Published online May 16, 2023. doi: 10.12998/wjcc.v11.i14.3275
Peer-review started: January 3, 2023
First decision: January 30, 2023
Revised: February 27, 2023
Accepted: April 7, 2023
Article in press: April 7, 2023
Published online: May 16, 2023
Processing time: 133 Days and 7.9 Hours
Here, we present a unique case of mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes (MELAS) syndrome, which initially appeared to be autoimmune encephalitis and was ultimately confirmed as MELAS with the mitochondrial DNA 3243A>G mutation.
A 58-year-old female presented with acute-onset speech impediment and auditory hallucinations, symmetrical bitemporal lobe abnormalities, clinical and laboratory findings, and a lack of relevant prodromal history, which suggested diagnosis of autoimmune encephalitis. Further work-up, in conjunction with the patient’s medical history, family history, and lactate peak on brain lesions on magnetic resonance imaging, suggested a mitochondrial disorder. Mitochondrial genome analysis revealed the m.3243A>G variant in the MT-TL1 gene, which led to a diagnosis of MELAS syndrome.
This case underscores the importance of considering MELAS as a potential cause of autoimmune encephalitis even if patients are over 40 years of age, as the symptoms and signs are atypical for MELAS syndrome.
Core Tip: Mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes (MELAS) syndrome is a multimitochondrial disease caused by DNA mutations and respiratory chain defects that is frequently misdiagnosed. Here, we describe a 58-year-old patient with MELAS syndrome who initially presented with acute cognitive impairment, tinnitus, and headache and was subsequently misdiagnosed with autoimmune encephalitis. The final diagnosis was based on MELAS mutation blood tests and magnetic resonance imaging results. The patient was treated with appropriate medication and gradually improved. This case shows that MELAS syndrome should be diagnosed only after other causes, including autoimmune encephalitis, have been ruled out and the atypical clinical features of MELAS syndrome, such as older age of onset, have been considered.
- Citation: Wang JW, Yuan XB, Chen HF. Late-onset mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes syndrome with mitochondrial DNA 3243A>G mutation masquerading as autoimmune encephalitis: A case report. World J Clin Cases 2023; 11(14): 3275-3281
- URL: https://www.wjgnet.com/2307-8960/full/v11/i14/3275.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i14.3275
Mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) syndrome is a multisystemic mitochondrial disorder[1]. MELAS is caused by mutations in mitochondrial DNA and subsequent respiratory chain deficiency[2]. In most cases, MELAS syndrome is characterized by severe aches, stroke-like episodes, short stature, sensorineural deafness, cognitive decline, and exercise intolerance. Conversely, hypertrophic cardiomyopathy, ataxia, ophthalmoplegia, and diabetes mellitus are rare features of MELAS[3,4]. MELAS syndrome presenting with the features of acute encephalitis is rare and has been described in only a few case reports[5,6]. Among these few cases, nonviral encephalitis is even rarer and may therefore pose a diagnostic challenge. Of note, the above clinical manifestations generally occur before the age of 40[5].
Here, we report the unique case of a 58-year-old female whose condition initially appeared to be autoimmune encephalitis and who was ultimately diagnosed with MELAS syndrome in the presence of the m.3243A>G mutation.
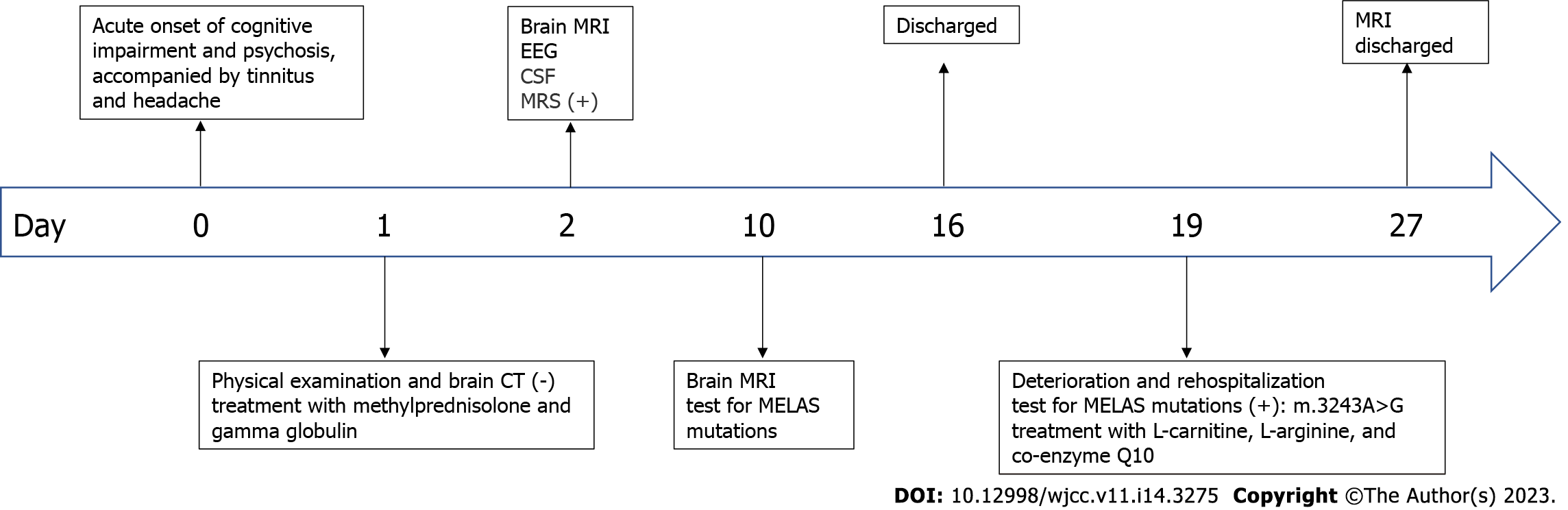
Speech impediment and auditory hallucination, accompanied by tinnitus and headache for 3 d.
A 58-year-old female presented with sudden cognitive dysfunction, auditory hallucinations, nonsensical behavior, inability to communicate normally with others, and complaints of headache and tinnitus. The patient had no fever, seizures, or consciousness disturbance.
The patient had a history of bilateral hearing loss.
The patient had no history of mental retardation or cognitive decline up to the time of her acute illness. Moreover, the patient denied a family history of neuromuscular disease, encephalitis, or mitochondrial disease.
On admission, the patient's height was 158 cm, and her weight was 45 kg. On physical examination, her body temperature was 36.5 °C and a history of previous infection and fever were denied. Chest auscultation revealed normal respiratory sounds and a normal heart rate with no murmur. The patient’s neurological examination, limited by the above symptoms, was otherwise normal.
Routine laboratory studies, including blood glucose, hepatic and renal function, coagulation testing, glycosylated hemoglobin, autoantibodies, autoantibody spectrum associated with anti-cardiolipin antibodies, thyroid function, homocysteine, serum tumor markers, human immunodeficiency virus antibody test and syphilis spirochete hemagglutination test, were all unremarkable. The patient’s serum white cell count was 14.38 × 109/L, and her C-reactive protein level was 7 mg/L. It is worth noting that her arterial blood lactate level was 4.7 mmol/L.
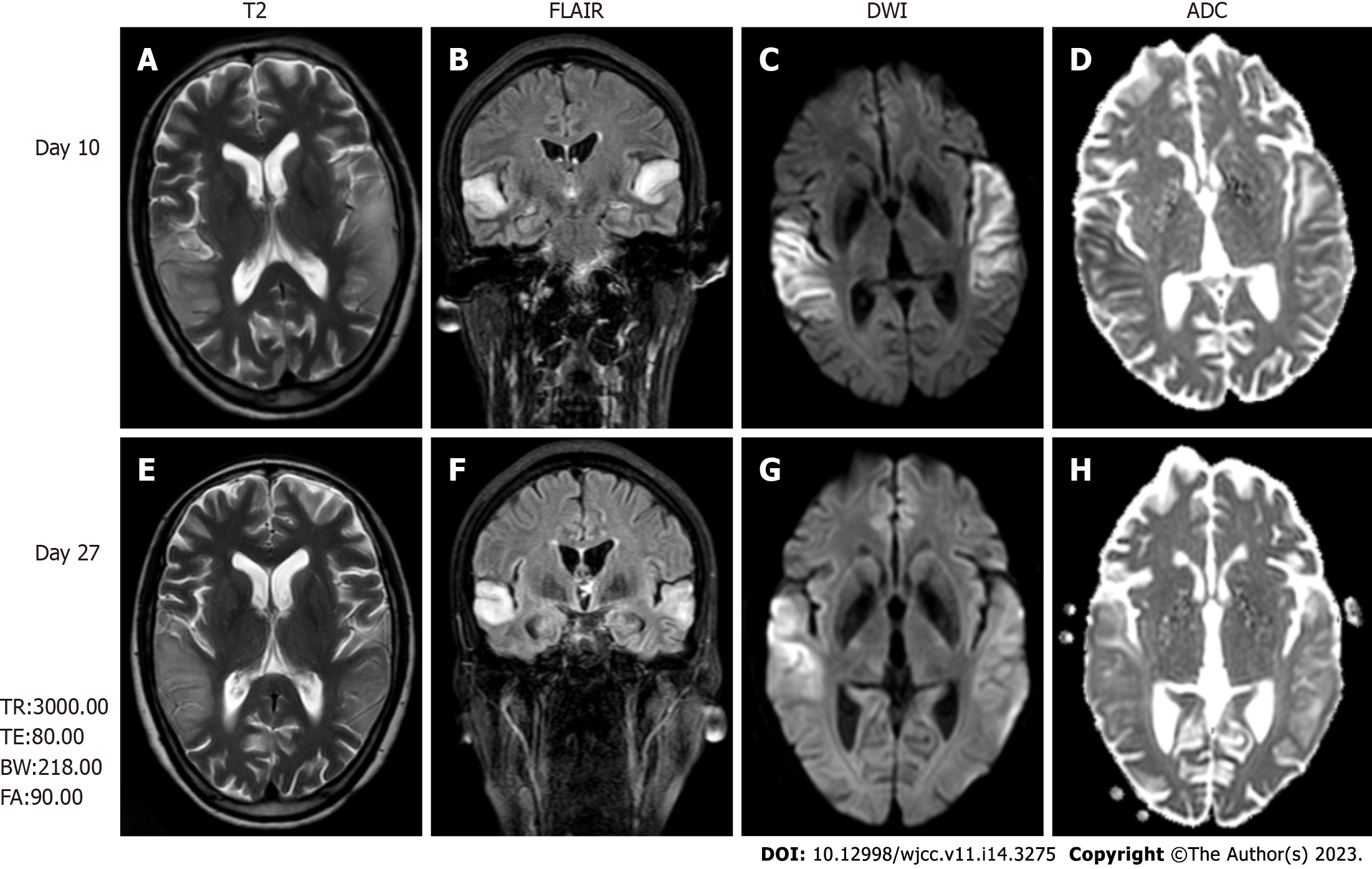
Magnetic resonance imaging (MRI) revealed high-intensity lesions in the bitemporal lobe on T2-weighted images (Figure 1A), fluid-attenuated inversion recovery (FLAIR) (Figure 1B) images, and diffusion-weighted images (DWI) (Figure 1C and E). The parts of the lesions involving the cortex appeared hyperintense on DWI (Figure 1C) and hypointense on apparent diffusion coefficient (ADC) maps (Figure 1D), features consistent with cytotoxic edema. Follow-up brain MRI obtained on Day 27 showed an extensive reduction in FLAIR/DWI signals in the left temporal lobe (Figure 1F and G), without an apparent reduction in T2 signals.
The electroencephalogram only indicated a slight increase in fast waves.
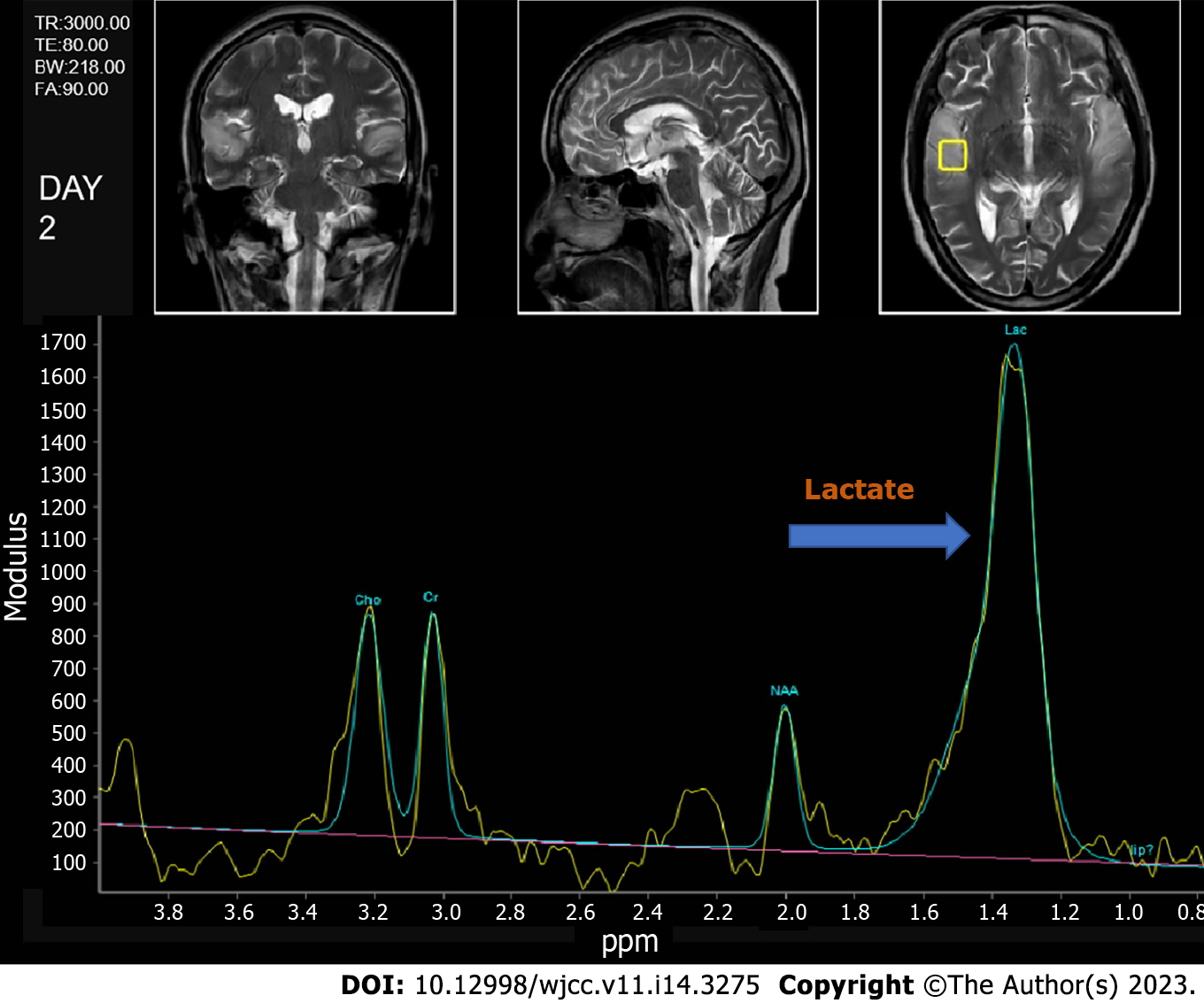
Magnetic resonance spectroscopy also revealed a prominent doublet and elevated lactate peak with reduced N-acetyl-aspartate levels (Figure 2). Cerebrospinal fluid (CSF) showed a white cell count of 4/μL and a protein level of 0.668 g/L with a CSF pressure of 140 mmH2O.
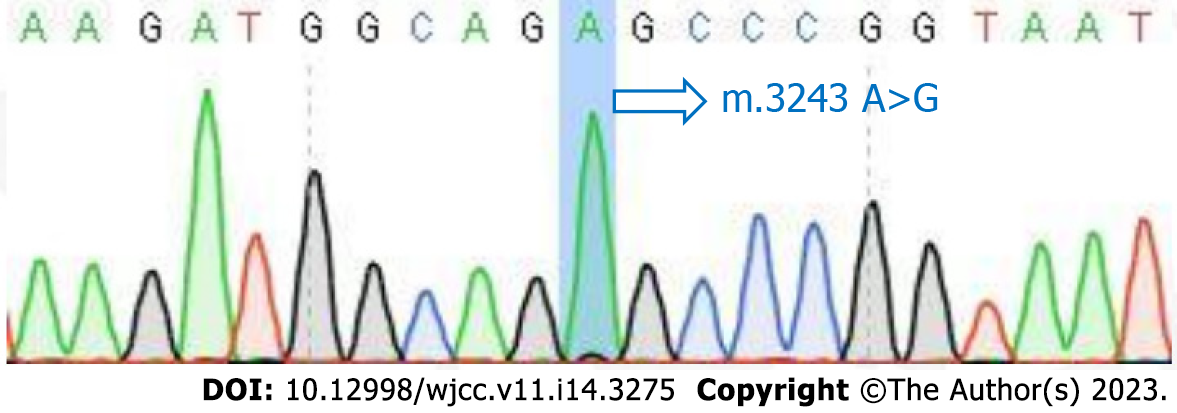
The mitochondrial DNA (mtDNA) 3243A>G mutation detected in the patient’s blood led to the final diagnosis of MELAS syndrome.
The patient was initially misdiagnosed with autoimmune encephalitis and treated with gamma globulin (18 mg/d × 5 d) therapy and intravenous methylprednisolone (1000 mg/d × 3 d to 500 mg/d × 3 d to 60 mg/d × 9 d). Then, the patient was discharged with slowly tapered oral methylprednisolone (44 mg/d × 2 wk followed by a dosage reduction of 4 mg every 2 wk). When the diagnosis of MELAS was confirmed by the presence of the 3243A>G mutation (Figure 3), we immediately stopped intravenous methylprednisolone and started therapy with L-carnitine (oral 1 g/d × 8 d), L-arginine (oral 4.5 g/d × 8 d), and coenzyme Q10 (oral 60 mg/d × 8 d) (Figure 4).
The patient gradually improved before being discharged from the hospital. At the outpatient follow-up a few months later, the patient's cognitive function had recovered well, and she was basically able to take care of herself.
Based on some classic features of MELAS syndrome, such as repeated headaches, previous history of hearing impairment, lactic acidosis, peak lactic acid on brain MRI, and the m.3243A>G mutation detected in serum, the diagnosis of MELAS was clear in our case. The clinical presentation of MELAS depends on the existence of heteroplasmy[6], which refers to the ratio of mutant to normal mtDNA, divided into four different transcription stages of 0%, 20%-30%, 50%-90%, and 100%[7]. It is generally believed that the heteroplasmy rate of typical MELAS is 50%-90%, with higher heterogeneity being associated with earlier onset time. The age of MELAS patients is mostly 10-30 years, and there are few reports of MELAS syndrome onset after 40 years of age[1,8-10]. Our patient’s late age of onset and some other atypical symptoms were related to the low heteroplasmy.
This case initially appeared to be autoimmune encephalitis. Few cases of MELAS appearing as acute encephalitis have been reported[11-15]. Most of these cases appear to be herpes simplex encephalitis (HSE). Johns et al[11] described three cases with different onset symptoms, all of which involved 3243A>G mutations and increased serum lactic acid levels. The intracranial lesions in all three patients were located in the unilateral temporal or parietal lobes. The case reported by Sharfstein et al[12] was characterized by aphasia and delirium; the lesions were located in the left temporal and parietal lobes, and the patient carried the 3243A>G mutation. Hsu et al[13] described a patient with acute-onset pyrexia, headache, and seizures who showed aberrant pleocytosis in the CSF but no obvious abnormalities on MRI. The lesions described by both Gieraerts et al[14] and Caldarazzo Ienco et al[16] were bilateral, but in the study by Gieraerts et al[14], the lesions were widely distributed; in the study by Caldarazzo Ienco et al[16], they were confined to the bilateral temporal lobes. All of the cases in these abovementioned studies were characterized by the mitochondrial 3243A>G mutation. Of note, 80% of MELAS patients carry the m.3243A>G mutation in the MT-TL1 gene, whereas the frequency of this mutation in the general population is approximately 1:15000[17]. Diseases related to other types of mutations are also misdiagnosed as encephalitis in approximately 20% of MELAS patients. Yokota et al[15] described a patient with the mtDNA 14453G→A mutation and acute cognitive impairment, psychosis, headache, and pyrexia who showed mild pleocytosis in the CSF and a lesion in the right temporoparietal lobe.
Based on the above cases, the distribution of lesions in MELAS syndrome patients can be diverse, and the symmetry of MELAS lesions is becoming gradually recognized[18,19]. However, the signal distribution of MELAS lesions on DWI and ADC is relatively unique, especially when compared with ischemic stroke lesions. MELAS lesions most often occur due to vasogenic edema; thus, the signal intensity on ADC maps is not or only mildly reduced[20,21]. In contrast, ischemic areas are primarily caused by cytotoxic edema and generally present as restricted diffusion and low signals on ADC maps[22,23]. This phenomenon is consistent with the imaging in our case.
HSE was not the first diagnosis we considered for our patient. First, the patient’s CSF was normal, and the gold standard for diagnosing HSE was not detected. Second, the lesions in the bilateral temporal lobes on brain MRI reduced the likelihood of HSE[24,25], though the negative results for the presence of antibodies against neuronal surface antigens in the CSF could not rule out the possibility of autoimmune encephalitis. Graus et al[26] reported clinical diagnostic criteria for autoimmune encephalitis and specifically pointed out that mitochondrial diseases can result in a diagnosis different from autoimmune encephalitis. When the present patient visited one of our physicians, we accepted the original diagnosis of autoimmune encephalitis and discharged her. When she subsequently developed deteriorated mental status under normal medication, combined with the findings of magnetic resonance spectroscopy and serum lactic acid, we then considered the possibility of metabolic disorders, especially MELAS. The final diagnosis of our patient was confirmed by molecular genetic testing of mitochondrial DNA. This case highlights the importance of deferring a diagnosis of autoimmune encephalitis until alternative causes, including MELAS syndrome, have been excluded, especially in antibody-negative encephalitis. It should be recognized that some atypical clinical symptoms of MELAS, such as onset at an advanced age, and deviation from the classic brain MRI features, including symmetry of the lesion location, are being increasingly reported. In particular, when a disease cannot be clearly diagnosed, we should decisively turn our attention to patient characteristics that do not conform to “classic” features.
This case shows that late-onset MELAS syndrome is rare but should be carefully considered in patients presenting with relevant symptoms as a crucial step in the diagnosis and treatment of such patients.
The authors acknowledge the medical staff at Affiliated Jinhua Hospital, Zhejiang University School of Medicine for their dedicated care of the patient and the cooperation. We sincerely thank the patient.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Clinical neurology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Mathieu C, France; Sijens PE, Netherlands; Vyshka G, Albania S-Editor: Yan JP L-Editor: A P-Editor: Guo X
| 1. | Sproule DM, Kaufmann P. Mitochondrial encephalopathy, lactic acidosis, and strokelike episodes: basic concepts, clinical phenotype, and therapeutic management of MELAS syndrome. Ann N Y Acad Sci. 2008;1142:133-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 230] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 2. | Deschauer M, Tennant S, Rokicka A, He L, Kraya T, Turnbull DM, Zierz S, Taylor RW. MELAS associated with mutations in the POLG1 gene. Neurology. 2007;68:1741-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Goto Y, Horai S, Matsuoka T, Koga Y, Nihei K, Kobayashi M, Nonaka I. Mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS): a correlative study of the clinical features and mitochondrial DNA mutation. Neurology. 1992;42:545-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 261] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 4. | McFarland R, Taylor RW, Turnbull DM. A neurological perspective on mitochondrial disease. Lancet Neurol. 2010;9:829-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 219] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 5. | Hirano M, Pavlakis SG. Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes (MELAS): current concepts. J Child Neurol. 1994;9:4-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 233] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | Crimmins D, Morris JG, Walker GL, Sue CM, Byrne E, Stevens S, Jean-Francis B, Yiannikas C, Pamphlett R. Mitochondrial encephalomyopathy: variable clinical expression within a single kindred. J Neurol Neurosurg Psychiatry. 1993;56:900-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Picard M, Zhang J, Hancock S, Derbeneva O, Golhar R, Golik P, O'Hearn S, Levy S, Potluri P, Lvova M, Davila A, Lin CS, Perin JC, Rappaport EF, Hakonarson H, Trounce IA, Procaccio V, Wallace DC. Progressive increase in mtDNA 3243A>G heteroplasmy causes abrupt transcriptional reprogramming. Proc Natl Acad Sci U S A. 2014;111:E4033-E4042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 234] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 8. | Zhang Z, Zhao D, Zhang X, Xiong H, Bao X, Yuan Y, Wang Z. Survival analysis of a cohort of Chinese patients with mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes (MELAS) based on clinical features. J Neurol Sci. 2018;385:151-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Kaufmann P, Engelstad K, Wei Y, Kulikova R, Oskoui M, Sproule DM, Battista V, Koenigsberger DY, Pascual JM, Shanske S, Sano M, Mao X, Hirano M, Shungu DC, Dimauro S, De Vivo DC. Natural history of MELAS associated with mitochondrial DNA m.3243A>G genotype. Neurology. 2011;77:1965-1971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 10. | Yatsuga S, Povalko N, Nishioka J, Katayama K, Kakimoto N, Matsuishi T, Kakuma T, Koga Y; Taro Matsuoka for MELAS Study Group in Japan. MELAS: a nationwide prospective cohort study of 96 patients in Japan. Biochim Biophys Acta. 2012;1820:619-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 198] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 11. | Johns DR, Stein AG, Wityk R. MELAS syndrome masquerading as herpes simplex encephalitis. Neurology. 1993;43:2471-2473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Sharfstein SR, Gordon MF, Libman RB, Malkin ES. Adult-onset MELAS presenting as herpes encephalitis. Arch Neurol. 1999;56:241-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Hsu YC, Yang FC, Perng CL, Tso AC, Wong LJ, Hsu CH. Adult-onset of mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) syndrome presenting as acute meningoencephalitis: a case report. J Emerg Med. 2012;43:e163-e166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Gieraerts C, Demaerel P, Van Damme P, Wilms G. Mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) syndrome mimicking herpes simplex encephalitis on imaging studies. J Comput Assist Tomogr. 2013;37:279-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Yokota Y, Hara M, Akimoto T, Mizoguchi T, Goto YI, Nishino I, Kamei S, Nakajima H. Late-onset MELAS syndrome with mtDNA 14453G→A mutation masquerading as an acute encephalitis: a case report. BMC Neurol. 2020;20:247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Caldarazzo Ienco E, Orsucci D, Simoncini C, Montano V, LoGerfo A, Siciliano G, Bonuccelli U, Mancuso M. Acute encephalopathy of the temporal lobes leading to m.3243A>G. When MELAS is not always MELAS. Mitochondrion. 2016;30:148-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Goodfellow JA, Dani K, Stewart W, Santosh C, McLean J, Mulhern S, Razvi S. Mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes: an important cause of stroke in young people. Postgrad Med J. 2012;88:326-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Miyahara H, Matsumoto S, Mokuno K, Dei R, Akagi A, Mimuro M, Iwasaki Y, Yoshida M. Autopsied case with MERRF/MELAS overlap syndrome accompanied by stroke-like episodes localized to the precentral gyrus. Neuropathology. 2019;39:212-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Bhatia KD, Krishnan P, Kortman H, Klostranec J, Krings T. Acute Cortical Lesions in MELAS Syndrome: Anatomic Distribution, Symmetry, and Evolution. AJNR Am J Neuroradiol. 2020;41:167-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Geberhiwot T, Chakrapani A, Hendriksz C. Case 36-2005: a woman with seizure, disturbed gait, and altered mental status. N Engl J Med. 2006;354:1096-7; author reply 1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Farrar MA, Lin CS, Krishnan AV, Park SB, Andrews PI, Kiernan MC. Acute, reversible axonal energy failure during stroke-like episodes in MELAS. Pediatrics. 2010;126:e734-e739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Tzoulis C, Bindoff LA. Serial diffusion imaging in a case of mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes. Stroke. 2009;40:e15-e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Xu W, Wen J, Sun C, Cao J, Li Y, Geng D. Conventional and Diffusional Magnetic Resonance Imaging Features of Mitochondrial Encephalomyopathy, Lactic Acidosis, and Stroke-Like Episodes in Chinese Patients: A Study of 40 Cases. J Comput Assist Tomogr. 2018;42:510-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Pauli W, Zarzycki A, Krzyształowski A, Walecka A. CT and MRI imaging of the brain in MELAS syndrome. Pol J Radiol. 2013;78:61-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Chow FC, Glaser CA, Sheriff H, Xia D, Messenger S, Whitley R, Venkatesan A. Use of clinical and neuroimaging characteristics to distinguish temporal lobe herpes simplex encephalitis from its mimics. Clin Infect Dis. 2015;60:1377-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 26. | Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, Cortese I, Dale RC, Gelfand JM, Geschwind M, Glaser CA, Honnorat J, Höftberger R, Iizuka T, Irani SR, Lancaster E, Leypoldt F, Prüss H, Rae-Grant A, Reindl M, Rosenfeld MR, Rostásy K, Saiz A, Venkatesan A, Vincent A, Wandinger KP, Waters P, Dalmau J. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2328] [Cited by in RCA: 2667] [Article Influence: 296.3] [Reference Citation Analysis (0)] |