Published online May 6, 2023. doi: 10.12998/wjcc.v11.i13.3010
Peer-review started: October 7, 2022
First decision: December 26, 2022
Revised: January 22, 2023
Accepted: March 31, 2023
Article in press: March 31, 2023
Published online: May 6, 2023
Processing time: 199 Days and 19.7 Hours
Malignant glaucoma, caused by aqueous misdirection, is a challenging post-surgical complication presented with normal/high intraocular pressure and shallowing of the central and peripheral anterior chambers. Its incidence is about 0.6%-4.0%. It can be secondary to filtering surgeries, laser iridotomy, and cataract surgery. Short axial length and a history of angle closure glaucoma are its main risk factors. Here, we report a bilateral malignant glaucoma with bullous keratopathy in the patient’s left eye.
We present a case of bilateral malignant glaucoma. The cause of malignant glaucoma for each eye of this patient was different. Hence, the management strategy and selection of surgical methods were also different. However, the normal anterior chamber was ultimately maintained, and maximum visual function was preserved. Even though the left eye received multiple surgeries and corneal endothelial decompensation occurred, the formation of a retroendothelial fibrous membrane partially compensated for the function of the corneal endothelium.
The formation of a retroendothelial fibrous membrane partially compensated for the function of the corneal endothelium.
Core Tip: Malignant glaucoma, caused by aqueous misdirection, is a challenging post-surgical complication presented with normal/high intraocular pressure and shallowing of the central and peripheral anterior chambers. Its incidence is about 0.6%-4.0%. It can be secondary to filtering surgeries, laser iridotomy, and cataract surgery. Short axial length and a history of angle closure glaucoma are its main risk factors. Here, we report bilateral malignant glaucoma with bullous keratopathy in the patient’s left eye. Interestingly, the best corrected visual acuity of the left eye improved to 20/70, and bullous keratopathy was relieved after the migration and implant of lens epithelial cells into the corneal endothelium.
- Citation: Ma YB, Dang YL. Bilateral malignant glaucoma with bullous keratopathy: A case report. World J Clin Cases 2023; 11(13): 3010-3016
- URL: https://www.wjgnet.com/2307-8960/full/v11/i13/3010.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i13.3010
Malignant glaucoma, caused by aqueous misdirection, is a challenging post-surgical complication presented with normal/high intraocular pressure (IOP) and shallowing of the central and peripheral anterior chambers. Its incidence is about 0.6%-4.0%[1-3]. It can be secondary to filtering surgeries, laser iridotomy, and cataract surgery. Short axial length and a history of angle closure glaucoma are its main risk factors[4,5]. Here, we reported bilateral malignant glaucoma with bullous keratopathy in the patient’s left eye. Interestingly, the best corrected visual acuity (BCVA) of her left eye improved to 20/70, and bullous keratopathy was relieved after the migration and implant of lens epithelial cells into the corneal endothelium.
A 36-year-old woman complained of “blurred vision in both eyes” and was diagnosed with chronic angle-closure glaucoma.
She received bilateral laser peripheral iridotomy and was prescribed with timolol, brimonidine, and brinzolamide twice per day and latanoprost daily. Her IOP remained high (38 mmHg-45 mmHg), and visual field worsened.
The patient did not report any past illnesses.
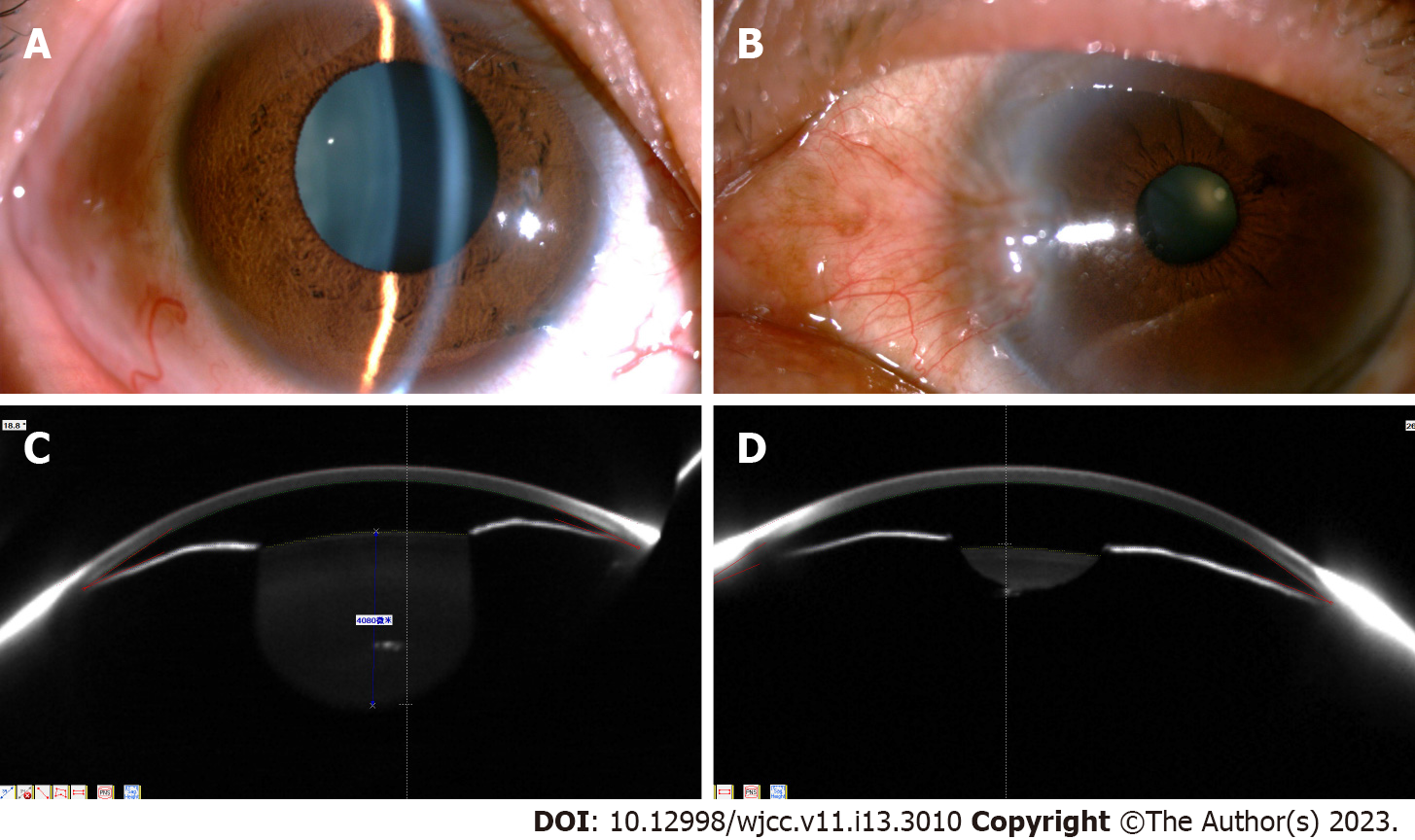
The BCVA of both eyes was 20/20. The central and peripheral anterior chambers were shallow. The anterior chamber angles were partially closed. The anterior chamber depth was 1.86 mm (OD) and 1.61 mm (OS). The pupil diameter was 6.5 mm (OD) and 2.8 mm (OS). The pupillary light reflex in her right eye was delayed (Figure 1). The lenses were transparent. Fundoscopy revealed that the right cup-disc ratio was 0.9, and the disc border was narrow. The left optic disc boundaries were clear and pale red in color. No apparent abnormalities were observed in either retina.
The right eye showed a tubular visual field. The contrast sensitivity in the left eye was decreased and accompanied with a visual field defect. The central corneal thicknesses were 535 µm (OD) and 556 µm (OS), and the IOPs were 45 mmHg (OD) and 40 mmHg (OS). The axial lengths were 19.55 mm (OD) and 19.59 mm (OS). The corneal endothelial cell density was 2488/mm2 (OD) and 2890/mm2 (OS).
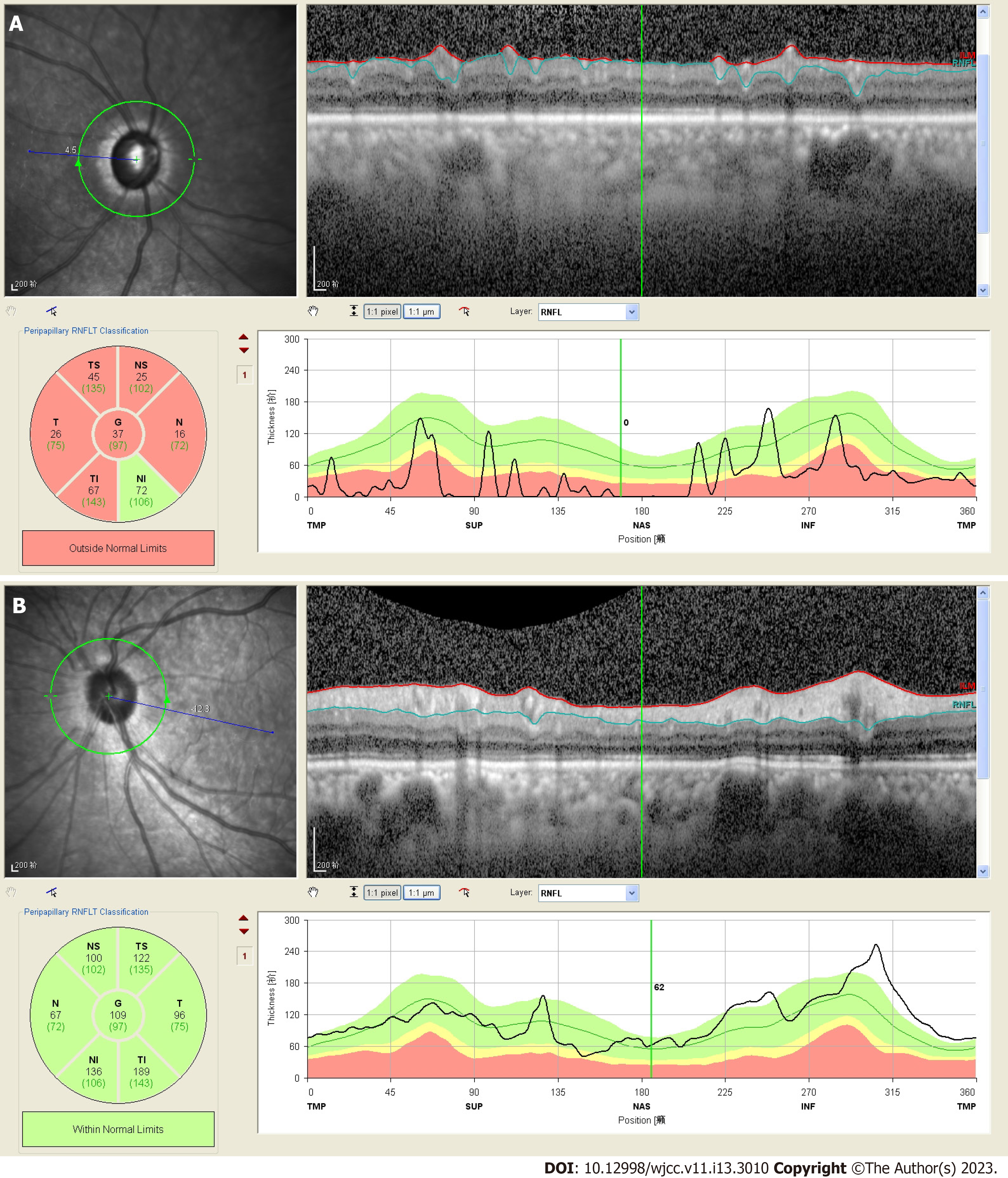
OCT revealed that the thickness of retinal nerve fiber layer in her right eye reduced (Figure 2A). No abnormality was observed in the left eye (Figure 2B).
Bilateral chronic angle-closure glaucoma
For her right eye, we performed cataract phacoemulsification combined with intraocular lens implantation. Postoperatively, the patient had a grade II shallow anterior chamber, intraocular lens protrusion, and IOP of 42 mmHg. We considered malignant glaucoma in her right eye. Therefore, periorbital injection of methylprednisolone and atropine mydriasis were continued for 3 d; however, there was no improvement. Subsequently, the patient underwent a capsulotomy and an anterior vitrectomy. Postsurgery, there was significant anterior chamber deepening with a central ACD of 2.89 mm and IOP fluctuating between 25 and 35 mmHg. Considering that this might be an instance of chronic angle closure in the right eye, we implanted an Ahmed drainage valve. Post-surgery, the IOP stabilized at 13 mmHg–19 mmHg, and BCVA was 20/25. The surgery had achieved the expected outcomes.
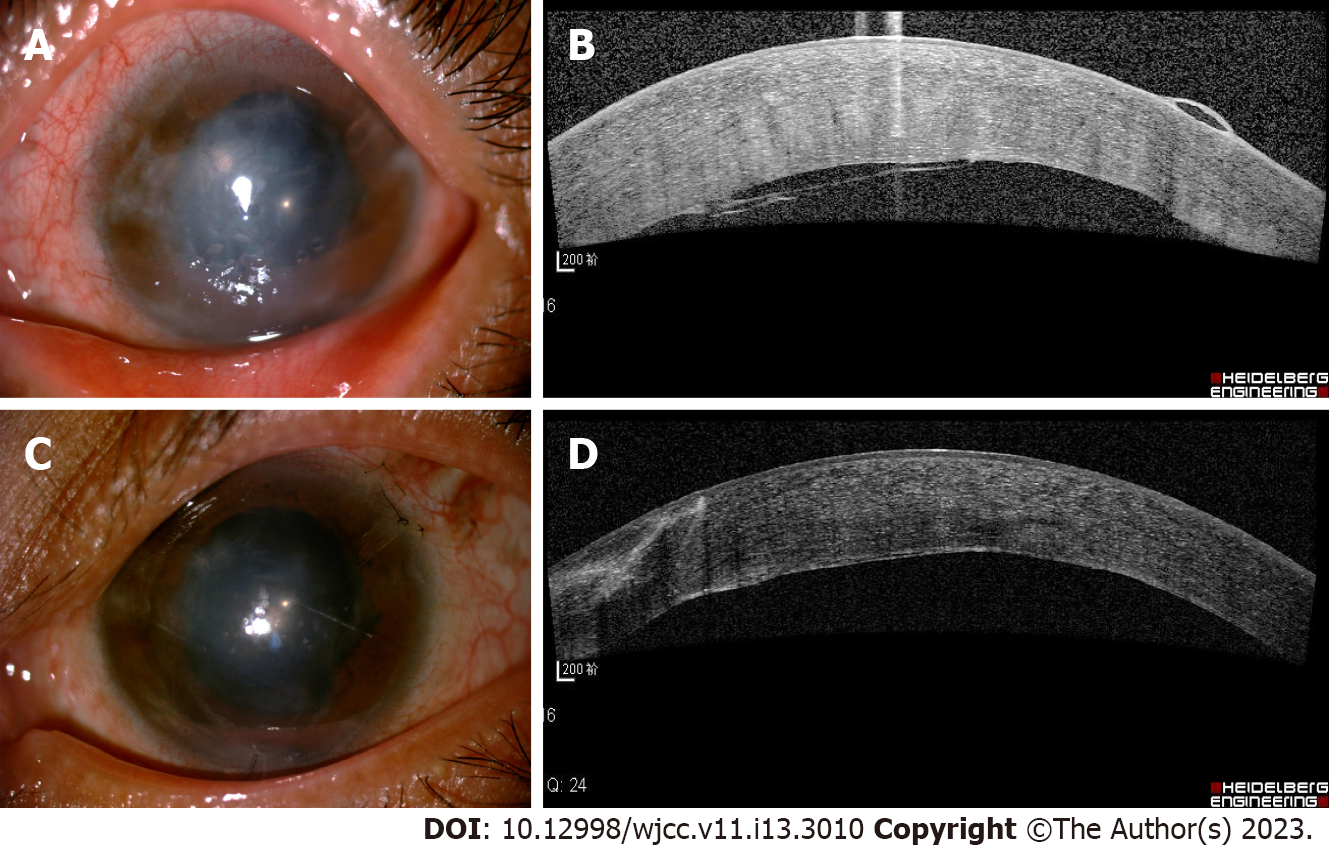
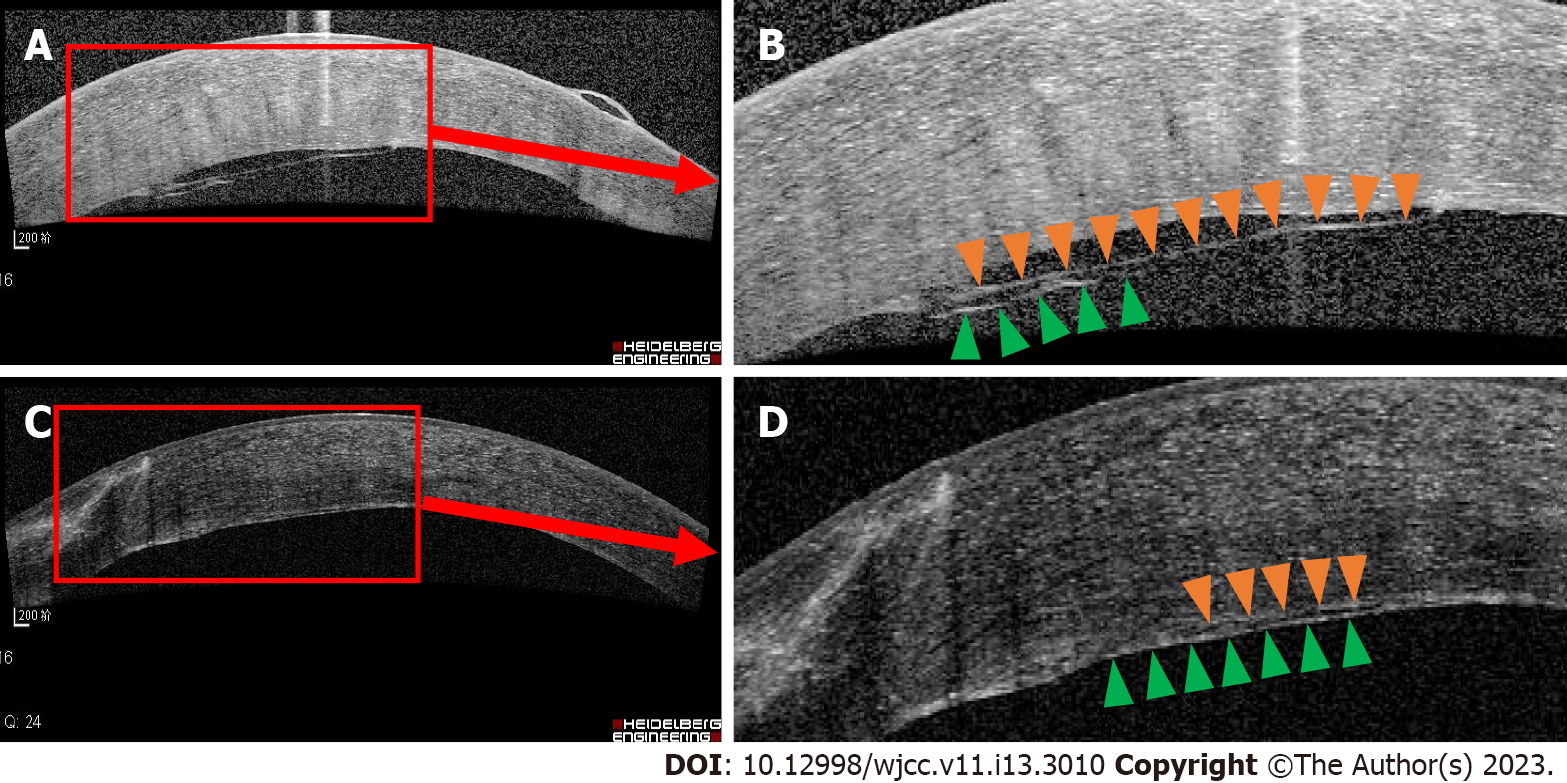
Due to the short axial length in her left eye and malignant glaucoma in her right eye, we performed cataract phacoemulsification and intraocular lens implantation, combined with anterior vitrectomy for her left eye. Post-surgery, the ACD was deepened at approximately 2.51 mm, but the IOP remained at 30 mmHg. One week later, considering the angle closure, we implanted an Ahmed valve. Postsurgery, the patient’s anterior chamber gradually disappeared, and a grade III shallow anterior chamber was detected. There was significant corneal edema. IOP was 20 mmHg, and the Seidel test was negative. We considered the malignant glaucoma in her left eye. We administered periorbital injections of methylprednisolone and atropine eye drops twice per day. However, these treatments were ineffective. A viscoelastic agent was injected into the anterior chamber, and laser posterior capsulotomy was performed. However, a grade III shallow anterior chamber recurred. A peripheral iridectomy with zonulo-capsulo-hyaloidotomy was performed through the pars plana route. Tension sutures were used to reform the anterior chamber. On day 2 postsurgery, the ACD was significantly increased to 2.75 mm. The IOP was 15 mmHg. One-week post-surgery, corneal edema was still significant, and bullous changes could be seen in the epithelium (Figure 3A). The OCT and slit lamp examination suggested that nearly one-third of the cornea endothelial detached, and a translucent membrane attached and stretched the endothelial layer (Figures 3B and 4A and B). The slit lamp revealed a bundle of white fibers that proliferated behind the corneal endothelium near the pupillary limbus at the 10 o’clock position and were pulling on the intraocular lens surface. Yttrium-aluminum-garnet (YAG) laser was immediately carried out to remove the proliferative fibers. We saw that fiber bundles had high tension and significant rebound.
Two weeks after the YAG laser, the corneal edema had abated, the corneal bullae had disappeared, and the peripheral cornea was transparent (Figure 3C). The OCT revealed that the corneal endothelial detachment gradually resolved, and a highly reflective membranous mass attached to the medial endothelial layer (Figures 3D and 4C and D). At the 1-year follow-up, the IOP was 17 mmHg, and ACD was normal. The peripheral cornea was partially transparent. The corneal edema persisted (central corneal thickness: 756 µm), and a fibrous proliferative membrane could be seen in the epithelium. The corneal epithelial bullae disappeared, and BCVA was 20/70.
We presented a case of bilateral malignant glaucoma after phacoemulsification and intraocular lens implantation combined with anterior vitrectomy and Ahmed drainage valve implantation. The patient’s right anterior chamber and IOP fully recovered after anterior vitrectomy. Although we realized that the incidence of malignant glaucoma in her left eye was extremely high and carried out anterior vitrectomy during stage I, grade III shallow anterior chamber and corneal lens contact still occurred, resulting in corneal endothelial decompensation. Fortunately, the anterior chamber reformed, and the IOP stabilized after peripheral iridectomy with zonulo-capsulo-hyaloidotomy and a tension suture fixation in the anterior chamber. It is worth noting that 1 wk after the left intraocular lens was disengaged from the cornea, a bundle of tense fiber formed between the lens surface and the corneal endothelium. This was suspected to be caused by epithelial cells migrating and transdifferentiating into myofibroblasts in the corneal endothelium. These myofibroblasts were attached to the surface of the corneal endothelium, blocking the entry of aqueous humor into the subcorneal epithelium and thereby preventing bullous keratopathy.
Short axial length and anterior segment crowding are risk factors for malignant glaucoma[6]. Wang et al[6] analyzed 1183 patients with angle-closure glaucoma and found that the axial length and ACD of those patients were significantly lower than of those with primary angle-closure glaucoma (axial length: 21.44 ± 1.18 mm vs 22.17 ± 0.97 mm, ACD: 2.12 ± 0.41 mm vs 2.49 ± 0.48 mm). As malignant glaucoma often involves both eyes[7,8], we performed a three-step lens resection combined with vitrectomy on the patient’s left eye[9]. First, the central vitreous was removed via the pars plana vitrectomy to relieve crowding of the anterior segment. Second, lens phacoemulsification, combined with intraocular lens implantation, was carried out. Finally, anterior vitrectomy was performed. Postsurgery, the anterior chamber was stable and deep, and aqueous misdirection did not occur. However, as the patient had chronic angle closure, the IOP remained high. Considering that trabeculectomy has poor results after vitrectomy, we opted for Ahmed drainage valve implantation[10].
Postsurgery, the patient had a grade III shallow anterior chamber and intraocular lens protrusion, with the intraocular lens coming into contact with the corneal endothelium. Although the IOP did not exceed 21 mmHg, aqueous misdirection had occurred. The diagnosis of malignant glaucoma was confirmed. The reasons for this were: (1) Excessive aqueous humor drainage after valve implantation; and (2) Postoperative inflammation promotes the proliferation, adhesion, and transformation of lens epithelial cells and ciliary body edema, resulting in a blockage of normal aqueous humor circulation[11]. Therefore, we carried out a peripheral iridectomy with zonulo-capsulo-hyaloidotomy at the 6 o’clock position, combined with tension suture fixation in the anterior chamber[12]. The postoperative anterior chamber reformation was good.
After the malignant glaucoma occurred, the patient’s left eye developed a grade III shallow anterior chamber, the intraocular lens was in contact with the corneal endothelium, and there was moderate-severe inflammation[11]. Inflammatory responses can promote epithelial-mesenchymal transformation of the lens epithelial cell[13]. In this patient, a fibrous bundle could be seen between the corneal endothelium and surface of the intraocular lens at the 10 o’clock 1 wk after anterior chamber reformation had been successfully performed.
The fibrous bundle had tension between the corneal endothelium and the intraocular lens. It pulled the corneal endothelium, resulting in partial detachment. After the fibrous bundle was resected with YAG laser, the corneal endothelium and fibrous membrane gradually attached to the posterior surface of the cornea. Even though the density of corneal endothelial cells was low (797 cells/mm2) and the function was poor (6A cells accounted for 20%), the corneal bullae gradually disappeared, together with the formation of the fibrous membrane behind the corneal endothelium. This suggests that fibrous membrane derived from lens epithelial transformation is somewhat impermeable to water.
We presented a case of bilateral malignant glaucoma. The cause of malignant glaucoma for each eye of this patient was different. Hence, the management strategy and selection of surgical methods were also different. However, the normal anterior chamber was ultimately maintained, and maximum visual function was preserved. Even though the left eye received multiple surgeries and corneal endothelial decompensation occurred, the formation of a retroendothelial fibrous membrane partially compensated for the function of the corneal endothelium.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tanabe S, Japan; Wang WW, China S-Editor: Liu GL L-Editor: Filipodia P-Editor: Liu GL
| 1. | Chandler PA, Simmons RJ, Grant WM. Malignant glaucoma. Medical and surgical treatment. Am J Ophthalmol. 1968;66:495-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 61] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Simmons RJ. Malignant glaucoma. Br J Ophthalmol. 1972;56:263-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Lowe RF. Clinical types of primary angle closure glaucoma. Aust N Z J Ophthalmol. 1988;16:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Jacoby B, Reed JW, Cashwell LF. Malignant glaucoma in a patient with Down's syndrome and corneal hydrops. Am J Ophthalmol. 1990;110:434-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Cohen RG, Wu HK, Schuman JS. Glaucoma with inflammatory precipitates on the trabecular meshwork: a report of Grant's syndrome with ultrasound biomicroscopy of precipitates. J Glaucoma. 1996;5:266-270. [PubMed] |
| 6. | Wang M, Tan Q, Jiang H, Xia X, Wang P, Jiang J, Liu D. [Clinical analysis of malignant glaucoma after glaucoma surgery]. Zhongnan Daxue Xuebao Yixueban. 2015;40:543-548. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Saunders PP, Douglas GR, Feldman F, Stein RM. Bilateral malignant glaucoma. Can J Ophthalmol. 1992;27:19-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Stan C. Glaucom malign bilateral--prezentare de caz. Conexiuni Medicale. 2007;45-47. [DOI] [Full Text] |
| 9. | Sharma A, Sii F, Shah P, Kirkby GR. Vitrectomy-phacoemulsification-vitrectomy for the management of aqueous misdirection syndromes in phakic eyes. Ophthalmology. 2006;113:1968-1973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Wang MH, Li QM, Dong HT, Dong SQ, Li Y, Zheng CY. Ahmed Valves vs Trabeculectomy Combined with Pans Plana Vitrectomy for Neovascular Glaucoma with Vitreous Hemorrhage. Eur J Ophthalmol. 2017;27:774-780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Martínez-de-la-Casa JM, García-Feijoó J, Castillo A, Polo V, Larrosa JM, Pablo L, García-Sánchez J. Glaucoma maligno tras cirugía combinada de implante valvular de Ahmed y facoemulsificación en el tratamiento del glaucoma crónico por cierre angular. Arch Soc Esp Oftalmol. 2005;80:11. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Balekudaru S, Choudhari NS, Rewri P, George R, Bhende PS, Bhende M, Lingam V, Lingam G. Surgical management of malignant glaucoma: a retrospective analysis of fifty eight eyes. Eye (Lond). 2017;31:947-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Gao L, Zhou Y, Li N, Liu X, Cong F. Lens Epithelial Mesenchymal Transition and Long-Term Chronic Inflammation After Cataract Surgery. Ocul Immunol Inflamm. 2022;1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |