Published online May 16, 2023. doi: 10.12998/wjcc.v11.i14.3267
Peer-review started: December 4, 2022
First decision: February 7, 2023
Revised: February 17, 2023
Accepted: April 6, 2023
Article in press: April 6, 2023
Published online: May 16, 2023
Processing time: 162 Days and 19.3 Hours
The development of immune checkpoint inhibitors (ICIs) has heralded a new era in cancer treatment, enabling the possibility of long-term survival in patients with metastatic disease. Unfortunately, ICIs are increasingly implicated in the deve
We present a man with squamous cell carcinoma of the oropharynx on a combination of teriprizumab, docetaxel, and cisplatin therapy who developed autoimmune polyendocrine syndrome type II (APS-2) including thyroiditis and type 1 diabetes mellitus and Crohn’s disease (CD). He developed thirst, abdominal pain, and fatigue after two-week treatment with the protein 1 ligand inhibitor teriprizumab. Biochemistry confirmed APS-2 and thyrotoxicosis. He was commenced on an insulin infusion. However, his abdominal pain persisted. Follow-up surgery confirmed CD and his abdominal pain was relieved by mesalazine. He was continued on insulin and mesalazine therapy.
Immunotherapy can affect all kinds of organs. When clinical symptoms cannot be explained by a single disease, clinicians should consider the possibility of multisystem damage.
Core Tip: We report a rare case of multi-system damage induced by cancer therapy with protein 1 ligand inhibitor teriprizumab. A man with squamous cell carcinoma of oropharynx on a combination regimen of teriprizumab, docetaxel, and cisplatin developed autoimmune polyendocrine syndrome type II (APS-2) including thyroiditis and type 1 diabetes mellitus (T1DM) and Crohn’s disease (CD). This case report highlights the possibility of chronic immune toxicities and the long-term implications of cancer immunotherapy. To the best of our knowledge, this is the first reported case of concurrent atypical APS-2 (including T1DM and thyrotoxicosis) and CD in a patient receiving immunotherapy for metastatic nasopharyngeal carcinoma.
- Citation: Gao MJ, Xu Y, Wang WB. Immune checkpoint inhibitor therapy-induced autoimmune polyendocrine syndrome type II and Crohn’s disease: A case report. World J Clin Cases 2023; 11(14): 3267-3274
- URL: https://www.wjgnet.com/2307-8960/full/v11/i14/3267.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i14.3267
Immune checkpoint inhibitors (ICIs) such as programmed cell death protein 1 (PD1) or its ligand (PDL1) and cytotoxic T-lymphocyte antigen-4 (CTLA-4) have revolutionized the treatment of many malignancies, such as melanoma, non-small cell lung cancer, and renal cell carcinoma. ICI therapy enhances the immune system and has been shown to improve survival in some cancer patients. However, ICIs occasionally induce immune-related adverse events (irAEs), which commonly affect the gastrointestinal tract, liver, skin, and endocrine system, but can affect virtually any organ system[1,2]. Thyroid disorders are common endocrinopathies associated with irAEs[3]. Hypophysitis is another well-recognized endocrine side effect[4]. Autoimmune diabetes mellitus especially type 1 diabetes mellitus (T1DM) has occasionally been reported[5], while adrenal insufficiency is observed even less frequently[6]. However, to the best of our knowledge, ICI-induced autoimmune polyendocrine syndrome type II (APS-2) including T1DM combined with Crohn’s disease (CD), has not yet been reported.
Here we report an elderly man who developed partial features of APS-2 combined with CD shortly after initiation of treatment with PDL1 antibody teriprizumab for squamous cell carcinoma of the oropharynx. This patient had an acute onset of T1DM with diabetic ketoacidosis (DKA), short-term thyrotoxicosis, and typical CD. This case report is supplemented by a literature review of PD1/PD-L1 inhibitor-associated APS-2 and CD. Our study may help improve the understanding of endocrinologists, oncologists, and gastroenterologists about rare irAEs.
A 61-year-old man presented to the emergency department of Shougang Hospital with fatigue, vomiting, polyuria, polydipsia, and abdominal pain for a few days.
The patient had earlier received treatment for squamous cell carcinoma of the oropharynx at the Peking University Tumor Hospital. About two weeks after discharge from that hospital, he developed thirst, fatigue, and abdominal pain. Five days later (September 21, 2021), he presented to the emergency department with abrupt onset of extreme fatigue, vomiting, polyuria, polydipsia, bellyache, hyperglycemia, severe acidosis, and the presence of ketones bodies in urine. DKA was diagnosed by an emergency medicine specialist. Treatment protocol for DKA with continuous intravenous insulin was initiated with good response within 8 h. Plasma amylase level was substantially increased. According to the Common Terminology Criteria for Adverse Events of the National Institute of Health, ICI-induced pancreatic injury was suspected although abdominal computed tomography (CT)-scan was unremarkable. After comprehensive treatment, his blood biochemical indicators and blood glucose returned to normal, but abdominal pain persisted. He was admitted to the inpatient department for further evaluation.
His biochemical parameters confirmed recovery, but abdominal pain persisted along with persistent positive stool occult blood and elevated C-reactive protein (CRP). The abdominal pain was mostly around umbilicus, sometimes in the right abdomen, and typically occurred approximately 30 to 45 min after meals; there was no rectal urgency. Sometimes, the pain was relieved after defecation. His dietary intake was reduced due to the pain.
He had been diagnosed with squamous cell carcinoma of the oropharynx which invaded the right medial pterygoid and right-sided neck lymph nodes. The carcinoma was surgically excised along with associated lymph nodes clearance (confirming T4N2M0) on July 22, 2021. One month later (2021-8-31), he received the first cycle of a three-drug chemotherapy regimen (docetaxel, cisplatin, and tigisay) combined with PDL1 targeted antibody (teriprizumab) therapy. The dosing regimen was: Docetaxel 120 mg qd, one day; cisplatin 120 mg qd, one day; tigisay 40 mg bid, two weeks; and teriprizumab 240 mg qd one day, three weekly. Full blood counts, biochemistry, and thyroid function tests were within normal limits prior to the initiation of therapy.
He had a history of hypertension and cerebral infarction, but there was no past or family history of autoimmune or endocrine disease.
On examination, he was conscious and oriented but looked unwell and was clinically dehydrated. Body temperature was normal. Heart rate was regular at 82 per minute, respiratory rate was 19 per minute with oxygen saturation of 97% on ambient air, and blood pressure was 118/76 mmHg. His body mass index was 20.9 kg/m2 (height 168 cm, weight 59 kg). He had hoarseness of voice and complained of abdominal pain. Abdominal examination revealed tenderness and active bowel sounds. Other systemic examination was unremarkable.
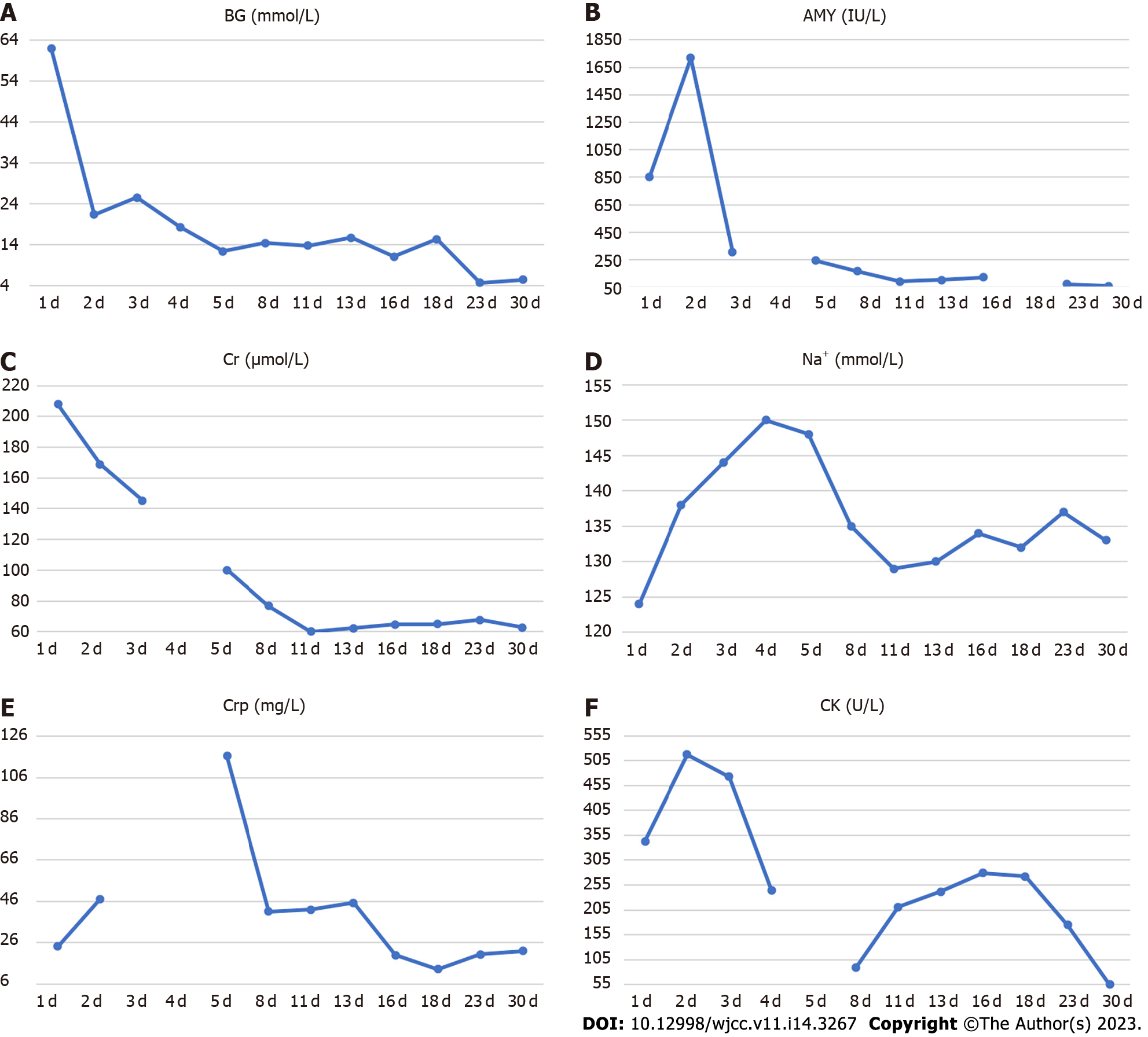
His blood parameters on admission in the emergency room were: Blood glucose 62.09 mmol/L; pH 7.008; urinary ketone body 4+; serum amylase 854 U/L; serum creatinine 207.8 µmol/L; and creatine kinase 343 U/L (Table 1). After following comprehensive treatment, his blood biochemical indicators returned to normal. He was administered multiple daily insulin injections and blood glucose was in an ideal range (Figure 1). Further workup revealed positive glutamic acid decarboxylase antibody insulin autoantibody and low C-peptide level (< 0.01 nmol/L), which was consistent with autoimmune T1DM (Table 2). His glycated hemoglobin (HbA1c) and glycated albumin were 8.3% and 36%, respectively. There were no signs of diabetes-related complications or microangiopathy; the urinary albumin-to-creatinine ratio and fundus retinal examination were all normal. The pituitary function, gonadal function, and adrenal function were within the normal range (Supplementary Table 1). Thyroid function tests showed overt thyrotoxicosis [Thyroid stimulating hormone < 0.01 mU/L, FT4 24.1 pmol/L, FT3 5.27 pmol/L, Thyroglobulin (TG)-Ab > 1000 IU/mL, TG-Ab 226.08 IU/mL, normal thyrotropin receptor antibody (TRAb)] but there were no symptoms of hyperthyroidism. The clinical picture was consistent with thyroiditis, deemed to be an irAE related to the medication teriprizumab (Table 3).
| Investigation | Value | Reference range |
| Blood gas analysis | ||
| pH | 7.008 | 7.35 to 7.45 |
| base excess (mmol/L) | -26.2 | -3 to 3 |
| lactic acid (mmol/L) | 3.7 | 0.4 to 2.2 |
| Blood biochemical analysis | ||
| Blood glucose (mmol/L) | 62.09 | 3.89 to 6.11 |
| Blood potassium (K+, mmol/L) | 5.58 | 3.5 to 5.5 |
| Blood sodium (Na+, mmol/L) | 124 | 137 to 147 |
| Serum creatinine (µmol/L) | 207.8 | 20.0 to 98.0 |
| Urea nitrogen (BUN, mmol/L) | 18.03 | 1.7 to 7.5 |
| Creatine kinase (U/L) | 343 | 24 to 195 |
| Amylase (IU/L) | 854 | 40 to 132 |
| C-reactive protein (mg/L) | 24.24 | 0.00 to 6.00 |
| Routine blood test | ||
| White blood cell count (109/L) | 6.7 | 3.5 to 9.5 |
| Neutrophils (109/L) | 3.7 | 1.8 to 6.3 |
| Red blood cell count (1012/L) | 5.23 | 4.3 to 5.8 |
| Platelet count (109/L) | 255 | 125 to 350 |
| Urinalysis | ||
| Urine gravity | 1.015 | 1.006 to 1.030 |
| pH | 5 | 5.5 to 8 |
| Protein | 1+ | Negative |
| Urine sugar | 4+ | Negative |
| Urinary ketone body | 4+ | Negative |
| Time (min) | BG (mmol/L) | Insulin (µIU/mL) | C-peptide (0.78-5.19 ng/mL) | 3 mo later C-peptide |
| 0 | 15.36 | 10.9 (2.5 to 9.4) | < 0.01 | < 0.01 |
| 60 | 25.89 | 6.6 (11.9 to 43.5) | < 0.01 | < 0.01 |
| 120 | 26.99 | 5.6 (8.7 to 29.7) | < 0.01 | < 0.01 |
| Investigation | On admission | 2 wk later | 3 mo later | Reference range |
| T3 (nmol/L) | 1.57 | 1.14 | 2.25 | 0.89 to 2.45 |
| T4 (nmol/L) | 191.37 | 128.27 | 132.69 | 62.68 to 150.84 |
| TSH (µIU/mL) | < 0.01 | 0.01 | 3.84 | 0.35 to 4.94 |
| FT3 (pmol/L)) | 5.27 | 3.87 | 4.71 | 2.63 to 5.71 |
| FT4 (pmol/L) | 24.1 | 16.27 | 10.91 | 9.1 to 19.24 |
| TPO-Ab (IU/mL) | > 1000.00 | > 1000.00 | 363 | 0 to 5.61 |
| TG-Ab (IU/mL) | 226.08 | 140.84 | 218 | 0 to 4.11 |
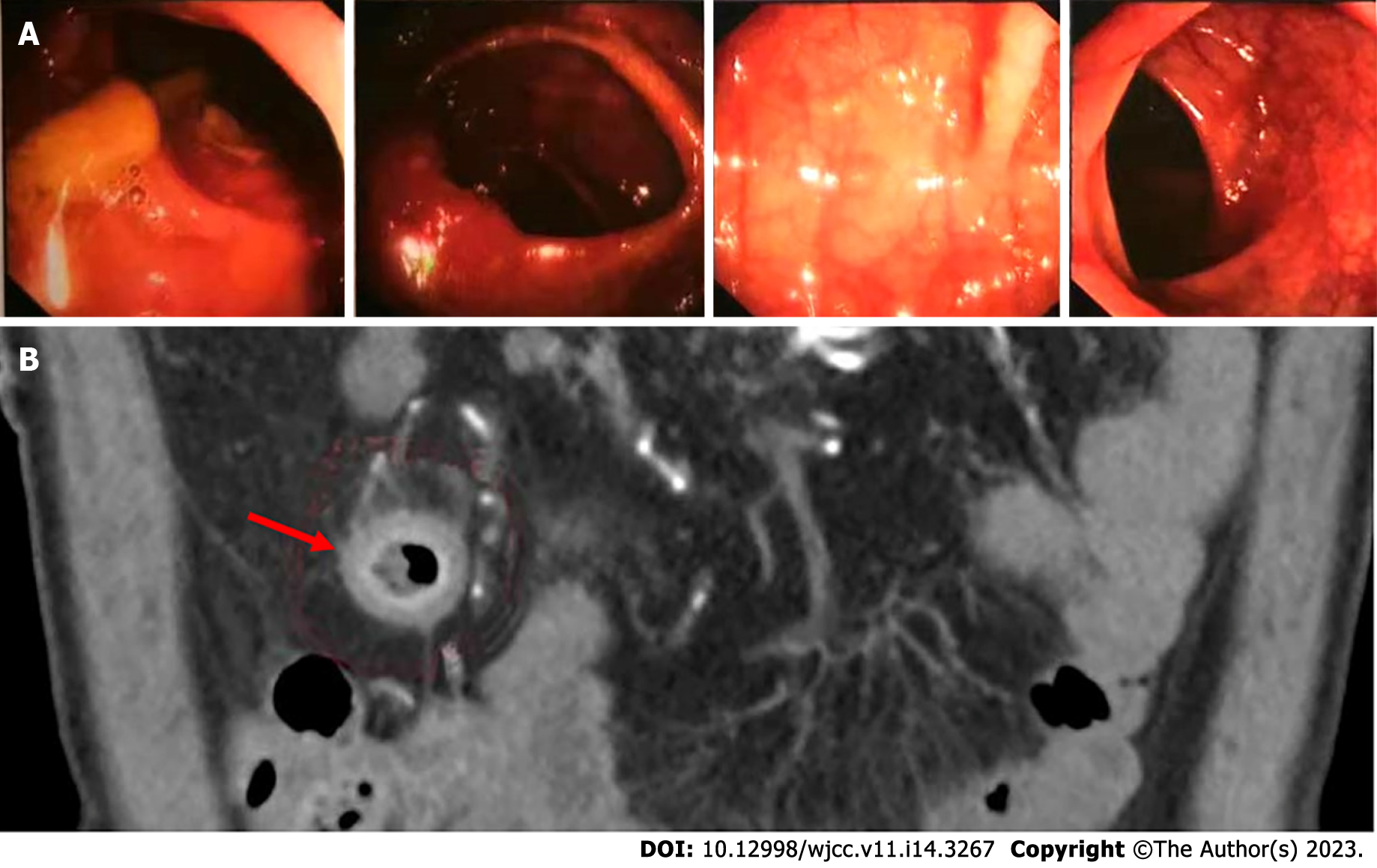
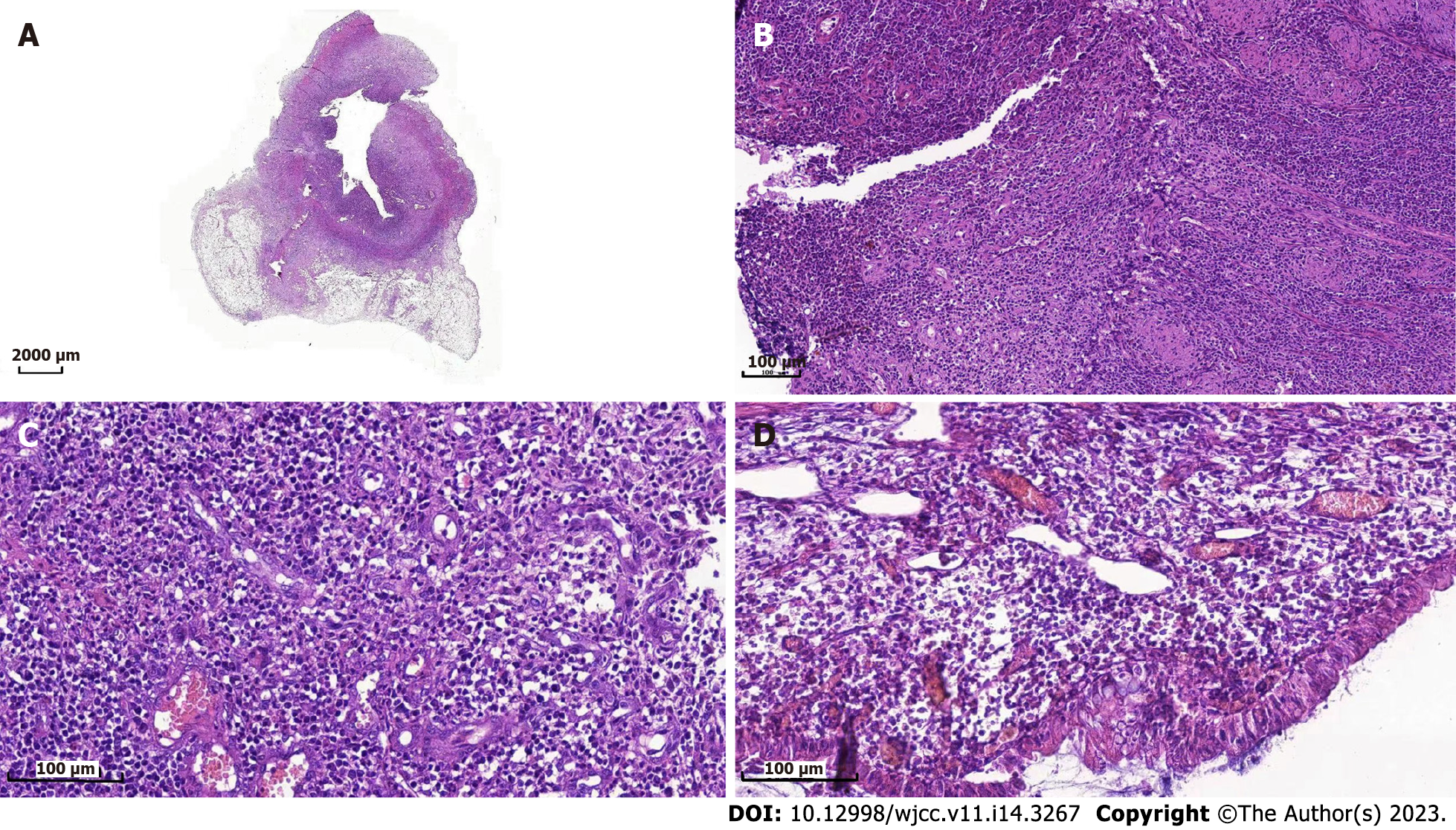
Owing to the recovery of biochemical indices, the persistent abdominal pain could not be attributed to DKA; therefore, the possibility of ICI-related gastrointestinal irAEs was considered. Moreover, due to persistent positive stool occult blood and mildly elevated CRP, endoscopy and imaging (electronic colonoscopy) were performed to directly visualize and inspect the bowel lumen. There were no remarkable findings apart from a single colon polyp (Figure 2A), which could not explain the abdominal pain, CRP and hematochezia. Consequently, abdominal CT-scan was performed which showed blurring and thickened of the wall of distal ileum which was presumed to be inflammatory (Figure 2B). He was scheduled for enteroscopy. Unfortunately, one month later, he had to undergo surgical treatment for intestinal obstruction (abdominal pain and distension, no passage of feces or flatus). The resected terminal ileum (about 5 cm) was sent for pathological examination, and there was transmural inflammation at the stenosis of intestinal canal. Postoperative histopathological staining showed extensive infiltration of lymphocytes, plasma cells, and neutrophils, along with simple ulcers in the affected bowel loops. There was destruction of crypt structure along with atrophy of intestinal villi in surrounding bowel loops and no lesion in upper or lower intestine, immunohistochemistry: CD3 (+), CD4 (+), CD5 (+), CD8 (+), CD10 (+++), CD79a (+++), MUM1 (+++), ki-67 (20%+), in situ hybridization: EBER (+), all of which were consistent with inflammatory bowel disease (IBD), especially CD (Figure 3).
The patient was diagnosed with APS 2 and CD.
Postoperatively, his blood glucose was difficult to control, then he was suggested to use immune modulators such as methotrexate or biological agents such as adalimumab for his CD, according to the serious adverse reactions induced by earlier treatment of nasopharyngeal carcinoma, he refused these medications. At last, he was administered mesalazine 4 g/d. The patient was discharged on oral mesalazine sustained-release granules with a dosage of 1 g quarter in die for 8 wk and then maintained at 1 g twice daily and daily insulin dosage of 36-42 IU administered via four injections per day.
After about two weeks of mesalazine treatment, there was complete remission of abdominal pain and the stool occult blood test was negative. Three months after withdrawal of teriprizumab, his thyroid hormone levels had returned to normal (Table 3). He received more than 20 sessions of local radiotherapy (nasopharyngeal) and cisplatin chemotherapy after his discharge from Shougang Hospital. In the first one-year follow-up, he showed good recovery with disappearance of hoarseness of voice and nasopharyngeal carcinoma. He was taking mesalazine and insulin intensive therapy, with CRP of 6.73 mg/L, negative stool occult blood and HbA1c of 7.8%.
Our patient developed low C-peptide level and modestly elevated HbA1c with positive T1DM auto-antibody two weeks after initiation of PDL1 antibody therapy pointing to acute, rapid destruction of -islets in pancreas. Increased amylase level indicated damage to the pancreatic exocrine gland. After admission, he was found to have thyroiditis and thyrotoxicosis. In addition, the increase in creatine kinase level was suggestive the possibility of rheumatic immune system damage. During following treatment, the patient developed IBD, especially CD. The overall clinical picture was suggestive of multi-system damage caused by teriprizumab. To the best of our knowledge, this is the first reported case of concurrent atypical APS-2 (including T1DM and thyrotoxicosis) and CD in a patient on immunotherapy for metastatic nasopharyngeal carcinoma.
The discovery of Checkpoint inhibitors has changed the landscape of cancer therapeutics. With the growing use of ICIs, the frequency of autoimmune complications has become increasingly apparent. The most common ICI-associated endocrinopathies are thyroid disorders and hypophysitis[7], but hypophysitis is nearly unique to patients receiving CTLA-4, which occurs more often (5% to 10%) and with an earlier onset (median 9 to 12 wk) in patients receiving ipilimumab-based regimens compared to those receiving anti-PD1/PD-L1 antibodies[8]. ICI-induced T1DM is uncommon, with a reported frequency ranging from 0.2% in randomized clinical studies[9] to 1.27% in real-life setting[5]. Primary adrenal insufficiency is less common, albeit with a similar presentation with hypophysitis, although this endocrinopathy may also involve mineralocorticoid deficiency and present with hypotension[10].
There are two definitions of APS-2, with some defining it as occurrence of any two of the three conditions, i.e., T1DM, autoimmune thyroiditis, and primary hypoadrenalism, while others specify that hypoadrenalism must exist, with at least one of the other two conditions. Spontaneous APS-2 is very rare, and anti-PD1/PDL1 antibodies-induced APS-2 has occasionally been reported. Out of the 14 cases reported in literature, three cases experienced primary hypoadrenalism and only one had all three features of APS-2[6]. Our case only presented T1DM and autoimmune thyroiditis, and his adrenocortical function needs further observation.
Regarding gastrointestinal irAEs, colitis occurs in up to 5% of patients receiving anti-PD-1/PD-L1 antibodies, and generally presents as diarrhea and less often as abdominal pain and hematochezia[11,12]. In addition, these irAEs-related chronic sequelae can also affect the rheumatological, pulmonary, neurological, and other organ systems[6]. These damages typically occur in one system and do not involve several systems at the same time. In our case, the damage involved the endocrine system and gastrointestinal system including T1DM, thyroiditis, and CD.
Abdominal pain was the chief complaint of our patient during treatment and the pain did not correlate with the severity of hyperglycemia. While DKA was cured, abdominal pain persisted. The pain aggravated after meals and was associated with increased CRP and positive occult blood in stool. These symptoms were suspicious of teriprizumab-induced gastrointestinal irAEs, especially IBD. However, no obvious lesions were observed on colonoscopy. Abdominal CT showed edema and thickening of small intestinal wall, and the subsequent pathological examination of the small intestine was consistent with CD, which made the case unique.
In conclusion, our patient had a few irAEs associated with PDL1 antibody teriprizumab treatment, especially APS-2 and CD. To the best of our knowledge, this is the first reported case of multi-organ damage associated with teriprizumab. Physicians should be aware of the chronic immune toxicities and the long-term implications of immunotherapy for cancer; especially, when clinical symptoms are not explained by a single disease, the possibility of multisystem damage should be considered.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sitkin S, Russia; Souza JLS, Brazil S-Editor: Li L L-Editor: A P-Editor: Yu HG
| 1. | Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev. 2016;44:51-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 637] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 2. | Johnson DB, Nebhan CA, Moslehi JJ, Balko JM. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol. 2022;19:254-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 593] [Article Influence: 197.7] [Reference Citation Analysis (0)] |
| 3. | Barroso-Sousa R, Barry WT, Garrido-Castro AC, Hodi FS, Min L, Krop IE, Tolaney SM. Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-analysis. JAMA Oncol. 2018;4:173-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 756] [Article Influence: 126.0] [Reference Citation Analysis (1)] |
| 4. | Di Dalmazi G, Ippolito S, Lupi I, Caturegli P. Hypophysitis induced by immune checkpoint inhibitors: a 10-year assessment. Expert Rev Endocrinol Metab. 2019;14:381-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 5. | Liu J, Zhou H, Zhang Y, Fang W, Yang Y, Huang Y, Zhang L. Reporting of Immune Checkpoint Inhibitor Therapy-Associated Diabetes, 2015-2019. Diabetes Care. 2020;43:e79-e80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Gunjur A, Klein O, Kee D, Cebon J. Anti-programmed cell death protein 1 (anti-PD1) immunotherapy induced autoimmune polyendocrine syndrome type II (APS-2): a case report and review of the literature. J Immunother Cancer. 2019;7:241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 7. | Muir CA, Clifton-Bligh RJ, Long GV, Scolyer RA, Lo SN, Carlino MS, Tsang VHM, Menzies AM. Thyroid Immune-related Adverse Events Following Immune Checkpoint Inhibitor Treatment. J Clin Endocrinol Metab. 2021;106:e3704-e3713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 8. | Faje A, Reynolds K, Zubiri L, Lawrence D, Cohen JV, Sullivan RJ, Nachtigall L, Tritos N. Hypophysitis secondary to nivolumab and pembrolizumab is a clinical entity distinct from ipilimumab-associated hypophysitis. Eur J Endocrinol. 2019;181:211-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 9. | Tsang VHM, McGrath RT, Clifton-Bligh RJ, Scolyer RA, Jakrot V, Guminski AD, Long GV, Menzies AM. Checkpoint Inhibitor-Associated Autoimmune Diabetes Is Distinct From Type 1 Diabetes. J Clin Endocrinol Metab. 2019;104:5499-5506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 10. | Grouthier V, Lebrun-Vignes B, Moey M, Johnson DB, Moslehi JJ, Salem JE, Bachelot A. Immune Checkpoint Inhibitor-Associated Primary Adrenal Insufficiency: WHO VigiBase Report Analysis. Oncologist. 2020;25:696-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 11. | Nishijima TF, Shachar SS, Nyrop KA, Muss HB. Safety and Tolerability of PD-1/PD-L1 Inhibitors Compared with Chemotherapy in Patients with Advanced Cancer: A Meta-Analysis. Oncologist. 2017;22:470-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 218] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 12. | Wang DY, Mooradian MJ, Kim D, Shah NJ, Fenton SE, Conry RM, Mehta R, Silk AW, Zhou A, Compton ML, Al-Rohil RN, Lee S, Voorhees AL, Ha L, McKee S, Norrell JT, Mehnert J, Puzanov I, Sosman JA, Chandra S, Gibney GT, Rapisuwon S, Eroglu Z, Sullivan R, Johnson DB. Clinical characterization of colitis arising from anti-PD-1 based therapy. Oncoimmunology. 2019;8:e1524695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |