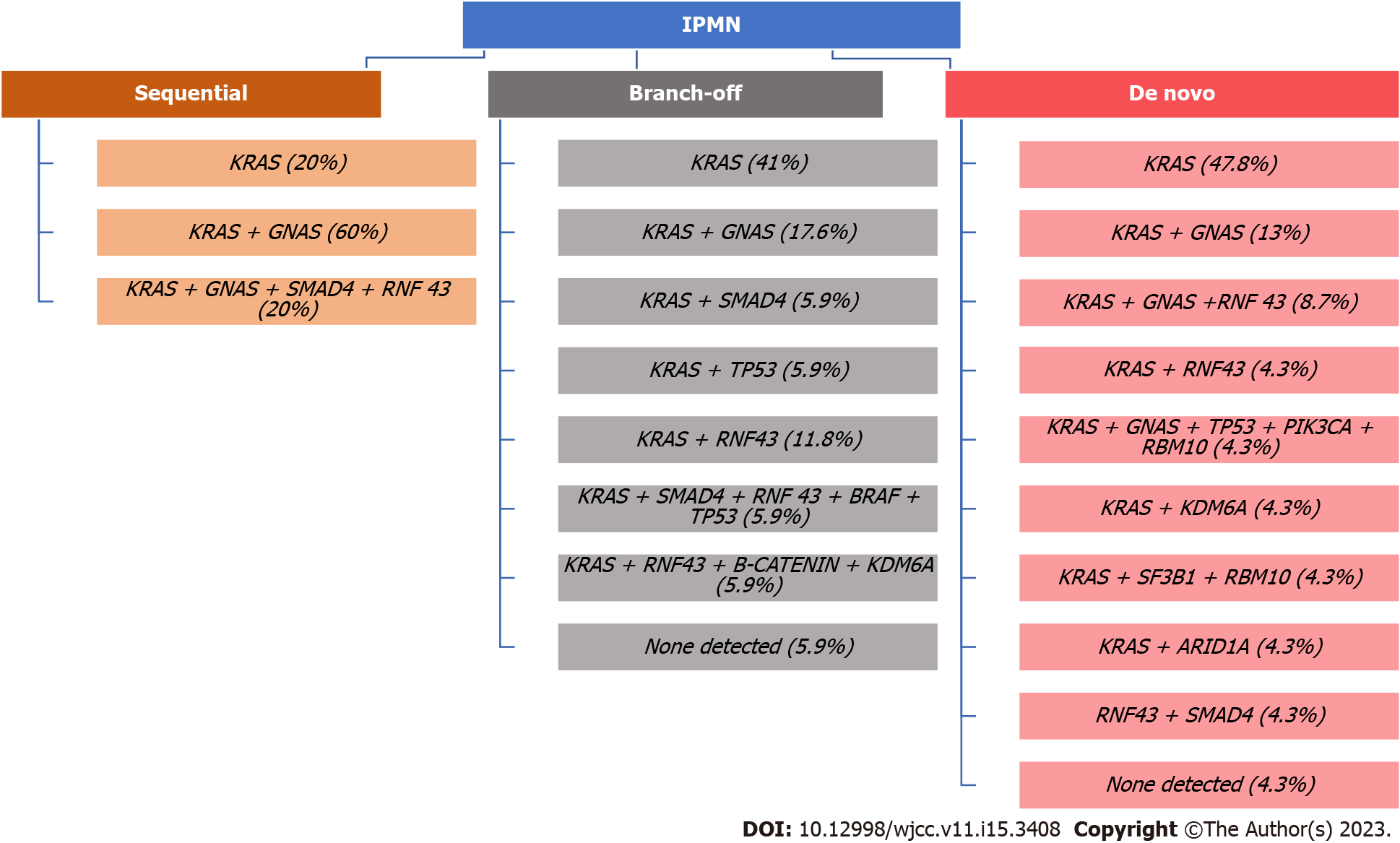
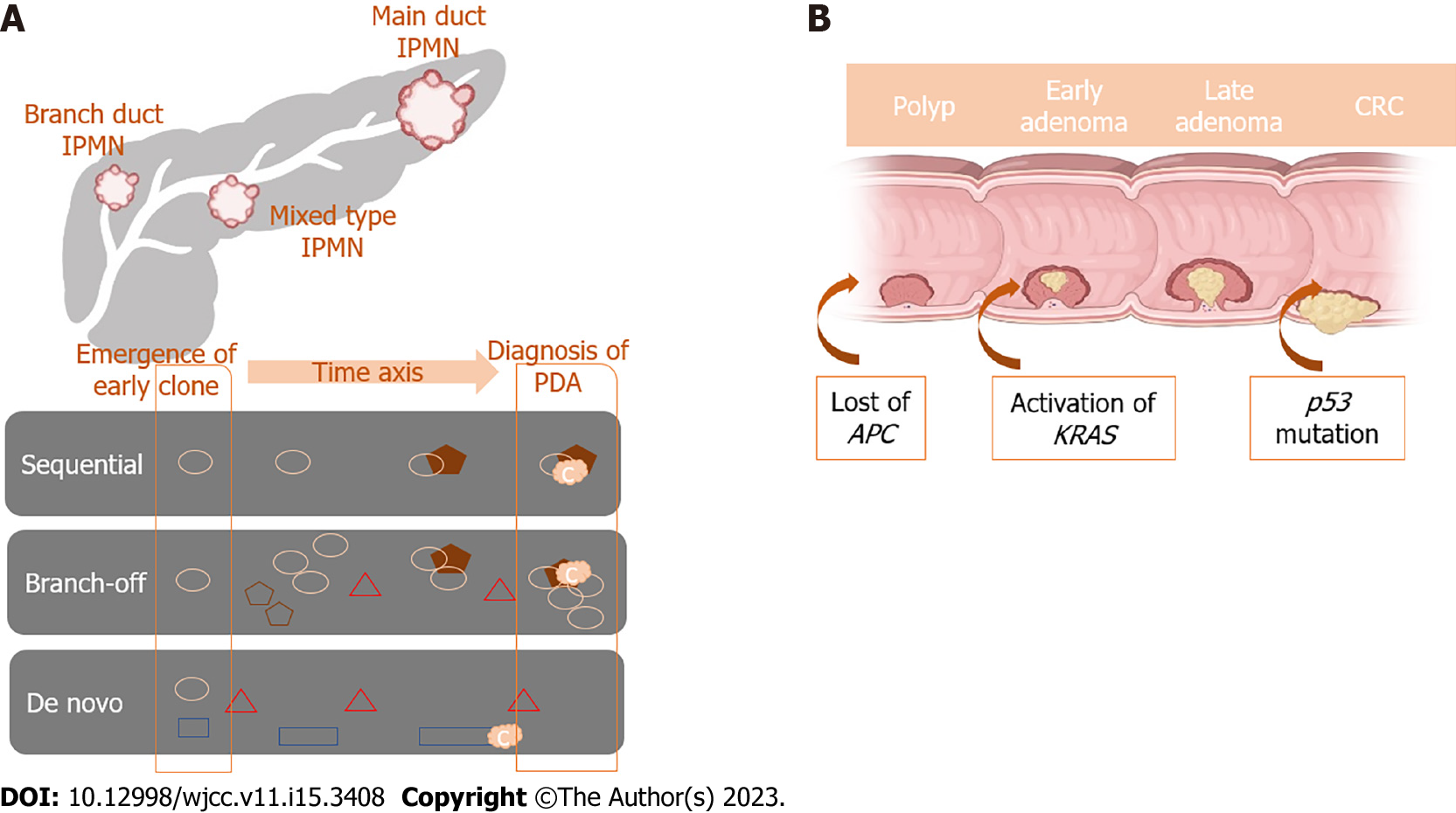
Pancreatic carcinogenesis is a result of somatic and germline mutations. For instance, inactivating mutations in tumor suppressor genes TP53, CDKN2A, and SMAD4 and activating gene mutations in the oncogene KRAS[14] are known contributors. Omori et al[4] investigated genetic alterations that occur in IPMN lesions leading to PC based on genetic and histologic analyses. According to the authors, there are three subtypes of malignant transformation from IPMN to PC. The first subtype is called the sequential pathway, which has a specific sequential acquisition of driver and tumor suppressor gene mutations, with frequent GNAS mutations that lead to PC development. The second type is called branch-off pathway, characterized by certain KRAS mutations that are common among PC and GNAS mutations that are more prevalent in IPMN. The third subtype has de novo driver mutations not found in concurrent IPMN and has substantial heterogeneity among early clones. Additionally, it was proven that PC resulting from the sequential or branch-off pathways has a worse prognosis compared to the de novo PC[15].
An interesting study by Ren et al[16] highlighted that GNAS and KRAS mutations are frequently observed in IPMN and are very specific for this entity. We want to underline that mutations in the aforementioned genes are one of the initial steps in the malignant transformation. However, this process is more complex, and additional genetic alterations, occurring in a stepwise manner, are needed to proceed with the process of malignant transformation of IPMN[17,18].
To summarize, the precise manner of malignant transformation of IPMN to PC is a more complex process due to the high genetic heterogeneity characterized by IPMN lesions (Figure 1).
CRC was one of the first genetically characterized tumors in which a stepwise progression was discovered and several molecular pathways for its formation have been proposed[19]. In the widely popular adenoma-carcinoma sequence, a gradual transition from normal colonic mucosa through aberrant crypt foci, small, medium, and large adenoma to carcinoma is accompanied by the accumulation of driver gene mutations, namely APC, KRAS, PIK3CA, SMAD4, and TP53[20]. This is the so-called chromosomal instability pathway, responsible for approximately 80% of sporadic CRC cases, which was proposed in the late 1990s by Fearon and Vogelstein[21].
It is believed that APC and KRAS mutations are early events in the progression from normal epithelium to adenoma, while PIK3CA mutation and SMAD4 and TP53 loss of function are late events in the adenoma-carcinoma sequence, enabling tumor cells to invade adjacent tissues and metastasize[22]. Also, the co-occurrence of APC, KRAS, and TP53 dysfunction is associated with distant metastasis and poor outcome, whereas mutations of PIK3CA and SMAD4 are absent in advanced disease[23].
An important co-player is the transforming growth factor-beta, acting in conjunction with B-catenin, which enters the cell nucleus when not regulated by the mutant APC gene. Together, they stimulate the Wnt pathway, leading to the induction of epithelial-mesenchymal transition and enhanced mobility and invasiveness of cells in CRC[24].
Around 5%-10% of CRC cases are due to heredity. The most common syndromes include hereditary non-polyposis CRC (HNPCC), familial adenomatous polyposis (FAP), attenuated FAP, Peutz-Jeghers syndrome (PJS), juvenile polyposis syndrome, and others[25].
The other mechanism of paramount importance for CRC formation is the microsatellite instability pathway. Microsatellites are short repetitive DNA sequences composed of 1-6 nucleotides, scattered throughout the human genome. They have no specific function but are prone to replication errors[26]. When this occurs, a special system, called the mismatch repair system (MMR), is activated and identifies and corrects base mismatches caused by DNA replication errors[27,28].
Furthermore, a third model for colorectal carcinogenesis has evolved: The CpG island methylator phenotype. It is characterized by dense promoter hypermethylation, leading to gene inactivation. Transcriptional silencing of essential tumor suppressor genes, caused by aberrant DNA methylation, could promote neoplastic growth. This mechanism is thought to be important in the serrated pathway in CRC[29].
Defective DNA MMR emerges from genetic or epigenetic alterations that most frequently lead to the inactivation of the genes hMLH1 and hMSH2. This genetic abnormality is thought to promote tumorigenesis by the accumulation of mutations in oncogenes and tumor suppressor genes. This pathway was reported in the tumorigenesis of CRC and later in other malignancies as well[30].
HNPCC, also known as Lynch syndrome, is characterized by germline mutations in MMR genes
To our knowledge, there are scarce data in the literature about the concomitant diagnosis of IPMN and Lynch syndrome. Only two case reports have been published so far. The first one was reported on patients with Lynch syndrome and IPMN, with a confirmed germline mutation in the MSH2 gene. It was confirmed that both tumors (colorectal and IPMN) showed identical loss of expression of MSH2 and MSH6 as well as a high level of microsatellite instability. Based on this fact it can be suggested that IPMN could be part of the variations of Lynch syndrome[34].
The second case was about a patient who met Amsterdam criteria for Lynch syndrome with a germline mutation in MSH2 and had a synchronous IPMN. More interestingly, in that case, the IPMN specimen did not demonstrate microsatellite instability and had preserved MSH2 expression[35].
In general, both case reports provided evidence for a potential relationship between Lynch syndrome and IPMN. Emerging new approaches revealed that mutations in MMR genes could play a role in the development of IPMN.
Although the accurate nature of the relationship between IPMN and Lynch syndrome is not yet understood, an enhanced level of suspicion for patients with Lynch syndrome and concomitant pancreatic cystic lesions is warranted. Further studies have to be conducted to identify the relationship between IPMN and HNPCC (Figure 2).
PJS is an autosomal dominant hereditary syndrome characterized by intestinal hamartomatous polyposis and mucocutaneous pigmentation[36]. It was established that patients with PJS have a higher risk of malignancies such as GI tumors (CRC, esophagus), and breast, ovarian and urinary cancers[37]. This syndrome is due to germline mutations in the STK11 gene. Recently, it was discovered that this gene exerts an impact on p53-dependent apoptosis[38].
To shed light on the role of genetic alterations and molecular features of IPMN, Sato et al[39] published a comparative study analyzing 22 IPMNs among patients with and without PJS. The authors revealed that STK11/LKB1 mutations were present in 100% (2/2) of samples from PJS patients vs only 25% (5/20) of samples from patients without PJS. In general, these data suggest that STK11/LKB1 mutations could play an important role in IPMN development.
A study by Resta et al[40] observed that STK11/LKB1 mutations in IPMNs among PJS patients may predispose this patient population to higher rates of progression to CRC compared to the general population. Therefore, such patients have a high risk of developing a variety of malignancies, including IPMN and CRC, and strict screening should be advised for these patients.
FAP is an autosomal dominant syndrome characterized by germline mutations in the APC gene on chromosome 5q21. This entity is associated with the development of hundreds to thousands of adenomas in the GI tract[41]. Polyps often develop at an early age and harbor a 100% lifetime risk of CRC. Patients with FAP have a high risk of developing other malignancies, including gastric, duodenal, pancreatic, and desmoid tumors[42].
IPMN is an extremely rare extracolonic manifestation associated with FAP, with few published cases in the medical literature so far. A case report by Maire et al[43] demonstrated an association between FAP and IPMN in a 41-year-old patient. The authors performed genetic analysis and found the absence of APC protein, which is typical of the adenomas in FAP patients, and wildtype APC in IPMN. These findings supported the idea that APC mutation leading to the transformation of normal mucosa into CRC could also play a role in the carcinogenesis of IPMN.
In addition to the above, another case report published by Chetty et al[44] provided a correlation between FAP and IPMN. The patient had an extracolonic manifestation IPMN of the pancreas and polypoid gastric heterotopia in the duodenum. The immunohistochemical analysis confirmed that duodenal adenomas and IPMN of the pancreas were pathogenetically related, with the IPMN being an unusual extracolonic manifestation of FAP and/or attenuated FAP.
In 1999, the first study related to the concomitant manifestation of IPMN and other extrapancreatic malignancies (EPMs) was published. Afterward, many additional studies were conducted to determine the frequency of prevalent or incident EPMs in patients with IPMN[12]. The results of these publications indicated that the frequency of EPM was around 24.6%-39.0%[7,45]. However, the majority of these studies were retrospective, based on a small group of patients, or featured a recruitment bias. Over the last few years, several meta-analyses and large studies confirmed an increased frequency of GI malignancy in IPMN patients in comparison to the general population. Thus, most research teams suggest special CRC surveillance programs for these patients.
A large meta-analysis, reviewing 16 studies and a total of 8240 patients, was published in 2022. The authors quantified the association between IPMN and EPM. They found that compared to the general population patients with IPMNs faced a greater risk of EPM. The subgroup analysis for GI malignancies in patients harboring IPMN provided an odds ratio of 12.9[46]. In 2022, Zelnik et al[47]published a matched cross-sectional historical study comparing the prevalence of colorectal polyps and CRC among 310 IPMN patients in comparison to sex- and age-matched middle-risk patients. The authors established a significantly higher prevalence of polyps with advanced dysplasia and CRC in the IPMN group than in matched controls, while the occurrence of polyps was similar in both groups. This study had some limitations such as a lack of IPMN histopathological specimens, genetic tests to determine shared molecular mechanisms for IPMN and CRC, and the absence of information regarding the time relationship between the appearance of findings in the pancreas and colon[47].
A study from the Mayo Clinic in 2010 confirmed that patients with IPMN had an increased risk of harboring EPM, in particular, CRC. In addition, the authors observed an increased incidence of colorectal adenomas in the IPMN group. The association between IPMN and colorectal polyps still remains uncertain. A prospective study published by Panic et al[48] suggested that the elevated risk of CRC among patients with IPMN was not related to accelerated adenoma formation.
In 2006, Eguchi et al[49] investigated the incidence of synchronous or metachronous extrapancreatic cancer among 69 patients who underwent surgery for IPMN. The authors reported that CRC occurred 5.37 times more frequently in IPMN patients than in the general population. In a population-based study, Rial et al[45] reported a 1.66 times higher rate of CRC in patients with invasive IPMN compared to the general population.
More recently, Larghi et al[50] published a multicentric study that included 390 IPMN patients. The authors reported an increased prevalence of EPM, notably for CRC and thyroid cancer. The reported incidence of EPM was 23.6% overall and 12.6% for CRC. A single-center study conducted by Panic et al[48] on 198 patients with IPMN sought to find any EPMs diagnosed previously, synchronously, or after the IPMN diagnosis. One of the frequently diagnosed EPMs was CRC (12 patients, 6.1%).
Another population-based study published in 2019 analyzed a diverse patient population from a large geographic area. The results confirmed that patients with IPMN were more likely to acquire EPM than the general population. The authors concluded that the advanced IPMN stage and the short latency period over which the second neoplasm developed were predictors of higher mortality[51].
Despite all these findings, it is still debatable whether there is a clear association between IPMN and CRC. In a critical systematic review of 15 studies from 2015, Pugliese et al[52] highlighted that the majority of available studies relied on weak levels of evidence and were retrospective. The authors concluded that the available data were not unanimous. A large European multicentric observational study questioned the significant association between CRC and IPMN, raising the question of whether IPMNs are a risk factor for the emergence of EPMs[53]. Analyzing data from a 5-year follow-up of 51 resected IPMN cases, Kato et al[54] determined that the EPM risk differed significantly in different types of IPMNs. The researchers established a 4-fold higher prevalence of ЕРM for malignant IPMN cases, while benign cases featured a similar risk to the general population. Another observational study focused on side branch IPMN did not show a strong correlation between IPMNs and EPM development (Table 1)[54].
| Ref. | Study design | Year | Patients | Prevalence of EPM, % | Prevalence of CRC, % |
| Zelnik et al[47] | Cross-sectional historical study | 2022 | 310 | NA | 5.2 |
| Panic et al[48] | Single-center study | 2018 | 198 | 31.8 | 6.1 |
| Larghi et al[50] | Multicentric study | 2013 | 198 | 23.6 | 12.4 |
| Lubezky et al[59] | Retrospective study | 2012 | 82 | 20.0 | 31.0 |
| Reid-Lombardo et al[9] | Retrospective study | 2010 | 471 | 52.0 | 4.0 |
| Yoon et al[13] | Retrospective study | 2010 | 210 | 33.8 | 7.0 |
| Riall et al[45] | Population-based study | 2007 | 992 | 10.0 | 3.0 |
| Huang et al[51] | Population-based study | 2019 | 2850 | 44.1 | 6.8 |
| Eguchi et al[49] | Retrospective study | 2006 | 69 | 28.0 | 12.0 |
Detection of EPM may be explained by repeated imaging for IPMN surveillance. This could lead to incidental malignancy detection. Common immunological, environmental, or hereditary factors are also potential explanations[47].
Patients with benign IPMN have a lower frequency of EPM compared with patients with malignant IPMN. Thus, the precise histological assessment of IPMN lesions could be a useful tool to assess the potential EPM incidence in IPMN patients and thus improve patient management[55].
The correlation between IPMN and CRC has already been confirmed for advanced IPMN cases. The relevant data suggest a significant contrast in EPM risk for different IPMN groups. It is still unclear what type of follow-up to identify EPMs is recommended for IPMN patients who have undergone resection and those who have not. Most of the authors find it reasonable to conduct GI malignancy screening, including colonoscopy for patients harboring IPMN[56,57].
In the past few decades, the increased number of imaging studies in IPMN surveillance may have contributed to the high rates of incidental malignancy detection, in particular CRC[7]. Regarding the low incidence of synchronous clinical presentation of IPMN and CRC, comprehensive surveillance protocols and follow-up duration or time of screening initiation have not been established. Of course, factors such as age, female sex, and White race are thought to be risk factors for these entities[7,45]. Additionally, the prognosis for patients with synchronous benign IPMN and EPM is notably better compared to those with malignant IPMN[12]. It is of great importance for potential subjects for surveillance to be detected on time. In situations when patients have been diagnosed with IPMN, detailed personal and family histories should be obtained. Patients diagnosed with IPMN without a colonoscopy are indicated for this procedure because of the increased risk for associated colonic neoplasms.
A special emphasis should be placed on patients with hereditary syndromes such as PJS, Lynch syndrome, and FAP for active surveillance based on the established data related to their higher rate of association with the development of colonic and extracolonic malignancy[58].
In this review, we highlighted the genetic alterations that occur in IPMN and CRC and discussed common genetic or epigenetic risk factors that could explain their synchronous manifestation. Of course, the process of malignant transformation in both entities is complex. The carcinogenic course of IPMNs and adenomatous colorectal polyps is similar. Both entities are characterized by malignant transformation from adenoma with low-grade dysplasia through adenoma with high-grade dysplasia to an invasive tumor.
Some distinctive features of IPMNs are linked with their genetic heterogeneity. Specific mutations in the GNAS and KRAS genes are primarily expressed in IPMN. It must be highlighted that mutations in GNAS are found in 40%-70% of IPMNs, and a significantly lower frequency of mutations is detected in other cancer-related genes, such as SMAD4, PI3KCA, PTEN, and BRAF[59].
On the other hand, the genetics of CRC is associated with stepwise progression accompanied by mutations in the APC, KRAS, PIK3CA, SMAD4, and TP53 genes. Unfortunately, the exact mechanism of the frequent development of synchronous IPMN and CRC is unknown. This process could be related to common environmental cancer-risk factors and genetic/epigenetic alterations that lead to common pathways of malignant transformation.
There is a significantly increased prevalence of CRC in patients with IPMN compared to the average population. Unfortunately, there are scarce data aimed at elucidating the molecular mechanisms leading to CRC development among patients with IPMN. More studies are needed to clarify the underlying pathophysiology and common genetic events shared between these two lesions.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Bulgaria
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Emran TB, Bangladesh; Fujimori S, Japan S-Editor: Li L L-Editor: Filipodia P-Editor: Yu HG
| 1. | Fonseca AL, Kirkwood K, Kim MP, Maitra A, Koay EJ. Intraductal Papillary Mucinous Neoplasms of the Pancreas: Current Understanding and Future Directions for Stratification of Malignancy Risk. Pancreas. 2018;47:272-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Obara T, Maguchi H, Saitoh Y, Itoh A, Arisato S, Ashida T, Nishino N, Ura H, Namiki M. Mucin-producing tumor of the pancreas: natural history and serial pancreatogram changes. Am J Gastroenterol. 1993;88:564-569. [PubMed] |
| 3. | Furukawa T, Hatori T, Fujita I, Yamamoto M, Kobayashi M, Ohike N, Morohoshi T, Egawa S, Unno M, Takao S, Osako M, Yonezawa S, Mino-Kenudson M, Lauwers GY, Yamaguchi H, Ban S, Shimizu M. Prognostic relevance of morphological types of intraductal papillary mucinous neoplasms of the pancreas. Gut. 2011;60:509-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 214] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 4. | Omori Y, Ono Y, Tanino M, Karasaki H, Yamaguchi H, Furukawa T, Enomoto K, Ueda J, Sumi A, Katayama J, Muraki M, Taniue K, Takahashi K, Ambo Y, Shinohara T, Nishihara H, Sasajima J, Maguchi H, Mizukami Y, Okumura T, Tanaka S. Pathways of Progression From Intraductal Papillary Mucinous Neoplasm to Pancreatic Ductal Adenocarcinoma Based on Molecular Features. Gastroenterology. 2019;156:647-661.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 5. | Schmidt CM, White PB, Waters JA, Yiannoutsos CT, Cummings OW, Baker M, Howard TJ, Zyromski NJ, Nakeeb A, DeWitt JM, Akisik FM, Sherman S, Pitt HA, Lillemoe KD. Intraductal papillary mucinous neoplasms: predictors of malignant and invasive pathology. Ann Surg. 2007;246:644-51; discussion 651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 295] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 6. | Sugiyama M, Atomi Y, Kuroda A. Two types of mucin-producing cystic tumors of the pancreas: diagnosis and treatment. Surgery. 1997;122:617-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 92] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Choi MG, Kim SW, Han SS, Jang JY, Park YH. High incidence of extrapancreatic neoplasms in patients with intraductal papillary mucinous neoplasms. Arch Surg. 2006;141:51-6; discussion 56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Benarroch-Gampel J, Riall TS. Extrapancreatic malignancies and intraductal papillary mucinous neoplasms of the pancreas. World J Gastrointest Surg. 2010;2:363-367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Reid-Lombardo KM, Mathis KL, Wood CM, Harmsen WS, Sarr MG. Frequency of extrapancreatic neoplasms in intraductal papillary mucinous neoplasm of the pancreas: implications for management. Ann Surg. 2010;251:64-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Farooqi AA, de la Roche M, Djamgoz MBA, Siddik ZH. Overview of the oncogenic signaling pathways in colorectal cancer: Mechanistic insights. Semin Cancer Biol. 2019;58:65-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 11. | Nissim S, Idos GE, Wu B. Genetic markers of malignant transformation in intraductal papillary mucinous neoplasm of the pancreas: a meta-analysis. Pancreas. 2012;41:1195-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Sugiyama M, Atomi Y. Extrapancreatic neoplasms occur with unusual frequency in patients with intraductal papillary mucinous tumors of the pancreas. Am J Gastroenterol. 1999;94:470-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 110] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Yoon WJ, Ryu JK, Lee JK, Woo SM, Lee SH, Park JK, Kim YT, Yoon YB. Extrapancreatic malignancies in patients with intraductal papillary mucinous neoplasm of the pancreas: prevalence, associated factors, and comparison with patients with other pancreatic cystic neoplasms. Ann Surg Oncol. 2008;15:3193-3198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Cancer Genome Atlas Research Network. Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2017;32:185-203.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1247] [Cited by in RCA: 1390] [Article Influence: 173.8] [Reference Citation Analysis (0)] |
| 15. | Yamaguchi K, Kanemitsu S, Hatori T, Maguchi H, Shimizu Y, Tada M, Nakagohri T, Hanada K, Osanai M, Noda Y, Nakaizumi A, Furukawa T, Ban S, Nobukawa B, Kato Y, Tanaka M. Pancreatic ductal adenocarcinoma derived from IPMN and pancreatic ductal adenocarcinoma concomitant with IPMN. Pancreas. 2011;40:571-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 192] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 16. | Ren R, Krishna SG, Chen W, Frankel WL, Shen R, Zhao W, Avenarius MR, Garee J, Caruthers S, Jones D. Activation of the RAS pathway through uncommon BRAF mutations in mucinous pancreatic cysts without KRAS mutation. Mod Pathol. 2021;34:438-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Tan MC, Basturk O, Brannon AR, Bhanot U, Scott SN, Bouvier N, LaFemina J, Jarnagin WR, Berger MF, Klimstra D, Allen PJ. GNAS and KRAS Mutations Define Separate Progression Pathways in Intraductal Papillary Mucinous Neoplasm-Associated Carcinoma. J Am Coll Surg. 2015;220:845-854.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 146] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 18. | Furukawa T, Kuboki Y, Tanji E, Yoshida S, Hatori T, Yamamoto M, Shibata N, Shimizu K, Kamatani N, Shiratori K. Whole-exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci Rep. 2011;1:161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 334] [Cited by in RCA: 338] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 19. | Nguyen HT, Duong HQ. The molecular characteristics of colorectal cancer: Implications for diagnosis and therapy. Oncol Lett. 2018;16:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 128] [Article Influence: 18.3] [Reference Citation Analysis (1)] |
| 20. | Bardhan K, Liu K. Epigenetics and colorectal cancer pathogenesis. Cancers (Basel). 2013;5:676-713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 186] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 21. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8087] [Cited by in RCA: 8007] [Article Influence: 228.8] [Reference Citation Analysis (1)] |
| 22. | Müller MF, Ibrahim AE, Arends MJ. Molecular pathological classification of colorectal cancer. Virchows Arch. 2016;469:125-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 267] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 23. | Kawaguchi Y, Kopetz S, Newhook TE, De Bellis M, Chun YS, Tzeng CD, Aloia TA, Vauthey JN. Mutation Status of RAS, TP53, and SMAD4 is Superior to Mutation Status of RAS Alone for Predicting Prognosis after Resection of Colorectal Liver Metastases. Clin Cancer Res. 2019;25:5843-5851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 134] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 24. | Achyut BR, Yang L. Transforming growth factor-β in the gastrointestinal and hepatic tumor microenvironment. Gastroenterology. 2011;141:1167-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 159] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 25. | Siskova A, Cervena K, Kral J, Hucl T, Vodicka P, Vymetalkova V. Colorectal Adenomas-Genetics and Searching for New Molecular Screening Biomarkers. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 26. | Pemberton TJ, Sandefur CI, Jakobsson M, Rosenberg NA. Sequence determinants of human microsatellite variability. BMC Genomics. 2009;10:612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Garrido-Ramos MA. Satellite DNA: An Evolving Topic. Genes (Basel). 2017;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 271] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 28. | De' Angelis GL, Bottarelli L, Azzoni C, De' Angelis N, Leandro G, Di Mario F, Gaiani F, Negri F. Microsatellite instability in colorectal cancer. Acta Biomed. 2018;89:97-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 105] [Reference Citation Analysis (0)] |
| 29. | Peruhova M, Peshevska-Sekulovska M, Krastev B, Panayotova G, Georgieva V, Konakchieva R, Nikolaev G, Velikova TV. What could microRNA expression tell us more about colorectal serrated pathway carcinogenesis? World J Gastroenterol. 2020;26:6556-6571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Handra-Luca A, Couvelard A, Degott C, Fléjou JF. Correlation between patterns of DNA mismatch repair hmlh1 and hmsh2 protein expression and progression of dysplasia in intraductal papillary mucinous neoplasms of the pancreas. Virchows Arch. 2004;444:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Lynch HT, Lynch PM, Lanspa SJ, Snyder CL, Lynch JF, Boland CR. Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet. 2009;76:1-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 634] [Cited by in RCA: 588] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 32. | Liu B, Parsons R, Papadopoulos N, Nicolaides NC, Lynch HT, Watson P, Jass JR, Dunlop M, Wyllie A, Peltomäki P, de la Chapelle A, Hamilton SR, Vogelstein B, Kinzler KW. Analysis of mismatch repair genes in hereditary non-polyposis colorectal cancer patients. Nat Med. 1996;2:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 599] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 33. | Stoffel EM. Lynch Syndrome/Hereditary Non-polyposis Colorectal Cancer (HNPCC). Minerva Gastroenterol Dietol. 2010;56:45-53. [PubMed] |
| 34. | Sparr JA, Bandipalliam P, Redston MS, Syngal S. Intraductal papillary mucinous neoplasm of the pancreas with loss of mismatch repair in a patient with Lynch syndrome. Am J Surg Pathol. 2009;33:309-312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Flanagan MR, Jayaraj A, Xiong W, Yeh MM, Raskind WH, Pillarisetty VG. Pancreatic intraductal papillary mucinous neoplasm in a patient with Lynch syndrome. World J Gastroenterol. 2015;21:2820-2825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Hizawa K, Iida M, Matsumoto T, Kohrogi N, Kinoshita H, Yao T, Fujishima M. Cancer in Peutz-Jeghers syndrome. Cancer. 1993;72:2777-2781. [PubMed] [DOI] [Full Text] |
| 37. | Karuman P, Gozani O, Odze RD, Zhou XC, Zhu H, Shaw R, Brien TP, Bozzuto CD, Ooi D, Cantley LC, Yuan J. The Peutz-Jegher gene product LKB1 is a mediator of p53-dependent cell death. Mol Cell. 2001;7:1307-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 236] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 38. | Wei C, Amos CI, Stephens LC, Campos I, Deng JM, Behringer RR, Rashid A, Frazier ML. Mutation of Lkb1 and p53 genes exert a cooperative effect on tumorigenesis. Cancer Res. 2005;65:11297-11303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 39. | Sato N, Rosty C, Jansen M, Fukushima N, Ueki T, Yeo CJ, Cameron JL, Iacobuzio-Donahue CA, Hruban RH, Goggins M. STK11/LKB1 Peutz-Jeghers gene inactivation in intraductal papillary-mucinous neoplasms of the pancreas. Am J Pathol. 2001;159:2017-2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 208] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 40. | Resta N, Pierannunzio D, Lenato GM, Stella A, Capocaccia R, Bagnulo R, Lastella P, Susca FC, Bozzao C, Loconte DC, Sabbà C, Urso E, Sala P, Fornasarig M, Grammatico P, Piepoli A, Host C, Turchetti D, Viel A, Memo L, Giunti L, Stigliano V, Varesco L, Bertario L, Genuardi M, Lucci Cordisco E, Tibiletti MG, Di Gregorio C, Andriulli A, Ponz de Leon M; AIFEG. Cancer risk associated with STK11/LKB1 germline mutations in Peutz-Jeghers syndrome patients: results of an Italian multicenter study. Dig Liver Dis. 2013;45:606-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 41. | Giardiello FM, Offerhaus GJ, Lee DH, Krush AJ, Tersmette AC, Booker SV, Kelley NC, Hamilton SR. Increased risk of thyroid and pancreatic carcinoma in familial adenomatous polyposis. Gut. 1993;34:1394-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 247] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 42. | Kanth P, Grimmett J, Champine M, Burt R, Samadder NJ. Hereditary Colorectal Polyposis and Cancer Syndromes: A Primer on Diagnosis and Management. Am J Gastroenterol. 2017;112:1509-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 43. | Maire F, Hammel P, Terris B, Olschwang S, O'Toole D, Sauvanet A, Palazzo L, Ponsot P, Laplane B, Lévy P, Ruszniewski P. Intraductal papillary and mucinous pancreatic tumour: a new extracolonic tumour in familial adenomatous polyposis. Gut. 2002;51:446-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Chetty R, Salahshor S, Bapat B, Berk T, Croitoru M, Gallinger S. Intraductal papillary mucinous neoplasm of the pancreas in a patient with attenuated familial adenomatous polyposis. J Clin Pathol. 2005;58:97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | Riall TS, Stager VM, Nealon WH, Townsend CM Jr, Kuo YF, Goodwin JS, Freeman JL. Incidence of additional primary cancers in patients with invasive intraductal papillary mucinous neoplasms and sporadic pancreatic adenocarcinomas. J Am Coll Surg 2007; 204: 803-13; discussion 813-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 46. | Kumar R, Fraser RE, Garcea G. A meta-analysis: incidental intraductal papillary mucinous neoplasm and extra-pancreatic malignancy. Langenbecks Arch Surg. 2022;407:451-458. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 47. | Zelnik Yovel D, Bear L, Scapa E, Shnell M, Bar Yishay I, Bar N, ZIv Baran T, Younis F, Phillips A, Lubezky N, Shibolet O, Ben-Ami Shor D. Increased prevalence of colorectal neoplasia in patients with intraductal papillary mucinous neoplasms. Therap Adv Gastroenterol. 2022;15:17562848221104306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 48. | Panic N, Macchini F, Solito S, Boccia S, Leoncini E, Larghi A, Berretti D, Pevere S, Vadala S, Marino M, Zilli M, Bulajic M. Prevalence of Extrapancreatic Malignancies Among Patients With Intraductal Papillary Mucinous Neoplasms of the Pancreas. Pancreas. 2018;47:721-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 49. | Eguchi H, Ishikawa O, Ohigashi H, Tomimaru Y, Sasaki Y, Yamada T, Tsukuma H, Nakaizumi A, Imaoka S. Patients with pancreatic intraductal papillary mucinous neoplasms are at high risk of colorectal cancer development. Surgery. 2006;139:749-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 50. | Larghi A, Panic N, Capurso G, Leoncini E, Arzani D, Salvia R, Del Chiaro M, Frulloni L, Arcidiacono PG, Zerbi A, Manta R, Fabbri C, Ventrucci M, Tarantino I, Piciucchi M, Carnuccio A, Boggi U, Costamagna G, Delle Fave G, Pezzilli R, Bassi C, Bulajic M, Ricciardi W, Boccia S. Prevalence and risk factors of extrapancreatic malignancies in a large cohort of patients with intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann Oncol. 2013;24:1907-1911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 51. | Huang X, Zhang B, Zhao J, Sun C, Kong K, Deng L, Liu Y, Zheng J. Increased Risk of Second Primary Cancers Following Diagnosis of Malignant Intraductal Papillary Mucinous Neoplasms of the Pancreas: A Population-Based Study. Front Oncol. 2019;9:610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Pugliese L, Keskin M, Maisonneuve P, D'Haese JG, Marchegiani G, Wenzel P, Del Chiaro M, Ceyhan GO. Increased incidence of extrapancreatic neoplasms in patients with IPMN: Fact or fiction? Pancreatology. 2015;15:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 53. | Marchegiani G, Malleo G, D'Haese JG, Wenzel P, Keskin M, Pugliese L, Borin A, Benning V, Nilsson L, Oruc N, Segersvard R, Friess H, Schmid R, Löhr M, Maisonneuve P, Bassi C, Ceyhan GO, Salvia R, Del Chiaro M. Association between pancreatic intraductal papillary mucinous neoplasms and extrapancreatic malignancies. Clin Gastroenterol Hepatol. 2015;13:1162-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 54. | Kato T, Alonso S, Noda H, Miyakura Y, Tsujinaka S, Saito M, Muto Y, Fukui T, Ichida K, Takayama Y, Watanabe F, Kakizawa N, Perucho M, Rikiyama T. Malignant, but not benign, intraductal papillary mucinous neoplasm preferentially associates with prior extrapancreatic malignancies. Oncol Rep. 2016;35:3236-3240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 55. | Malleo G, Marchegiani G, Borin A, Capelli P, Accordini F, Butturini G, Pederzoli P, Bassi C, Salvia R. Observational study of the incidence of pancreatic and extrapancreatic malignancies during surveillance of patients with branch-duct intraductal papillary mucinous neoplasm. Ann Surg. 2015;261:984-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 56. | Baiocchi GL, Molfino S, Frittoli B, Pigozzi G, Gheza F, Gaverini G, Tarasconi A, Ricci C, Bertagna F, Grazioli L, Tiberio GA, Portolani N. Increased risk of second malignancy in pancreatic intraductal papillary mucinous tumors: Review of the literature. World J Gastroenterol. 2015;21:7313-7319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 57. | Ohmoto A, Yachida S, Morizane C. Genomic Features and Clinical Management of Patients with Hereditary Pancreatic Cancer Syndromes and Familial Pancreatic Cancer. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 58. | Schönleben F, Qiu W, Ciau NT, Ho DJ, Li X, Allendorf JD, Remotti HE, Su GH. PIK3CA mutations in intraductal papillary mucinous neoplasm/carcinoma of the pancreas. Clin Cancer Res. 2006;12:3851-3855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 135] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 59. | Lubezky N, Ben-Haim M, Lahat G, Marmor S, Solar I, Brazowski E, Nackache R, Klausner JM. Intraductal papillary mucinous neoplasm of the pancreas: associated cancers, family history, genetic predisposition? Surgery. 2012;151:70-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |