Published online May 16, 2023. doi: 10.12998/wjcc.v11.i14.3295
Peer-review started: January 7, 2023
First decision: March 24, 2023
Revised: April 2, 2023
Accepted: April 12, 2023
Article in press: April 12, 2023
Published online: May 16, 2023
Processing time: 128 Days and 21.4 Hours
Pulmonary mucormycosis is a rare but life-threatening invasive fungal infection that mostly affects immunocompromised patients. This disease usually develops acutely and progresses rapidly, often leading to a poor clinical prognosis. Chronic pulmonary mucormycosis is highly unusual in immunocompetent patients.
A 43-year-old man, who was a house improvement worker with a long history of occupational dust exposure, presented with an irritating cough that had lasted for two months. The patient was previously in good health, without dysglycemia or any known immunodeficiencies. Chest computed tomography revealed a mass in the left lower lobe, measuring approximately 6 cm in diameter, which was suspected to be primary lung carcinoma complicated with obstructive pneu
This article reports a rare case of chronic pulmonary mucormycosis caused by Rhizopus microsporus in a middle-aged male without dysglycemia or immunodeficiency. The patient's surgical outcome was excellent, reaffirming that surgery remains the cornerstone of pulmonary mucormycosis treatment.
Core Tip: Pulmonary mucormycosis is a rare yet life-threatening invasive fungal infection that typically affects immunocompromised patients. In this report, we present a case of chronic pulmonary mucormycosis in a 43-year-old immunocompetent house improvement worker. The patient initially presented with an irritating cough and a solitary mass in the left lower lobe, which raised concerns of primary lung carcinoma. A successful thoracoscopic-assisted left lower lobectomy was performed, and subsequent metagenomic next-generation sequencing analysis and specialized pathological staining of surgical specimens suggested Rhizopus microsporus as the causative agent of the infection. During a six-month postoperative follow-up, no signs of recurrence were observed.
- Citation: Guo XZ, Gong LH, Wang WX, Yang DS, Zhang BH, Zhou ZT, Yu XH. Chronic pulmonary mucormycosis caused by rhizopus microsporus mimics lung carcinoma in an immunocompetent adult: A case report. World J Clin Cases 2023; 11(14): 3295-3303
- URL: https://www.wjgnet.com/2307-8960/full/v11/i14/3295.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i14.3295
Pulmonary mucormycosis is a rare but aggressive and life-threatening fungal infection that mainly affects immunocompromised individuals[1]. The most common causative agents are Rhizopus species, particularly Rhizopus microsporus, which belong to the class Zygomycetes[2]. The infection typically occurs in patients with underlying conditions such as diabetes mellitus, hematologic malignancy, and solid organ or stem cell transplant[3]. Pulmonary mucormycosis is an aggressive and rapidly progressive infection that carries a poor clinical prognosis, with a mortality rate as high as 50%-60% worldwide[4,5]. Chronic pulmonary mucormycosis, lasting more than one month, is extremely rare, with most reported cases occurring in patients with diabetes[6-10]. In this case report, we present a rare case of chronic pulmonary mucormycosis caused by Rhizopus microsporus, which mimicked lung carcinoma in an immunocompetent middle-aged male. The patient underwent aggressive surgical resection, and achieved an excellent therapeutic response.
A 43-year-old man from China presented at the hospital with a history of coughing and sputum production for the past two months.
Two months prior to admission to our hospital, the patient visited a local hospital due to an irritative cough with a small amount of white, foamy sputum that lasted for two wk. The patient did not exhibit any obvious indications of accompanying symptoms such as blood in the sputum, fever, night sweats, weight loss, hoarseness, dyspnea, or discomforts. A chest computed tomography (CT) scan revealed a soft tissue mass, approximately 57 mm x 51 mm in size, in the left lower lobe that was suspected to be a fungal infection or tumor. Electronic bronchoscopy showed that the basal branch mucosa of the left lower lobe was swollen and hypertrophic, and the surface was not smooth. No active bleeding or new organisms were found. Bronchial mucosal biopsy revealed chronic inflammatory changes. Acid-fast stains of bronchoalveolar lavage fluid, as well as bacterial and fungal cultures, were negative. Since the specific cause of the symptoms could not be clarified, the patient was treated with empirical anti-infection medication, specifically cephalosporins, in the local hospital for two wk. However, the patient's cough symptoms did not improve significantly. As a result, the patient was admitted to our thoracic medicine department as an outpatient for further diagnosis and treatment.
The patient did not report any prior history of surgeries, trauma, severe infections, or significant medical conditions. Additionally, the patient denied any previous infection with coronavirus disease 2019.
The patient reported no significant family history of related illnesses. However, it was observed that the patient had been working as a house improvement worker and had a prolonged history of occupational dust exposure.
On physical examination, the patient's height was 176 cm, weight 70 kg, body temperature 36.2 °C, heart rate 94 beats per minute, respiratory rate 20 breaths per minute, and blood pressure 132/87 mmHg. The superficial lymph nodes in the supraclavicular and neck regions were non-palpable, and the chest wall was symmetrical with no deformities. The patient's breathing was regular, and apart from decreased breath sounds in the lower left lung, no other significant abnormalities were noted.
The laboratory test results were as follows: White blood cell count was 9.54 × 109/L, neutrophil percentage was 52%, hemoglobin was 151 g/L, hematocrit was 47.1%, fasting blood glucose was 5.5 mmol/L, total protein level was 81 g/L, and globulin level was 37 g/L. Additionally, twelve tumor markers including AFP, CEA, NSE, CA125, CA15-3, CA242, CA19-9, PSA, f-PSA, FER, β-HCG, and HGH were measured by protein biochip detection in the serum and found to be within the normal range. Pulmonary function test results showed forced vital capacity of 5.18 L (110% predicted) and forced expiratory volume in 1 s of 3.79 L (98% predicted). Other results of routine laboratory examinations were within normal limits, including urine and stool routine, liver and kidney function, and electrolytes. The patient's infectious disease screening, which included hepatitis B, hepatitis C, syphilis, and human immunodeficiencyvirus tests, also showed no abnormalities.
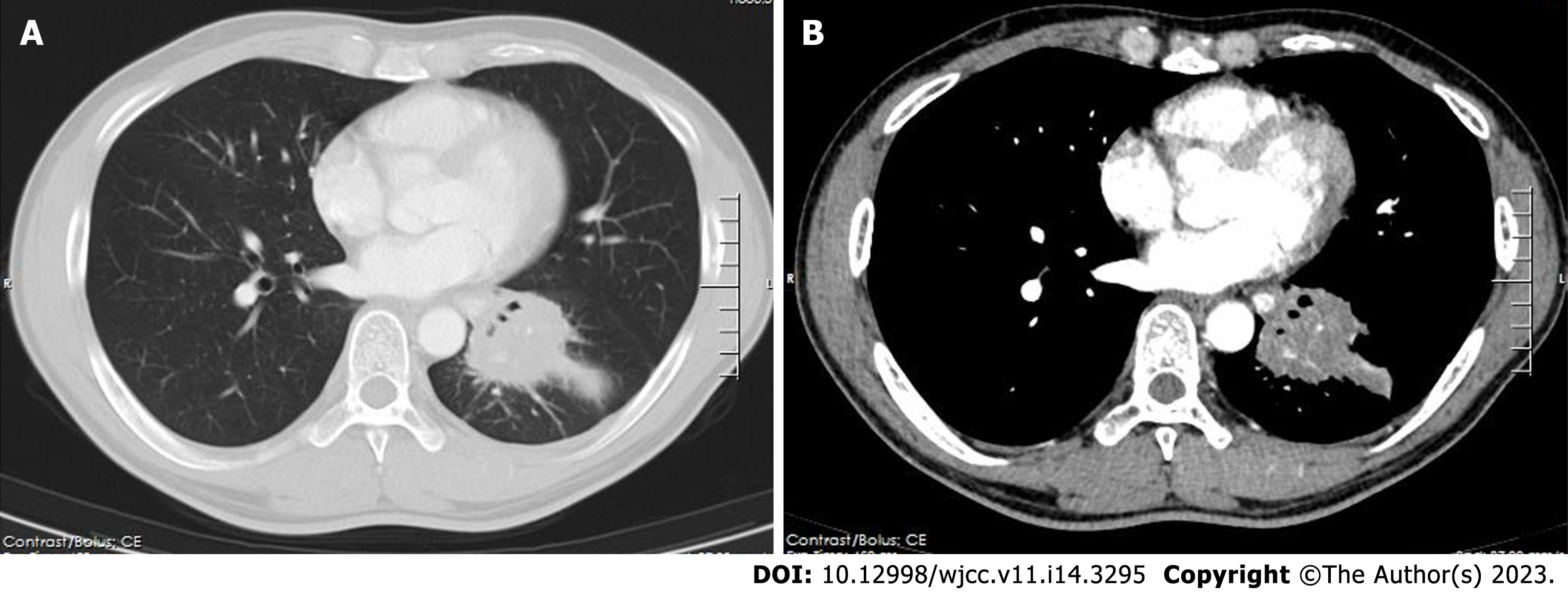
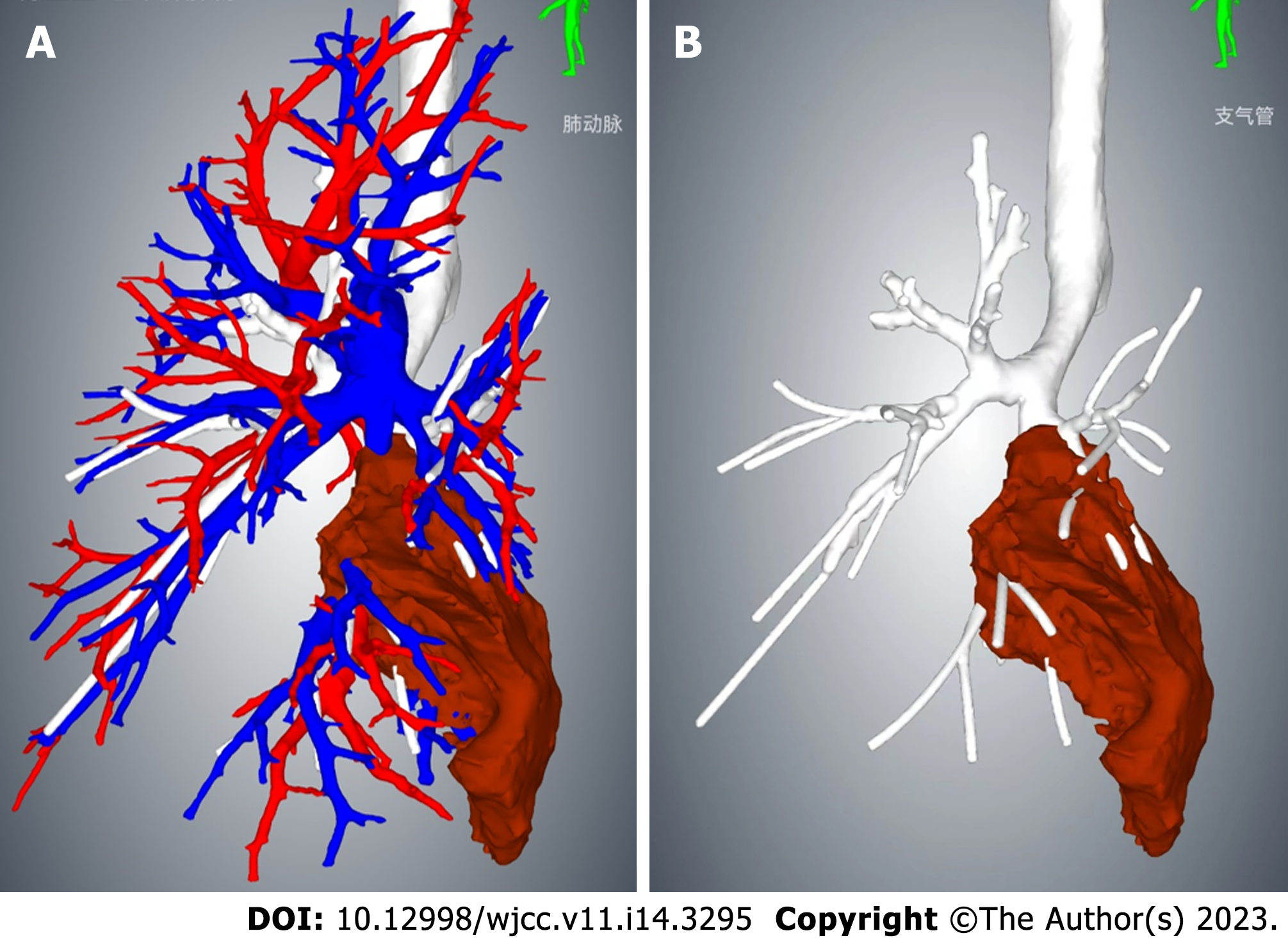
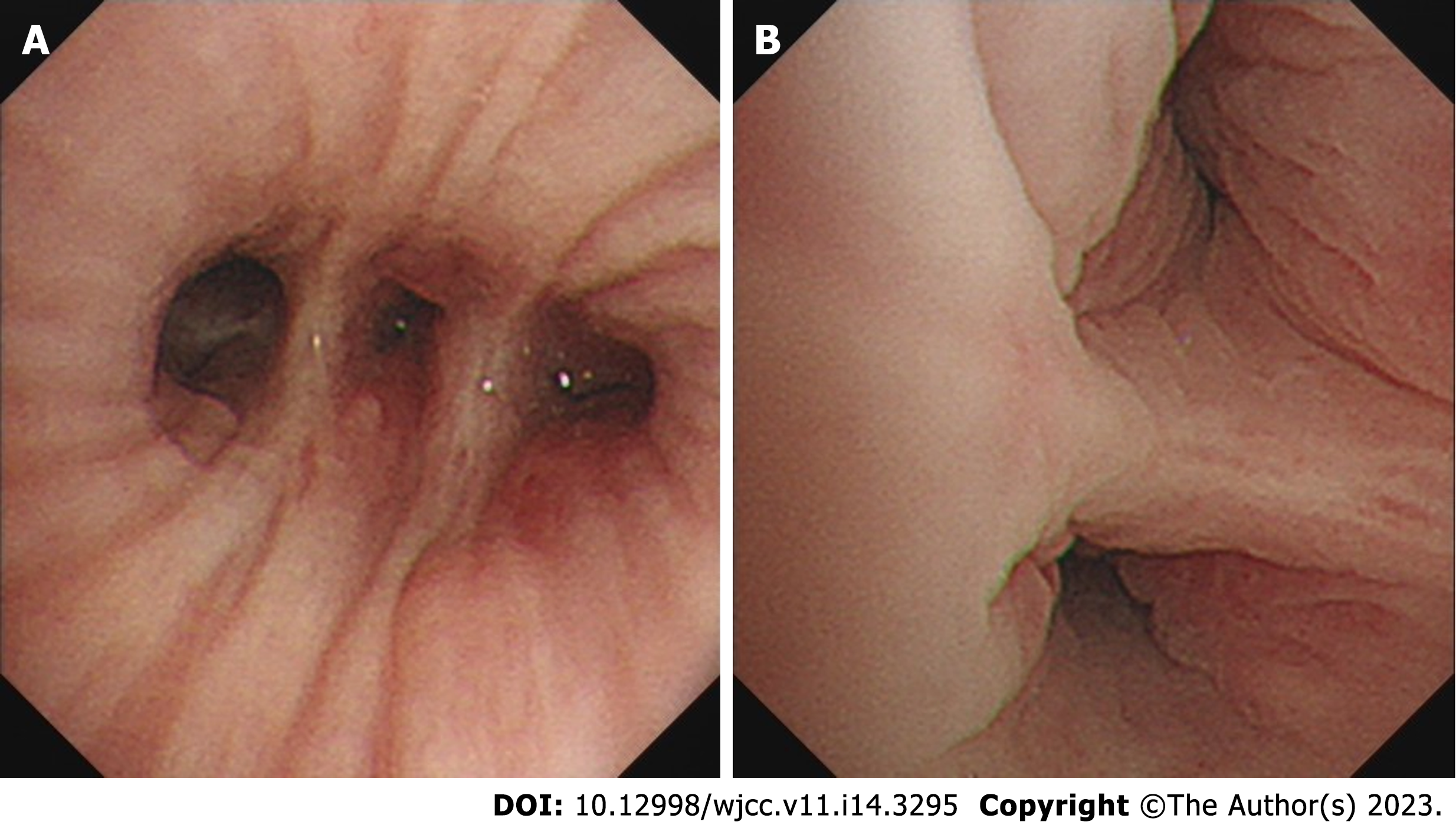
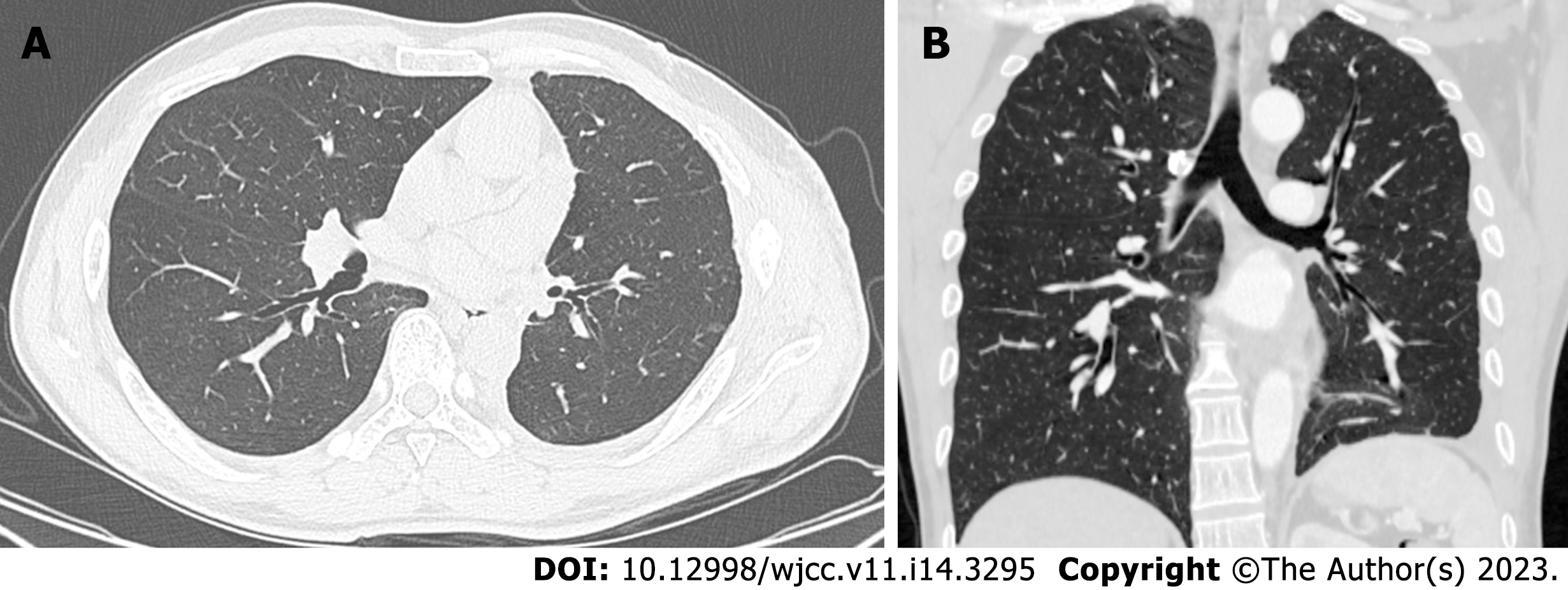
The contrast-enhanced chest CT taken at our hospital revealed a 6 cm mass in the left lower lobe, primarily located in the lateral segment (S9) and posterior segment (S10). The mass showed slight enhancement and obstructed the bronchi of the affected lung segments, with the pulmonary vasculature faintly visible within it (Figures 1 and 2). Bronchoscopy revealed narrowed lumens of the left lower lobe's lateral and posterior segmental bronchi, along with congested and swollen mucosa, without any detection of new organisms in the lumen (Figure 3). Magnetic resonance imaging of the head and CT scans of the neck and abdomen showed no abnormalities. The bone scan also had negative results.
The patient had undergone bronchoscopy twice at different hospitals, but both procedures failed to confirm the pathological diagnosis by forceps biopsy. The patient declined further biopsies, including percutaneous lung biopsy or endobronchial ultrasound-guided transbronchial needle aspiration (ebus tbna). However, the patient expressed a strong desire for surgery and was willing to accept the risks associated with surgical treatment.
Thoracic medical oncologists organized a multidisciplinary consultation. The experts unanimously recommended respecting the patient's wishes and scheduling a surgical operation to remove the left lower lung within a specific time frame. The mass tissue obtained during the operation would then be used to make a diagnosis and guide subsequent treatment steps. Following the experts’ advice, the patient was transferred to Department of Thoracic Surgery for surgical treatment.
Based on the patient's two-month medical history and imaging signs, primary bronchial lung cancer combined with obstructive pneumonia was suspected, and specific infectious lesions could not be entirely ruled out. No sign of distant metastasis of a malignant tumor was detected during the patient's systemic examination. If the mass in the patient's left lower lobe was malignant, the current clinical stage would be cT3N1M0, stage IIIA.
Due to the large size of the mass, we performed a thoracoscopic-assisted left lower lobectomy through an anterolateral incision approximately 10 cm long in the fourth intercostal space. The thoracoscopic hole was located in the seventh intercostal space on the posterior axillary line. Intraoperative exploration revealed that the visceral and parietal pleura were smooth without nodules, the oblique fissure was well developed, and the mass in the left lower lobe was hard, with the basal segment densely adhered to the lateral chest wall. After separating the adherent tissues, the pulmonary artery, vein, and bronchi belonging to the left lower lobe were exposed sequentially and then closed and separated using the endoscopic linear cutting stapler. During this surgical procedure, we found that the lymph nodes in the lung mass's drainage area were significantly enlarged and hardened, but their adventitia remained intact. Interestingly, these lymph nodes appeared purulent white instead of the conventional carbon black.
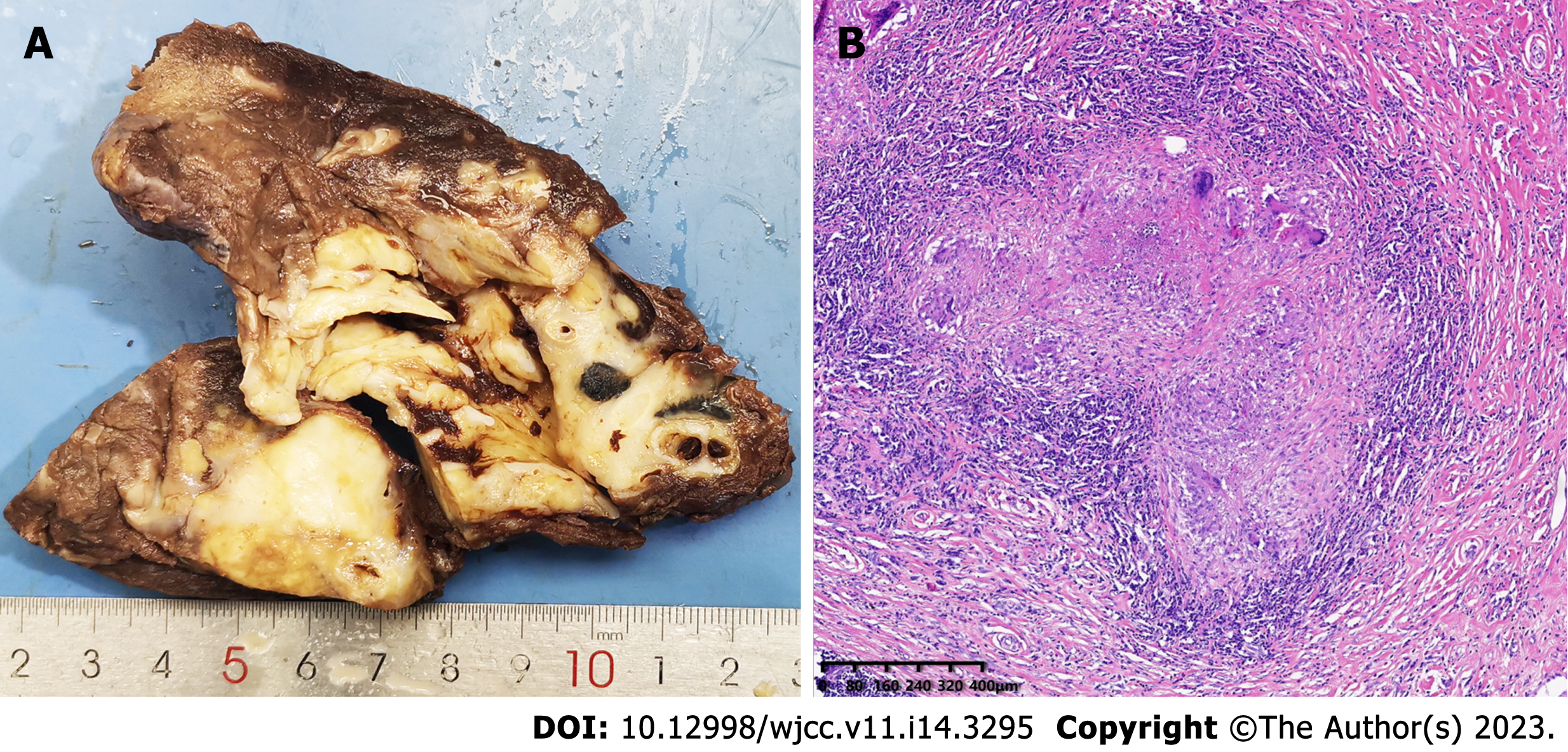
The resected left lower lobe was removed from the incision. The mass was firm, approximately 6 cm in diameter, and pale in color when sectioned. Subsequent intraoperative frozen rapid pathological examination results indicated that the mass was a granulomatous inflammatory lesion with no malignant components. It was suspected that this may have been a particular type of infection which needed to be further confirmed by routine pathological examination and special staining. To determine the cause of the granuloma, we excised the clean granuloma tissue approximately 0.5 cm3 in size and sent it for clinical metagenomic next-generation sequencing (mNGS). The 3-h operation went smoothly, and the blood loss was 100 mL. There were no postoperative complications. The drainage tube was removed on the fifth day after the operation, and the patient was discharged one day later.
The results of mNGS analysis for the left lower lobe granuloma were obtained on the second day after the operation. The analysis suggested an infection caused by Rhizopus microsporus, and no other pathogenic microorganisms, including bacteria, viruses, parasites, mycobacteria, mycoplasma, or chlamydia, were detected. The analysis was performed by Precision Genes Technology, Inc. on August 25, 2022.
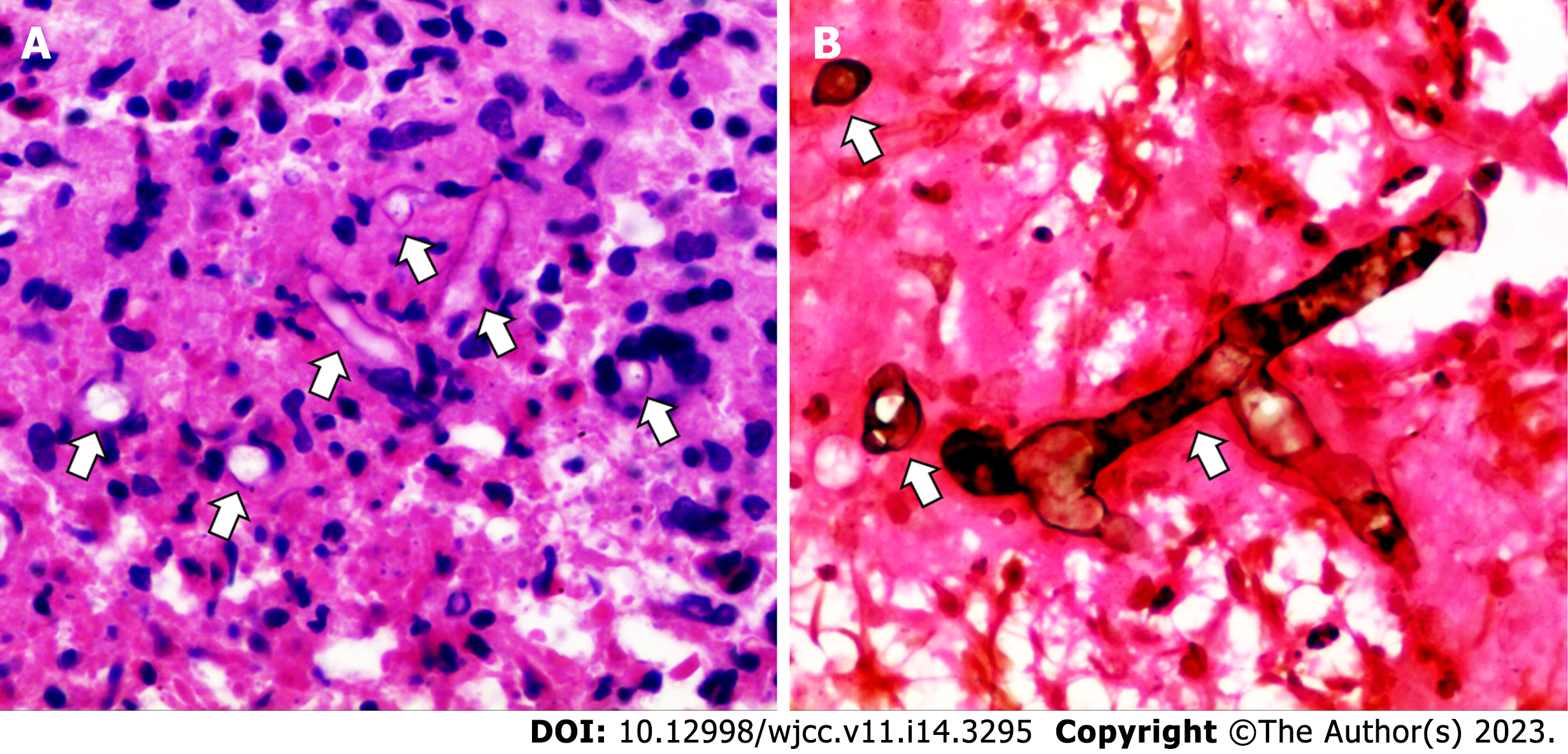
The postoperative routine pathological examination revealed that the left lower lobe mass was an inflammatory granuloma (Figure 4). Interstitial fibrous tissue hyperplasia, inflammatory cell infiltration, and multinucleated giant cell reaction were all observed under the microscope. In addition, hexamine silver staining of its histological sections revealed broad, right-angled branched aseptate hyphae, strongly suggesting the possibility of pulmonary mucormycosis (Figure 5).
Considering the patient's clinical manifestations, as well as the laboratory, imaging, mNGS, and histopathological examinations, the patient was finally diagnosed with chronic pulmonary mucormycosis caused by Rhizopus microsporus infection, which is very rare in immunocompetent nondiabetic patients.
After surgery, the patient's respiratory symptoms were relieved, and a CT re-examination three and six months after the operation showed that the lungs were in good condition, with no signs of recurrence (Figure 6).
Pulmonary mucormycosis represents a group of invasive fungal infections in the lungs caused by members of the order Mucorales, and it has a high mortality rate[1,5]. The causative fungal agent is commonly found on decaying food, soil, and animal excrement. During its asexual reproduction, its hyphae develop sporangium and release spores. Patients often become infected by inhaling these spores into the bronchioles and alveoli[1,11]. Pulmonary mucormycosis is a relatively uncommon opportunistic infection that primarily occurs in immunocompromised populations, with risk factors including diabetes mellitus, hematologic malignancy, neutropenia, or transplantation[12,13]. Very rarely, pulmonary mucormycosis also occurs in immunocompetent individuals and should not be entirely ignored[5,11,14,15]. In our report, we present a case of a 43-year-old Chinese man who developed pulmonary mucormycosis without dysglycemia or any known immunodeficiency. We learned from the consultation that the patient did not wear a mask at work to reduce dust inhalation. Therefore, as a house improvement worker, he may have inhaled mucormycosis spores during his occupational work, which may have induced this pulmonary mucormycosis infection.
The clinical and imaging manifestations of pulmonary mucormycosis are not specific[16]. Most patients present with an acute respiratory infection and have typical symptoms such as fever, cough, chest pain, dyspnea, and hemoptysis. Additionally, a few patients suffer a longer course of the disease and manifest as chronic lung lesions, which need to be differentiated from lung carcinoma[17]. In our case, the patient had a persistent left lower lung mass and mild clinical symptoms characterized by an irritating cough for at least two months. These chronic symptoms led us to consider lung cancer initially, but it was eventually ruled out by thoracoscopic excisional biopsy.
The definitive diagnosis of pulmonary mucormycosis relies on the histopathological finding of mucoraceous hyphae in affected tissues. Its pathological characteristics include broad, aseptate, and ribbon-like hyaline hyphae with wide-angle branching[18]. Although specimen fungal culture allows for species identification, it is time-consuming and has a low positive rate[5,19]. In recent years, polymerase chain reaction and mNGS, especially the latter, have become relatively precise and convenient methods for finding pathogens[3,20,21]. In our case, we first identified the only suspected pathogenic microorganism, Rhizopus microsporus, by mNGS detection of sterile surgical specimens, although its sequence number was only two. Then, the pathologist identified mucoraceous hyphae by special stains. Interestingly, corresponding to the low sequence number of mNGS results, the hyphae in the specimen were sparse, which may also be associated with chronic infection and a good prognosis.
Due to the high mortality rate associated with pulmonary mucormycosis, early identification and treatment of the disease are critical for an improved likelihood of survival. Surgical resection is the cornerstone treatment for pulmonary mucormycosis[22], and it should be strongly considered when feasible[23]. In addition, timely antifungal therapy with amphotericin B or posaconazole has also been shown to improve outcomes[1,24]. Despite our repeated insistence on the necessity of antifungal therapy, the patient refused any further antifungal treatment postoperatively. The patient cited mild preoperative infection symptoms, satisfactory postoperative recovery, concerns about potential drug side effects, and high follow-up treatment costs as reasons for declining the treatment. Nevertheless, follow-up CT scans of the patient's chest at three and six months after surgery showed satisfactory results without any signs of recurrence.
This article reports a rare case of chronic pulmonary mucormycosis caused by Rhizopus microsporus in a middle-aged male without dysglycemia or immunodeficiency. The patient presented with an irritating cough and a solitary left lower lobe mass that lasted for two months, similar to that of lung carcinoma. We performed a lobectomy, and the surgical specimen was subjected to mNGS detection and special pathological staining, which suggested rhizopus microsporus infection. The surgical outcome for the patient was excellent, reaffirming that surgery remains the cornerstone of treatment for pulmonary mucormycosis.
We would like to thank the patient for his participation and cooperation in this case report.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nasa P, United Arab Emirates; Rotondo JC, Italy S-Editor: Liu XF L-Editor: A P-Editor: Cai YX
| 1. | Agrawal R, Yeldandi A, Savas H, Parekh ND, Lombardi PJ, Hart EM. Pulmonary Mucormycosis: Risk Factors, Radiologic Findings, and Pathologic Correlation. Radiographics. 2020;40:656-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 2. | Jeong W, Keighley C, Wolfe R, Lee WL, Slavin MA, Kong DCM, Chen SC. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect. 2019;25:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 548] [Article Influence: 91.3] [Reference Citation Analysis (0)] |
| 3. | Chen L, Su Y, Xiong XZ. Rhizopus microsporus lung infection in an immunocompetent patient successfully treated with amphotericin B: A case report. World J Clin Cases. 2021;9:11108-11114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 4. | Muthu V, Agarwal R, Dhooria S, Sehgal IS, Prasad KT, Aggarwal AN, Chakrabarti A. Has the mortality from pulmonary mucormycosis changed over time? Clin Microbiol Infect. 2021;27:538-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 5. | Lee FY, Mossad SB, Adal KA. Pulmonary mucormycosis: the last 30 years. Arch Intern Med. 1999;159:1301-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 238] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 6. | Tojima H, Tokudome T, Otsuka T. [Chronic pulmonary mucormycosis that developed in preexisting cavities caused by tuberculosis in a patient with diabetes mellitus and liver cirrhosis]. Nihon Kyobu Shikkan Gakkai Zasshi. 1997;35:100-105. [PubMed] |
| 7. | Soltani A, Torabizadeh Z, Akha O, Godazandeh G, Mohammad G. A case of chronic pulmonary mucormycosis with indolent course complicating diabetic ketoacidosis. Respiratory Medicine Extra. 2006;2:105-107. [DOI] [Full Text] |
| 8. | Matsumura Y, Shibuya J, Kobayashi S, Handa M, Kondo T, Fujimura S. [Chronic pulmonary mucormycosis diagnosed by bronchoscopy: a case report]. Kyobu Geka. 1993;46:891-894. [PubMed] |
| 9. | Iqbal N, Irfan M, Jabeen K, Kazmi MM, Tariq MU. Chronic pulmonary mucormycosis: an emerging fungal infection in diabetes mellitus. J Thorac Dis. 2017;9:E121-E125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Agarwal R, Kumar V, Gupta D. Pulmonary mucormycosis: two of a kind. Eur J Intern Med. 2006;17:63-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Yang J, Zhang J, Feng Y, Peng F, Fu F. A case of pulmonary mucormycosis presented as Pancoast syndrome and bone destruction in an immunocompetent adult mimicking lung carcinoma. J Mycol Med. 2019;29:80-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH, Kontoyiannis DP, Walsh TJ. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1793] [Cited by in RCA: 1964] [Article Influence: 98.2] [Reference Citation Analysis (1)] |
| 13. | Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54 Suppl 1:S23-S34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 863] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 14. | Ng KL, Huan NC, Nasaruddin MZ, Muhammad NA, Daut UN, Abdul Rahaman JA. Pulmonary mucormycosis masquerading as endobronchial tumour in an immunocompetent pregnant young lady. Respirol Case Rep. 2021;9:e00704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | He J, Sheng G, Yue H, Zhang F, Zhang HL. Isolated pulmonary mucormycosis in an immunocompetent patient: a case report and systematic review of the literature. BMC Pulm Med. 2021;21:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Tsyrkunou AV, Ellison RT 3rd, Akalin A, Wiederhold N, Sutton DA, Lindner J, Fan H, Levitz SM, Zivna I. Multifocal Rhizopus microsporus lung infection following brush clearing. Med Mycol Case Rep. 2014;6:14-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Peng M, Meng H, Sun Y, Xiao Y, Zhang H, Lv K, Cai B. Clinical features of pulmonary mucormycosis in patients with different immune status. J Thorac Dis. 2019;11:5042-5052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Kantarcioğlu AS, Yücel A, Nagao K, Sato T, Inci E, Ogreden S, Kaytaz A, Alan S, Bozdağ Z, Edali N, Sar M, Kepil N, Oz B, Altas K. A Rhizopus oryzae strain isolated from resected bone and soft tissue specimens from a sinonasal and palatal mucormycosis case. Report of a case and in vitro experiments of yeastlike cell development. Med Mycol. 2006;44:515-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Leung CCD, Chan YH, Ho MY, Chan MC, Chen CH, Kwok CT, Yeung YC. First reported case of late recurrence of pulmonary mucormycosis in a renal transplant recipient with poorly controlled diabetes mellitus. Respirol Case Rep. 2021;9:e0877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Sun Y, Li H, Chen J, Ma Z, Han P, Liu Y, Wen J, Ren F, Ma X. Case Report: Metagenomics Next-Generation Sequencing Can Be Performed for the Diagnosis of Disseminated Mucormycosis. Front Med (Lausanne). 2021;8:675030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Gu Z, Buelow DR, Petraitiene R, Petraitis V, Walsh TJ, Hayden RT. Quantitative multiplexed detection of common pulmonary fungal pathogens by labeled primer polymerase chain reaction. Arch Pathol Lab Med. 2014;138:1474-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Choi H, Lee H, Jeon K, Suh GY, Shin S, Kim HK, Kim K, Jeong D, Kim H. Factors affecting surgical resection and treatment outcomes in patients with pulmonary mucormycosis. J Thorac Dis. 2019;11:892-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Multani A, Reveron-Thornton R, Garvert DW, Gomez CA, Montoya JG, Lui NS. Cut it out! Mycoses. 2019;62:893-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Yuan F, Chen J, Liu F, Dang YC, Kong QT, Sang H. Successful treatment of pulmonary mucormycosis caused by Rhizopus microsporus with posaconazole. Eur J Med Res. 2021;26:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |