Published online May 16, 2023. doi: 10.12998/wjcc.v11.i14.3204
Peer-review started: December 9, 2022
First decision: March 10, 2023
Revised: March 22, 2023
Accepted: April 6, 2023
Article in press: April 6, 2023
Published online: May 16, 2023
Processing time: 150 Days and 21.2 Hours
Neuroendoscopy is a very useful technique to Chronic Subdural Hematoma (CSH). But how to achieve the goal of treatment more minimally invasive?
To develop a simple, fast and accurate preoperative planning method in our way for endoscopic surgery of patients with CSH.
From June 2018 to May 2020, forty-two patients with CSH, admitted to our hospital, were performed endoscopic minimally invasive surgery; computed tomography (CT) imaging was employed to locate the intracerebral hematoma and select the appropriate endoscopic approach before the endoscopic surgery. The clinical data and treatment efficacy were analyzed.
According to the learning of CT scanning images, the surgeon can accurately design the best minimally invasive neuroendoscopic surgical approach and realize the precise positioning and design of the drilling site of the skull and the size of the bone window, so as to provide the most effective operation space with the smallest bone window. In this group, the average operation time was only about 1 h, and the clearance rate of hematoma was about 95%.
Patients with CSH can achieve good therapeutic effect by using our way to positioning and design to assist the operation of CSH according to CT scan and image, and our way is very useful and necessary.
Core Tip: Via minimally invasive neuroendoscopic surgery, one can use smaller surgical incisions and bone windows to achieve effective removal of intracranial hematoma, minimal trauma to brain tissue, and effective reduction of recurrence rate. However, due to variations in hematoma site, shape, size and degree of clots, the location of bone hole and approaches for minimally invasive endoscopy are also different for each patient. How to accurately locate the intracerebral hematoma in chronic subdural hematoma (cSDH) patients before surgery and design an individualized approach for minimally invasive endoscopy is one of the keys to success. To better treat cSDH patients using minimally invasive neuroendoscopy, we use computed tomography scanning to locate cSDH and select the best endoscopic micro-mirror approach before performing minimally invasive neuroendoscopic surgery and analyzed the clinical data and treatment efficacy.
- Citation: Wang XJ, Yin YH, Zhang LY, Wang ZF, Sun C, Cui ZM. Positioning and design by computed tomography imaging in neuroendoscopic surgery of patients with chronic subdural hematoma. World J Clin Cases 2023; 11(14): 3204-3210
- URL: https://www.wjgnet.com/2307-8960/full/v11/i14/3204.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i14.3204
Chronic subdural hematoma (cSDH) is one of the common diseases in neurosurgery, but its pathogenesis is still not fully understood[1-3]. cSDH can be caused by many convenient factors and presented with different manifestations and symptoms. Although many methods including drilling drainage, burr hole surgery and craniotomy have been used to treat cSDH[4-6], for patients with muscularized and separated hematoma, their treatment efficacy is poor, and the operation risk and recurrence rate are high[6-8]. With the development of microinvasive neurosurgical techniques and neuroendoscopy, cSDH evacuation with minimally invasive neuroendoscopy has become an important means of surgical treatment. Via minimally invasive neuroendoscopic surgery, one can use smaller surgical incisions and bone windows to achieve effective removal of intracranial hematoma, minimal trauma to brain tissue, and effective reduction of recurrence rate[1,2]. However, due to variations in hematoma site, shape, size and degree of clots, the location of bone hole and approaches for minimally invasive endoscopy are also different for each patient. How to accurately locate the intracerebral hematoma in cSDH patients before surgery and design an individualized approach for minimally invasive endoscopy is one of the keys to success. In clinical work, we need to find a simple and reliable way to design surgical incision and bone window, so as to more easily achieve minimally invasive surgery, which is very necessary[1]. To better treat cSDH patients using minimally invasive neuroendoscopy, surgeons in our Department, treated 42 cSDH patients from June 2018 to May 2020 using computed tomography (CT) scanning to locate cSDH and select the best endoscopic micro-mirror approach before performing minimally invasive neuroendoscopic surgery and analyzed the clinical data and treatment efficacy. The summary is as follows.
A total of 42 cSDH patients (26 males and 16 females) at age of 34–76 years old with average of 55.3 years old were enrolled in the study. The amount of hematoma in these patients was calculated according to the Tada formula. The average amount of blood loss was 64.3 ± 15.2 mL. The Glasgow Coma score at admission was 13–15 points for 29 patients and 9–12 points for 13 patients.
Based on the emergency CT results of cSDH patients at admission, the location, shape and the thickest part of the intracranial hematoma were estimated to initially design the possible approach of minimally invasive endoscopic surgery. The approach was used as the alternative bone window site for the minimally invasive surgery. Before operation, a 64-slice spiral CT scanner was used to perform the conventional head CT scan with the scan line parallel to the orbitomeatal line and the scan range from the base to the top of the skull.
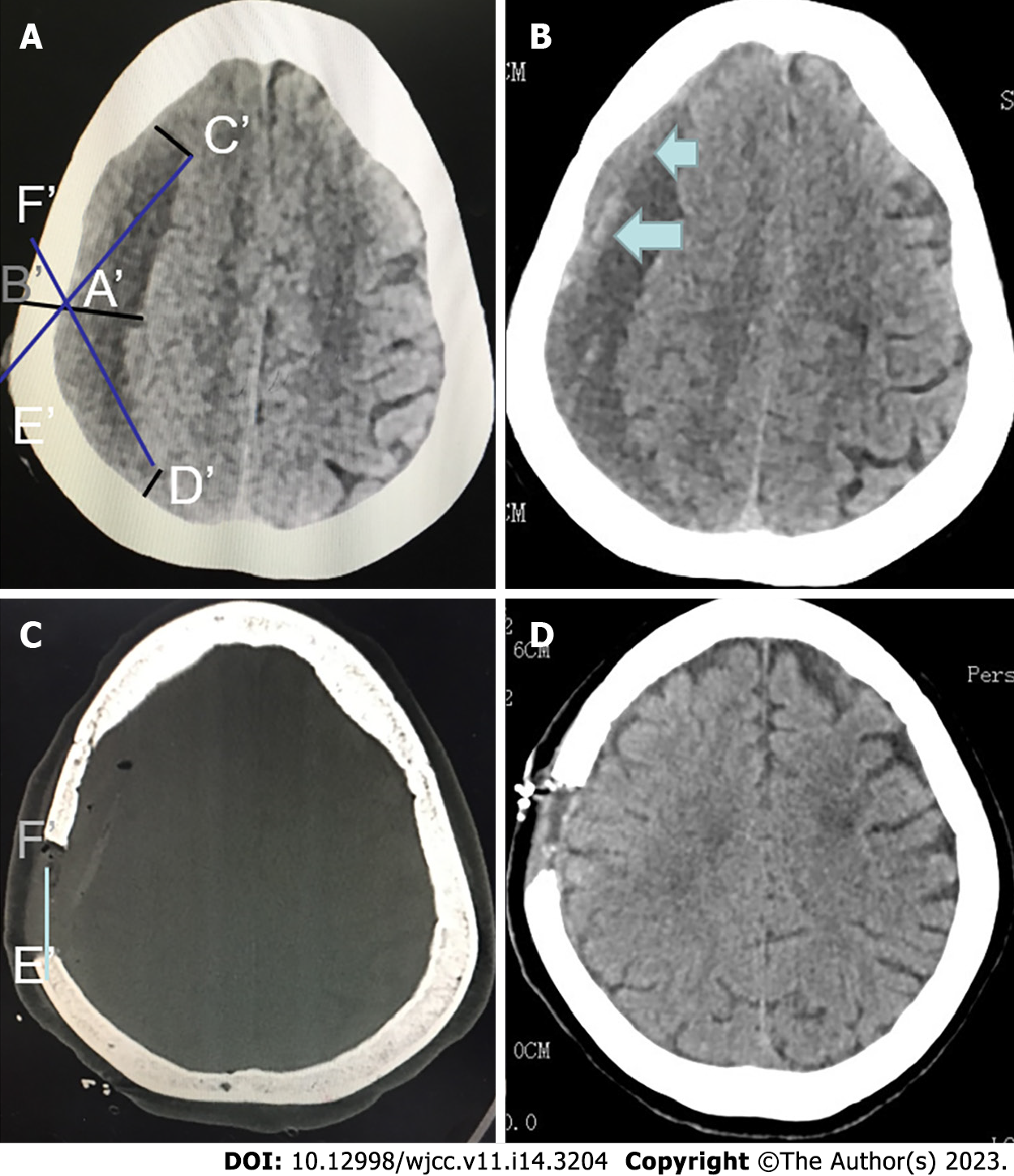
After the preoperative CT scan (Figure 1A and B), the thickest point of the hematoma was selected as the approximate location of the skull bone window for minimally invasive endoscopic surgery. (Figure 1A) On the thickest layer of the hematoma cavity in the CT image, make a straight line perpendicular to brain surface, which cross the skull at point A' at the inner side and point B' at the outer side. At the edge of the hematoma at the layer, find points C' and D' with distance to A' and B' equal to the thickness of the hematoma, respectively. Connect and extend C'A' and D'A', which intersect at points E' and F' with the surface of the skull, respectively (Figure 1A and C). The length and distance of E'F' were the optimal size and range of the bone window (Figure 1C and D). Similarly, the range of bone windows could also be found in the coronal position, so that a complete bone window can be formed.
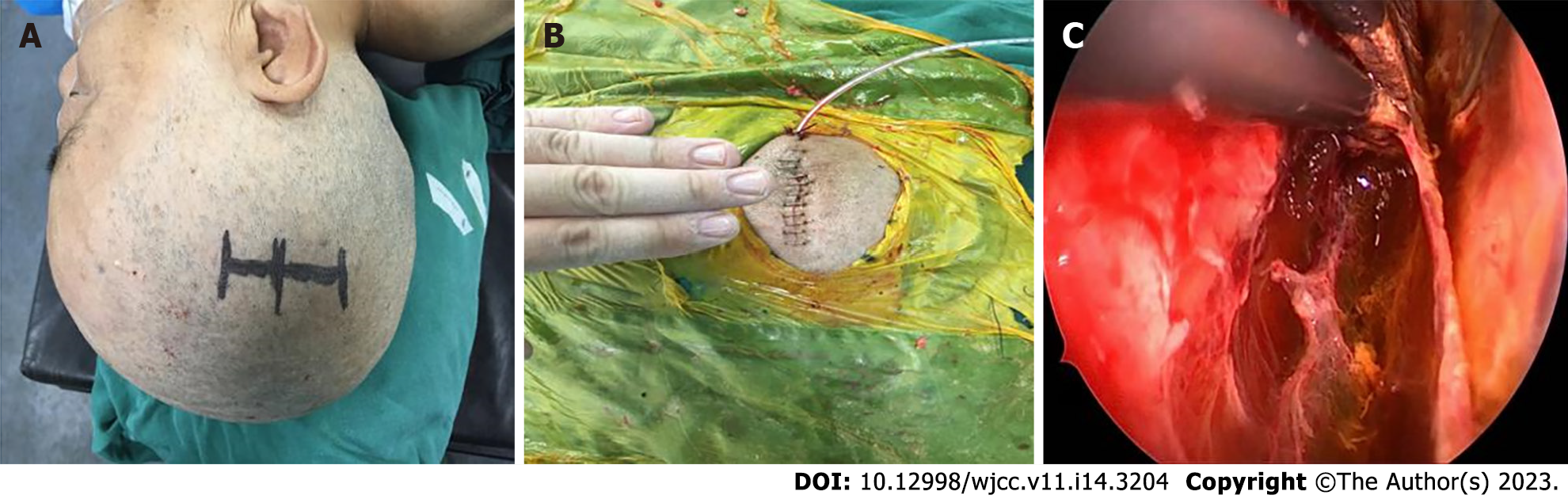
The operation was performed using a rigid Storz neuroendoscope with zero viewing angle along with special TV monitoring and video recording system, conventional endoscopic special surgical instruments and deep microsurgery instruments. In detail, (1) Place special locating marker on the surface of the scalp according to the range of bone window obtained previously; (2) Determine the position of the scalp incision; (3) Cut the scalp skin as needed and keep it open using an opener; (4) Drill a hole on the skull, and mill out the bone window using a milling cutter; (5) Radially cut the dura mater with a sharp knife; (6) Remove the hematoma and mechanized tissues using a attractor under the guide of the endoscope; (7) Coagulate the separating tissue using a bipolar electrocoagulation followed by suction, and if necessary, cut apart the separating tissue using scissors; (8) Once confirmed the surrounding area was cleaned under the direct vision of the endoscope, coagulate the local active bleeding points using a bipolar electrocoagulation method or special endoscopic bipolar coagulation; (9) Rinse the hematoma cavity repeatedly to make sure no further bleeding; and (10) At the end of the operation, place the drainage tube in the hematoma cavity under the direct view of the neuroendoscope, suture the dura mater, return the bone flap and suture the skin.
CT positioning and surgical planning methods can quickly and effectively determine the scope of surgical incisions and bone windows, effectively providing a surgical operation window for neuroendoscopic surgery. Figure 1 shows the design of bone window before head CT surgery for a female with cSDH. Figure 2 shows the results of surgical incision and intraoperative findings before and after minimally invasive surgery for this patient.
According to the surgical approach design, the surgeon can accurately design the optimal approach for minimally invasive endoscopic surgery, therefore achieving accurate positioning of the skull drilling site and accurate determination of the bone window size, and reducing the time for surgical preparation, anesthesia and operation. In this study, the average operation time was only about 1 h and the hematoma clearance rate was about 95%.
cSDH is one of the most common diseases in neurosurgery, with a prevalence rate of 8.2–13.1/100,000[9,10], accounting for about 10% of various intracranial hematomas. The disease is caused by many factors and can be treated using multiple methods including drilling drainage, cone craniotomy, and craniotomy hematoma removal. Among all cSDH patients, 25% have subdural hematoma and 14% bilateral hematoma. Studies have shown that head trauma is the main reason for the formation of cSDH, and is related to brain atrophy, low intracranial pressure and increased venous tension[6,11].
With the development of minimally invasive neuroendoscopic techniques and surgical instruments, cSDH can be visually and rapidly cleared via small bone hole and scalp incision through minimally invasive neuroendoscopic surgery[2]. As a minimally invasive, visual and rapid surgical treatment for direct removal of hematoma and capsule for cSDH patients, minimally invasive neuroendoscopic surgery has become an important alternative for surgical treatment of cSDH. Especially for those with muscularized and separated hematoma, it has advantage to avoid craniotomy of large bone flap[6].
Because the location, shape and size of intracranial hematoma in cSDH patients are different, it is necessary to design a personalized minimally invasive surgery for each cSDH patient. Designing a personalized minimally invasive surgical procedure is one of the key factors affecting the success of minimally invasive neuroendoscopic treatment of cSDH. At present, very few studies have investigated the design of minimally invasive neuroendoscopy for cSDH[1,7]. In traditional neurosurgery for cSDH, surgeons mainly determine the approximate location of cSDH and its surrounding structures based on the results of conventional CT scans, as well as physicians’ anatomic knowledge and experiences, thus selecting the point of drilling drainage or the incision of the craniotomy[3]. However, the position of the drilling drainage is required to be lower, and the traumatic lesions of craniotomy are large, while neuroendoscopy is required to be performed under the smallest minimally invasive incision to achieve the purpose of adequate surgical operation. Under the minimally invasive incision, sufficient surgical operation is achieved[8]. Therefore, in actual surgery, in order to avoid deviations in positioning, it is often necessary to make a relatively large scalp incision and bone window. Due to its large error, the traditional method often fails to meet the practical needs of precise positioning cSDH under a minimally invasive neuroendoscope[8]. With the development of imaging technology, stereotactic technology and neuron navigation system have become important means of neurosurgical positioning. However, stereotactic hematoma positioning needs a long time for surgery preparation. Although the neuron navigation system is a commonly used surgical positioning method in neurosurgery, it also needs many steps such as installing navigation and positioning frames, navigation registration and other steps before the operation, which also needs longer time for preparation[12,13]. In addition, stereotactic and neuron navigation techniques are only used to guide specific locations. For any incision and operational positioning, there is still need to design an adequate approach. Therefore, finding an accurate, reliable, intuitive, simple, fast, and inexpensive positioning method has become an important topic for minimally invasive neuroendoscopy for cSDH.
In order to explore a surgical positioning method for applying minimally invasive neuroendoscopy for cSDH, we firstly estimated the location and shape and its thickest part to the inner plate of the skull of cSDH based on the results of emergency CT. We then selected the CT at the thickest layer of the hematoma cavity to make a straight line perpendicular to the brain surface at the thickest part, which cross the skull at point A at the inner side and point B at the outer side. At the edge of the hematoma at the layer, the points C and D with distance to A and B equal to the thickness of the hematoma can be found, respectively. We then connected and extended CA and DA, which intersected at points E and F with the surface of the skull, respectively. The length and distance of EF were the optimal size and range of the bone window. In this study, we applied this technique for 42 cSDH patients and successfully completed the operation. The average operation time was only about 1 h, and the hematoma clearance rate was about 95%. Therefore, the positioning technology used in this study is simple and requires no stereotactic system, navigation system, no need to install a positioning head frame or a positioning frame before surgery. Over all, the technique needs shorter time for surgical preparation and anesthesia, and is less expensive, more flexible and more efficient, which not only saves the cost of hospitals and departments, but also reduces the medical expenses and economic burden of the patients.
Minimally invasive neuroendoscopy for cSDH as a minimally invasive surgery with high-efficiency, rapidness, reliable hemostasis and less bleeding in combination with CT imaging positioning will further improve the treatment efficacy and efficiency for cSDH. With the continuous improvement of neuroendoscopy and imaging technology, minimally invasive neuroendoscopy for cSDH will be more complete, accurate and popular.
Patients with Chronic Subdural Hematoma (CSH) can achieve good therapeutic effect by using our way to positioning and design to assist the operation of CSH according to CT scan and image, and our way is very useful and necessary.
This technology can be further promoted and applied in clinical practice, which will certainly achieve better clinical therapeutic effect, improve the curative effect of surgery, and be more minimally invasive and reasonable.
Patients with Chronic Subdural Hematoma (CSH) can achieve good therapeutic effect by using our way to positioning and design to assist the operation of CSH according to computed tomography (CT) scan and image, and our way is very useful and necessary.
We designed a new surgical incision method of neuroendoscopy under CT imaging technology for chronic subdural hematoma (cSDH), and achieved satisfactory therapeutic effect through the application of the above methods.
A minimally invasive surgical incision design suitable for cSDH under neuroendoscopy was designed by using neuroendoscopy technology, combined with the study of CT and other imaging technologies.
To study more convenient methods of surgical incision and bone window size for the treatment of cSDH under neuroendoscopy, so as to make the operation more minimally invasive.
The bone window for the treatment of cSDH under neuroendoscopy needs to vary from person to person; otherwise, the bone window may be too large or too small during the operation, which may affect the operation.
Neuroendoscopy is a very useful technique to CSH. But how to achieve the goal of treatment more minimally invasive? How can incisions be designed and positioned to be more minimally invasive?
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Senior Member of the American Society of Peripheral Neurosurgery.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Shariati MBH, Iran; Velnar T, Slovenia S-Editor: Liu XF L-Editor: A P-Editor: Yu HG
| 1. | Pevehouse BC, Bloom WH, Mckissock W. Ophthalmologic aspects of diagnosis and localization of subdural hematoma. An analysis of 389 cases and review of the literature. Neurology. 1960;10:1037-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Hashimoto N, Sakakibara T, Yamamoto K, Fujimoto M, Yamaki T. Two fluid-blood density levels in chronic subdural hematoma. Case report. J Neurosurg. 1992;77:310-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Masopust V, Netuka D, Häckel M. Chronic subdural haematoma treatment with a rigid endoscope. Minim Invasive Neurosurg. 2003;46:374-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Scotton WJ, Kolias AG, Ban VS, Crick SJ, Sinha R, Gardner A, Massey K, Minett T, Santarius T, Hutchinson PJ. Community consultation in emergency neurosurgical research: lessons from a proposed trial for patients with chronic subdural haematomas. Br J Neurosurg. 2013;27:590-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Ueba T, Yasuda M, Inoue T. Endoscopic burr hole surgery with a curettage and suction technique to treat traumatic subacute subdural hematomas. J Neurol Surg A Cent Eur Neurosurg. 2015;76:63-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Berhouma M, Jacquesson T, Jouanneau E. The minimally invasive endoscopic management of septated chronic subdural hematomas: surgical technique. Acta Neurochir (Wien). 2014;156:2359-2362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Callovini GM, Bolognini A, Callovini G, Gammone V. Primary enlarged craniotomy in organized chronic subdural hematomas. Neurol Med Chir (Tokyo). 2014;54:349-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Ducruet AF, Grobelny BT, Zacharia BE, Hickman ZL, DeRosa PL, Andersen KN, Sussman E, Carpenter A, Connolly ES Jr. Erratum to: The surgical management of chronic subdural hematoma. Neurosurg Rev. 2015;38:771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Asghar M, Adhiyaman V, Greenway MW, Bhowmick BK, Bates A. Chronic subdural haematoma in the elderly--a North Wales experience. J R Soc Med. 2002;95:290-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Kudo H, Kuwamura K, Izawa I, Sawa H, Tamaki N. Chronic subdural hematoma in elderly people: present status on Awaji Island and epidemiological prospect. Neurol Med Chir (Tokyo). 1992;32:207-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 200] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Haines DE, Harkey HL, al-Mefty O. The "subdural" space: a new look at an outdated concept. Neurosurgery. 1993;32:111-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Kim IS, Son BC, Lee SW, Sung JH, Hong JT. Comparison of frame-based and frameless stereotactic hematoma puncture and subsequent fibrinolytic therapy for the treatment of supratentorial deep seated spontaneous intracerebral hemorrhage. Minim Invasive Neurosurg. 2007;50:86-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Yadav YR, Ratre S, Parihar V, Bajaj J, Sinha M, Kumar A. Endoscopic Management of Chronic Subdural Hematoma. J Neurol Surg A Cent Eur Neurosurg. 2020;81:330-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |