Published online May 6, 2023. doi: 10.12998/wjcc.v11.i13.2992
Peer-review started: January 9, 2023
First decision: February 20, 2023
Revised: March 8, 2023
Accepted: March 27, 2023
Article in press: March 27, 2023
Published online: May 6, 2023
Processing time: 105 Days and 21.8 Hours
Total shoulder arthroplasty (TSA) results in a large amount of perioperative blood loss due to severe trauma.
To investigate the safety and efficacy of intravenous tranexamic acid (TXA) in TSA.
We searched the PubMed, Cochrane Library, Embase and Web of Science databases for randomized controlled trials (RCTs) on the use of TXA in TSA. And all the results were checked and assessed by Reference Citation Analysis (https://www.referencecitationanalysis.com/). A meta-analysis was performed with Review Manager 5.3 to calculate the odds ratio (OR) or weighted mean difference (WMD) of related outcome indicators.
A total of 5 RCTs with level 1 evidence were included. There were 369 cases, with 186 in the TXA group and 183 in the placebo group. The meta-analysis showed that TXA can significantly reduce total blood loss during the perioperative period [WMD = -249.56, 95% confidence interval (CI): -347.6 to -151.52, P < 0.0001], and the incidence of adverse reactions was low (OR = 0.36, 95%CI: 0.16-0.83, P = 0.02). Compared with the placebo group, the TXA group had significantly less total haemoglobin loss (WMD = -34.39, 95%CI: -50.56 to -18.22), less haemoglobin fluctuation before and after the operation (WMD = -0.6, 95%CI: -0.93 to -0.27) and less 24-h drain output (WMD = -136.87, 95%CI: -165.87 to -106.49). There were no significant differences in the operation time (P = 0.11) or hospital length of stay (P = 0.30) between the two groups.
The application of intravenous TXA in the perioperative period of TSA can significantly reduce the total volume of perioperative blood loss and reduce the incidence of adverse reactions, so TXA is worthy of widespread clinical use.
Core Tip: The development and application of total shoulder arthroplasty (TSA) have been slower than those of total knee and total hip arthroplasty, and there is still a lack of advanced evidence-based evidence about the application of tranexamic acid (TXA) in the perioperative period of TSA. Therefore, a meta-analysis was conducted to determine the efficacy and safety of intravenous TXA in the perioperative period of TSA.
- Citation: Deng HM. Efficacy and safety of intravenous tranexamic acid in total shoulder arthroplasty: A meta-analysis. World J Clin Cases 2023; 11(13): 2992-3001
- URL: https://www.wjgnet.com/2307-8960/full/v11/i13/2992.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i13.2992
Total shoulder arthroplasty (TSA) is commonly used in the treatment of end-stage rotator cuff arthropathy, irreparable rotator cuff tears, primary glenohumeral arthritis and traumatic shoulder arthritis[1-4]. When conservative treatment methods, such as analgesic drugs, local hormone injections, and physical therapy, cannot relieve pain or improve shoulder joint range of motion, TSA often significantly relieves pain, improves the range of motion and improves the quality of life of patients[5-7]. Due to developments and improvements in shoulder replacement medical technology and replacement materials, the number of total shoulder replacements is increasing[8]. Studies have shown that the volume of intraoperative blood loss during total shoulder replacement can reach between 354 mL and 361 mL[9,10]. For patients undergoing primary TSA, the probability of blood transfusion is between 2.4% and 9.5%[11,12]. The presence of anaemia and the need for blood transfusion after surgery may increase the incidence of complications. Common complications include angina pectoris, myocardial infarction, thrombosis, and even death[13,14].
Tranexamic acid (TXA) is a fibrinolytic inhibitor that can reversibly block the binding site of lysine, which is compatible with fibrinogen; inhibit fibrinolytic reactions; prevent blood clots from being dissolved by fibrinolytic enzymes; and reduce the extent of perioperative bleeding[15,16]. TXA has been shown to significantly reduce the amount of blood loss in total knee and total hip arthroplasty[17-19]. Therefore, TXA has been widely used in total joint replacement for perioperative blood management. However, because the development and application of TSA have been slower than those of total knee and total hip arthroplasty, there is still a lack of advanced evidence-based evidence about the application of TXA in the perioperative period of TSA. Therefore, through the inclusion of high-quality randomized controlled trials (RCTs), a meta-analysis was conducted to determine the efficacy and safety of intravenous TXA in the perioperative period of TSA, thereby providing a high-quality evidence-based basis for clinical application.
This meta-analysis was conducted in strict accordance with the preferred reporting items for systematic reviews and meta-analyses statement[20]. All the data used in this study are provided in the text and Supplementary materials.
PubMed, Embase, Cochrane Library and Web of Science were searched. The retrieval time was from the establishment of each database to November 15, 2022. The combination of MeSH terms and entry words to search the above four databases was used. The key words included "tranexamic acid", "tranexamic acid", "antimicrobial agents", "cyklokapron", "transamin", "total shoulder arthroplasty", "total shoulder replacement" and "shoulder replacement arthroplasty". Additionally, Reference Citation Analysis (https://www.referencecitationanalysis.com/) was used to check and supplement the search results. Supplementary material includes the search strategy used for each database.
The inclusion criteria were as follows: (1) All patients were treated with TSA or reverse TSA; (2) the experimental group was treated with intravenous TXA, and the control group was treated with a placebo; (3) the type of study was an RCT; and (4) one of the following outcome measures were reported: Total blood loss, adverse events, operative time, total haemoglobin loss, hospital length of stay, change in haemoglobin level and 24-h drain output. There were no language restrictions.
The exclusion criteria were as follows: (1) Studies with incomplete original data; and (2) duplicate studies including the same population.
The extracted data included basic information (first author, year of publication, country, research type, sample size, age, etc.), the primary outcome indicators, the secondary outcome indicators, and information related to the quality of the study.
The primary outcomes were as follows: Total blood loss and adverse events. The secondary outcomes were as follows: Operative time, total haemoglobin loss, hospital length of stay, change in haemoglobin level, and 24-h drain output.
Version 2.0 (Rob 2.0) of the risk of bias assessment tool recommended by Cochrane was used to evaluate the quality of the studies[21]. The evaluation tool evaluates the risk of bias in five areas. If the evaluation results of all five areas are low risk, then the overall risk of bias is low. If the assessment result of any one of the areas is high risk or the assessment results of multiple areas are possible risk, then the overall risk level is high.
Review Manager 5.3 software (Cochrane Collaboration, United Kingdom) was used for data analysis. The continuous variables are represented by weighted mean differences (WMDs) and 95% confidence intervals (CIs), while the categorical variables are represented by odds ratios (ORs) and 95%CIs. P < 0.05 was considered statistically significant. I2 was used to evaluate the heterogeneity of the consolidated data. I2 < 50% indicated low heterogeneity, and a fixed-effects model was used for these data; I2 > 50% indicated high heterogeneity, and a random-effects model was used for these data. The latter group of results should be interpreted carefully. Stata 14.0 software was used to perform Egger’s and Begg’s tests to quantitatively evaluate publication bias for the outcome indicators with data retrieved from 3 or more articles.
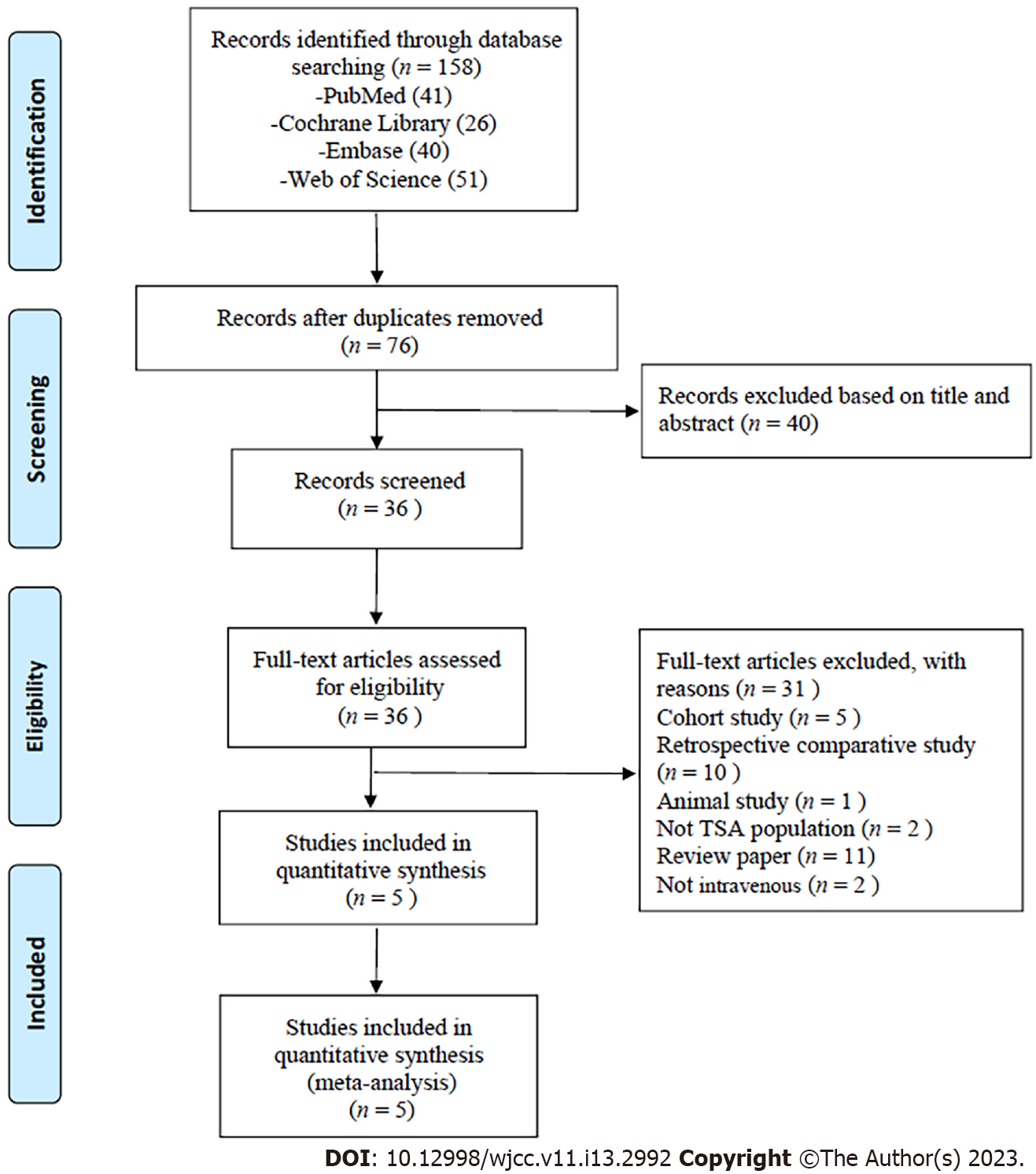
A total of 158 articles were retrieved, including 41 from PubMed, 26 from Cochrane Library, 40 from Embase and 51 from Web of Science. After duplicate studies were excluded and the full texts were read, five articles were included. The process of literature retrieval and the reasons for exclusion are shown in Figure 1. This meta-analysis included five RCTs[22-26] from four countries, two[23,26] of which were from the United States. The clinical evidence level of 5 studies[22-26] was 1. A total of 369 cases were included, including 186 cases in the experimental group and 183 cases in the placebo group. Among the five RCTs, only two studies reported that in the trial group and the placebo group, blood transfusion was needed due to excessive blood loss[25,26]. The basic characteristics of the studies included in this study are shown in Table 1.
| Ref. | Country | Study design (LOE) | Sample | Average age, yr | Intervention | Surgery | Transfusion | ||||
| TXA | Placebo | TXA | Placebo | TXA | Placebo | TXA | Placeo | ||||
| Cunningham et al[22], 2021 | Switzerland | RCT (Level I) | 31 | 29 | 72 ± 8 | 73 ± 9 | TXA, 2 g, IV | An equivalent volume of NS, IV | TSA or RTSA | 0 | 0 |
| Cvetanovich et al[23], 2018 | United States | RCT (Level I) | 52 | 56 | 67.7 ± 10.9 | 65.2 ± 9.2 | TXA, 1 g, IV | An equivalent volume of NS, IV | TSA | 0 | 0 |
| Pauzenberger et al[24], 2017 | Austria | RCT (Level I) | 27 | 27 | 70.3 ± 9.3 | 71.3 ± 7.9 | 100 mL NS infused with 1 g of TXA, IV | 100 ml NS, IV | TSA or RTSA | 0 | 0 |
| Garcia et al[25], 2022 | Portugal | RCT (Level I) | 23 | 22 | 76.7 ± 7.1 | 75.7 ± 5.7 | TXA, 1 g, IV | Without the TXA infusion | TSA or RTSA | 3 | 2 |
| Vara et al[26], 2017 | United States | RCT (Level I) | 53 | 49 | 67 ± 9 | 66 ± 9 | TXA, 10 mg/kg, IV | An equivalent volume of NS, IV | RTSA | 3 | 7 |
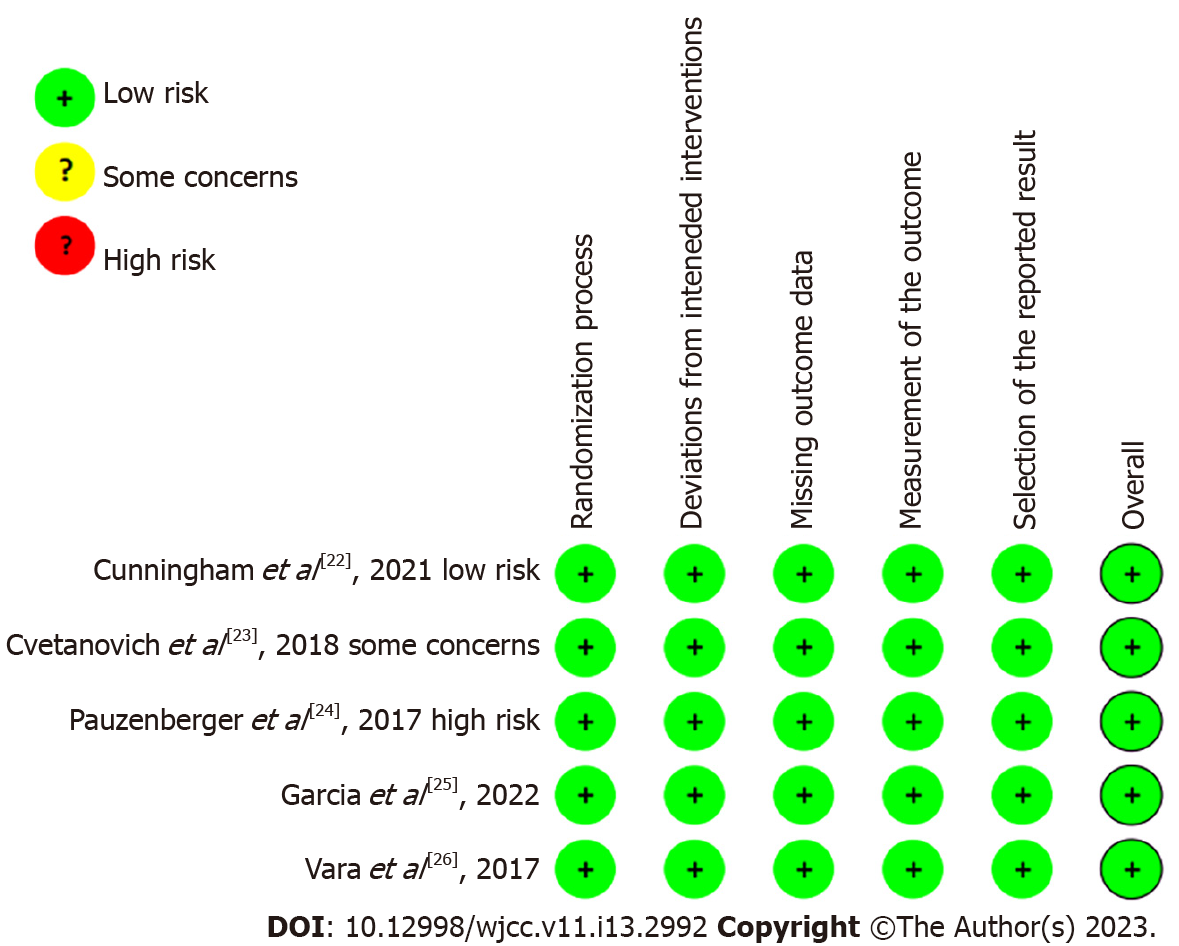
In this study, the Cochrane randomized controlled trial risk of bias assessment tool 2.0 was used to evaluate the quality of the 5 included articles. All five articles[22-26] were considered to have a low risk of bias. The above results of the literature quality evaluation showed that the methodological quality of the five studies[22-26] included in this study was very high. All the included studies used the double-blinding method for clinical research. The risk of bias results for each study are shown in Figure 2.
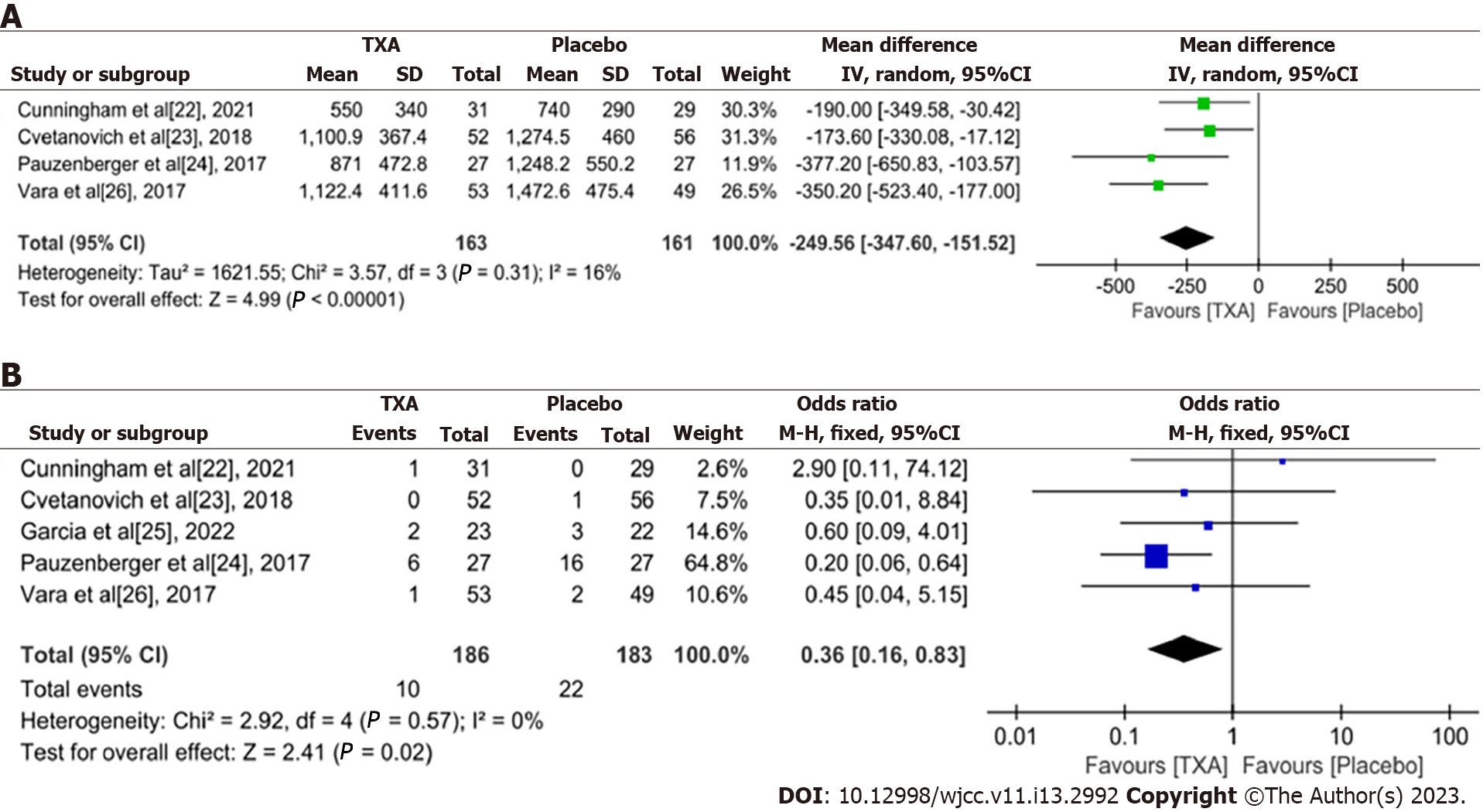
Total blood loss (mL): Four RCTs[22-24,26] reported total blood loss in TSA. There were 163 cases in the experimental group and 161 cases in the placebo group. The heterogeneity among the studies was large (P = 0.31, I2 = 16%), and a fixed-effects model was used for meta-analysis. The results showed that there was a significant difference in the total amount of bleeding between the two groups [weighted mean difference (WMD) = -249.56, 95%CI: -347.6 to -151.52, P < 0.0001], which indicated that TXA can significantly reduce bleeding in TSA (Figure 3A).
Adverse events: All the included studies[22-26] reported the occurrence of adverse reactions. There was no heterogeneity among the studies (P = 0.57, I2 = 0%), so a fixed-effects model was used. The meta-analysis showed that compared with the placebo group, the TXA group had significantly fewer adverse events and higher safety (OR = 0.36, 95%CI: 0.16-0.83, P = 0.02) (Figure 3B).
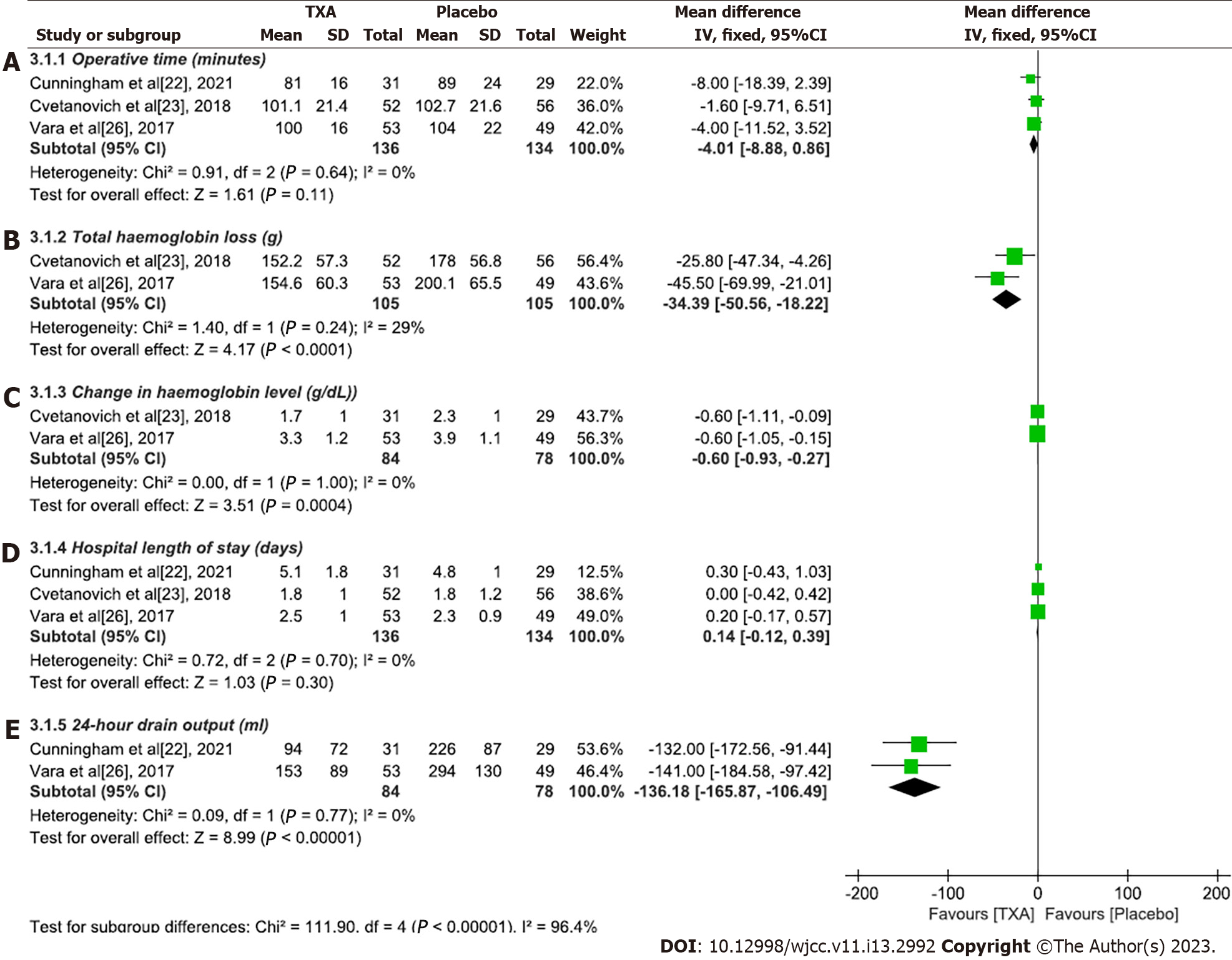
Operative time (minutes): Three studies[22,23,26] compared operation time. There was no heterogeneity among the studies (P = 0.64, I2 = 0%), so a fixed-effects model was used for statistical analysis. The results showed that there was no significant difference in operation time between the experimental group and the placebo group (WMD = -4.01, 95%CI: -8.88 to 0.86, P = 0.11) (Figure 4A).
Total haemoglobin loss (g): Two studies[23,26] compared total haemoglobin loss between the experimental and placebo groups. The heterogeneity between the two studies was small (P = 0.24, I2 = 29%), so a fixed-effects model was used for analysis. The meta-analysis showed that the experimental group had less haemoglobin loss than did the placebo group (WMD = -34.39, 95%CI: -50.56 to -18.22, P < 0.0001) (Figure 4B).
Change in haemoglobin level (g/dL): Two studies[23,26] compared haemoglobin levels before and after TSA. There was no heterogeneity among the three studies (P = 1.00, I2 = 0%), so a fixed-effects model was used for analysis. The results of the meta-analysis showed that the haemoglobin level of the experimental group fluctuated less before and after the operation (WMD = -0.6, 95%CI: -0.93 to -0.27, P < 0.0001), which indicated that TXA could significantly reduce bleeding in shoulder replacement patients (Figure 4C).
Hospital length of stay (days): A fixed-effects model was used to analyse the length of stay data of three studies[22,23,26] (P = 0.70, I2 = 0%). The results showed that there was no statistically significant difference in the length of hospital stay between the experimental group and the placebo group (WMD = 0.14, 95%CI: -0.12 to 0.39, P = 0.30) (Figure 4D).
Twenty-four-hour drain output (mL): A total of two studies[22,26] compared the 24-h postoperative drainage volume between the experimental group and the placebo group. The homogeneity of the two studies was good (P = 0.77, I2 = 0%), so a fixed-effects model was used for analysis. The results showed that the 24-h drainage volume of the experimental group was significantly less than that of the placebo group, indicating that TXA can reduce the drainage volume after TSA (WMD = -136.87, 95%CI: -165.87 to -106.49, P < 0.0001) (Figure 4E).
Begg’s and Egger’s tests were performed to assess the publication bias of the studies. No evidence of publication bias was found for the WMD of total blood loss (Begg’s test, P = 0.734, Egger’s test, P = 0.634) or the OR of adverse events (Begg’s test, P = 0.734, Egger’s test, P = 0.379). There was no publication bias in the WMD of the hospital length of stay or operational time. The statistical results of publication bias of each index are shown in Supplementary Table 1.
Whether TXA, an antifibrinolytic agent, can effectively reduce perioperative blood loss without increasing the risk of adverse reactions in TSA still lacks high-level evidence-based support. The efficacy of TXA in various surgical procedures has been confirmed, and it has higher efficacy and leads to fewer drug-related complications than other antifibrinolytic drugs[27,28]. The purpose of this study was to investigate the efficacy of intravenous TXA in reducing bleeding and its safety in the perioperative period of TSA through a meta-analysis of high-quality RCTs with level 1 evidence. The results of this meta-analysis showed that the application of intravenous TXA in TSA can not only significantly reduce the total volume of perioperative blood loss but is also safe. The secondary outcome measures also showed that TXA can significantly reduce total haemoglobin loss, reduce fluctuations in haemoglobin levels before and after the operation, and reduce postoperative drainage. In addition, this study showed that compared with a placebo, intravenous TXA did not significantly differ in terms of operation time or length of hospital stay. This study showed that TXA can significantly reduce the total volume of perioperative blood loss in TSA. Studies[29,30] have shown that TXA is a lysine derivative that cannot convert fibrinolysin into activated fibrinolysin by occupying the action site of fibrinolysin and cannot dissolve blood clots to promote haemostasis. With the widespread application of TXA in joint surgery[31,32], especially in total knee and total hip arthroplasty, the good haemostatic effect and safety of TXA have been recognized by the majority of scholars. Abildgaard et al[33] reviewed 168 cases and found that TXA can significantly reduce perioperative blood loss, haemoglobin fluctuations and postoperative drainage volume. Clay et al[34] analysed the blood loss and haematocrit of 435 patients who underwent shoulder replacement, and the results confirmed the above conclusion.
In addition, based on the secondary outcome indicators that were compared between the intravenous TXA and placebo groups, the perioperative application of TXA can significantly reduce the total haemoglobin loss, reduce the absolute value of haemoglobin fluctuations before and after surgery, and reduce the amount of postoperative drainage. These findings also confirm that intravenous TXA can reduce perioperative bleeding in TSA, which is very important. Operation time and blood loss are two factors that affect each other. An increase in the operation time increases the wound exposure time and blood loss. An increase in blood loss increases the operation time. The meta-analysis showed that there was no significant difference in the operation time between the intravenous TXA group and the placebo group. When the influence of operation time is excluded, the haemostatic effect of TXA can be more accurately assessed. The results of this meta-analysis also suggest that intravenous TXA is not an influencing factor of the length of hospital stay.
In terms of safety, TXA led to fewer adverse reactions than placebo (OR = 0.36, 95%CI: 0.16-0.83). By reviewing the 5 included studies[22-26], this study found that the main adverse reaction of TXA was haematoma, and no severe adverse reactions were reported. In contrast, adverse reactions such as skin allergies, haematoma and deep vein thrombosis occurred in the placebo group. Carbon et al[35] retrospectively analysed the data of 71174 patients retrieved from a national claims database and found that the use of TXA was not associated with an increased incidence of complications in patients who underwent TSA. Our results are consistent with those of Carbon et al[35], which supports the widespread use of TXA in the perioperative period of TSA.
The conclusions of this systematic review and meta-analysis come from only RCTs with level 1 evidence, and the heterogeneity was very low, which indicates that the above conclusions are supported by a very high level of evidence. This meta-analysis showed that TXA is efficacious and safe in TSA to a certain extent, but there are some limitations of the study: (1) Although the quality of the RCTs included in this meta-analysis was high, the total number of included studies and total sample size were relatively small; and (2) data on the total haemoglobin loss and 24-h drainage volume were retrieved from only two studies, which may have affected the reliability of the results. There is no doubt that multicentre, large-sample prospective RCTs are needed in the future to further verify the findings of this study. In TSA, the impact of intravenous TXA on medical costs and patient satisfaction in the postoperative period should also be evaluated, which will be conducive to comprehensive evaluation of the clinical value of intravenous TXA.
Our meta-analysis revealed that the application of intravenous TXA can significantly reduce total blood loss and is safe for application in TSA, so TXA is worthy of widespread clinical application. In addition, we also found that the application of TXA did not influence the operation time or length of hospital stay.
Total shoulder arthroplasty (TSA) results in a large amount of perioperative blood loss due to severe trauma.
Therefore, through the inclusion of high-quality randomized controlled trials (RCTs), a meta-analysis was conducted to determine the efficacy and safety of intravenous tranexamic acid (TXA) in the perioperative period of TSA, thereby providing a high-quality evidence-based basis for clinical application.
The purpose of this meta-analysis was to investigate the safety and efficacy of intravenous TXA in TSA.
Meta-analysis.
A total of 5 RCTs with level 1 evidence were included. There were 369 cases, with 186 in the TXA group and 183 in the placebo group. The meta-analysis showed that TXA can significantly reduce total blood loss during the perioperative period [WMD = -249.56, 95% confidence interval (CI): -347.6 to -151.52, P < 0.0001], and the incidence of adverse reactions was low (OR = 0.36, 95%CI: 0.16-0.83, P = 0.02). Compared with the placebo group, the TXA group had significantly less total haemoglobin loss (WMD = -34.39, 95%CI: -50.56 to -18.22), less haemoglobin fluctuation before and after the operation (WMD = -0.6, 95%CI: -0.93 to -0.27) and less 24-h drain output (WMD = -136.87, 95%CI: -165.87 to -106.49). There were no significant differences in the operation time (P = 0.11) or hospital length of stay (P = 0.30) between the two groups.
The application of intravenous TXA in the perioperative period of TSA can significantly reduce the total volume of perioperative blood loss and reduce the incidence of adverse reactions, so TXA is worthy of widespread clinical use.
Multicentre, large-sample prospective RCTs are needed in the future to further verify the findings of this study. In TSA, the impact of intravenous TXA on medical costs and patient satisfaction in the postoperative period should also be evaluated, which will be conducive to comprehensive evaluation of the clinical value of intravenous TXA.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arslan M, Turkey; Mahmoud MZ, Saudi Arabia S-Editor: Li L L-Editor: A P-Editor: Yu HG
| 1. | Kazley JM, Cole KP, Desai KJ, Zonshayn S, Morse AS, Banerjee S. Prostheses for reverse total shoulder arthroplasty. Expert Rev Med Devices. 2019;16:107-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Kim DM, Alabdullatif F, Aldeghaither M, Shin MJ, Kim H, Park D, Kholinne E, Jeon IH, Koh KH. Do Modern Designs of Metal-Backed Glenoid Components Show Improved Clinical Results in Total Shoulder Arthroplasty? Orthop J Sports Med. 2020;8:2325967120950307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | An KY, Park JY, Yoon TR. Subscapularis-sparing deltopectoral approach in reverse total shoulder arthroplasty. Int Orthop. 2022;46:2845-2851. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Hagen MS, Allahabadi S, Zhang AL, Feeley BT, Grace T, Ma CB. A randomized single-blinded trial of early rehabilitation vs immobilization after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2020;29:442-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 5. | Baumgarten KM. Is stemless total shoulder arthroplasty indicated in elderly patients? J Shoulder Elbow Surg. 2023;32:260-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | O'Keefe DS, Hao KA, Teurlings TL, Wright TW, Wright JO, Schoch BS, Farmer KW, Struk AM, King JJ. Survivorship analysis of revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 7. | Schiffman CJ, Prabhakar P, Hsu JE, Shaffer ML, Miljacic L, Matsen FA 3rd. Assessing the Value to the Patient of New Technologies in Anatomic Total Shoulder Arthroplasty. J Bone Joint Surg Am. 2021;103:761-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Best MJ, Aziz KT, Wilckens JH, McFarland EG, Srikumaran U. Increasing incidence of primary reverse and anatomic total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2021;30:1159-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 236] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 9. | Saltzman BM, Chalmers PN, Gupta AK, Romeo AA, Nicholson GP. Complication rates comparing primary with revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23:1647-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 10. | Chalmers PN, Gupta AK, Rahman Z, Bruce B, Romeo AA, Nicholson GP. Predictors of early complications of total shoulder arthroplasty. J Arthroplasty. 2014;29:856-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Seok HG, Park JJ, Park SG. Risk Factors for Periprosthetic Joint Infection after Shoulder Arthroplasty: Systematic Review and Meta-Analysis. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 12. | Gupta AK, Chalmers PN, Rahman Z, Bruce B, Harris JD, McCormick F, Abrams GD, Nicholson GP. Reverse total shoulder arthroplasty in patients of varying body mass index. J Shoulder Elbow Surg. 2014;23:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Mollon B, Mahure SA, Ding DY, Zuckerman JD, Kwon YW. The influence of a history of clinical depression on peri-operative outcomes in elective total shoulder arthroplasty: a ten-year national analysis. Bone Joint J. 2016;98-B:818-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Kirksey M, Chiu YL, Ma Y, Della Valle AG, Poultsides L, Gerner P, Memtsoudis SG. Trends in in-hospital major morbidity and mortality after total joint arthroplasty: United States 1998-2008. Anesth Analg. 2012;115:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 15. | Huang GP, Jia XF, Xiang Z, Ji Y, Wu GY, Tang Y, Li J, Zhang J. Tranexamic Acid Reduces Hidden Blood Loss in Patients Undergoing Total Knee Arthroplasty: A Comparative Study and Meta-Analysis. Med Sci Monit. 2016;22:797-802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Kim SH, Jung WI, Kim YJ, Hwang DH, Choi YE. Effect of Tranexamic Acid on Hematologic Values and Blood Loss in Reverse Total Shoulder Arthroplasty. Biomed Res Int. 2017;2017:9590803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Yen SH, Lin PC, Wu CT, Wang JW. Comparison of Effects of a Thrombin-Based Hemostatic Agent and Topical Tranexamic Acid on Blood Loss in Patients with Preexisting Thromboembolic Risk Undergoing a Minimally Invasive Total Knee Arthroplasty. A Prospective Randomized Controlled Trial. Biomed Res Int. 2021;2021:2549521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 18. | Sershon RA, Fillingham YA, Abdel MP, Malkani AL, Schwarzkopf R, Padgett DE, Vail TP, Nam D, Nahhas C, Culvern C, Della Valle CJ; Hip Society Research Group. The Optimal Dosing Regimen for Tranexamic Acid in Revision Total Hip Arthroplasty: A Multicenter Randomized Clinical Trial. J Bone Joint Surg Am. 2020;102:1883-1890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Shichman I, Shaked O, Ashkenazi I, Schwarzkopf R, Warschawski Y, Snir N. Tranexamic acid in non-elective primary total hip arthroplasty. Injury. 2021;52:1544-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Rethlefsen ML, Kirtley S, Waffenschmidt S, Ayala AP, Moher D, Page MJ, Koffel JB; PRISMA-S Group. PRISMA-S: an extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst Rev. 2021;10:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1182] [Cited by in RCA: 1173] [Article Influence: 293.3] [Reference Citation Analysis (0)] |
| 21. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 15220] [Article Influence: 2536.7] [Reference Citation Analysis (0)] |
| 22. | Cunningham G, Hughes J, Borner B, Mattern O, Taha ME, Smith MM, Young AA, Cass B. A single dose of tranexamic acid reduces blood loss after reverse and anatomic shoulder arthroplasty: a randomized controlled trial. J Shoulder Elbow Surg. 2021;30:1553-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Cvetanovich GL, Fillingham YA, O'Brien M, Forsythe B, Cole BJ, Verma NN, Romeo AA, Nicholson GP. Tranexamic acid reduces blood loss after primary shoulder arthroplasty: a double-blind, placebo-controlled, prospective, randomized controlled trial. JSES Open Access. 2018;2:23-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 24. | Pauzenberger L, Domej MA, Heuberer PR, Hexel M, Grieb A, Laky B, Blasl J, Anderl W. The effect of intravenous tranexamic acid on blood loss and early post-operative pain in total shoulder arthroplasty. Bone Joint J. 2017;99-B:1073-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 25. | Garcia T, Fragão-Marques M, Pimentão P, Pinto M, Pedro I, Martins C. Tranexamic acid in total shoulder arthroplasty under regional anesthesia: a randomized, single blinded, controlled trial. Braz J Anesthesiol. 2022;72:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Vara AD, Koueiter DM, Pinkas DE, Gowda A, Wiater BP, Wiater JM. Intravenous tranexamic acid reduces total blood loss in reverse total shoulder arthroplasty: a prospective, double-blinded, randomized, controlled trial. J Shoulder Elbow Surg. 2017;26:1383-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 27. | Sun L, Guo R, Feng Y. Efficacy and Safety of Tranexamic Acid in Bimaxillary Orthognathic Surgery. Plast Surg (Oakv). 2020;28:94-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Wright GP, Wolf AM, Waldherr TL, Ritz-Holland D, Laney ED, Chapman HA, Lane BR, Assifi MM, Chung MH. Preoperative tranexamic acid does not reduce transfusion rates in major oncologic surgery: Results of a randomized, double-blind, and placebo-controlled trial. J Surg Oncol. 2020;122:1037-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Colomina MJ, Koo M, Basora M, Pizones J, Mora L, Bagó J. Intraoperative tranexamic acid use in major spine surgery in adults: a multicentre, randomized, placebo-controlled trial†. Br J Anaesth. 2017;118:380-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 30. | Winter SF, Santaguida C, Wong J, Fehlings MG. Systemic and Topical Use of Tranexamic Acid in Spinal Surgery: A Systematic Review. Global Spine J. 2016;6:284-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 31. | Xie J, Hu Q, Huang Q, Ma J, Lei Y, Pei F. Comparison of intravenous vs topical tranexamic acid in primary total hip and knee arthroplasty: An updated meta-analysis. Thromb Res. 2017;153:28-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 32. | Wei W, Dang S, Duan D, Wei L. Comparison of intravenous and topical tranexamic acid in total knee arthroplasty. BMC Musculoskelet Disord. 2018;19:191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Abildgaard JT, McLemore R, Hattrup SJ. Tranexamic acid decreases blood loss in total shoulder arthroplasty and reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2016;25:1643-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Clay TB, Lawal AS, Wright TW, Patrick M, Struk AM, Farmer KW, King JJ. Tranexamic acid use is associated with lower transfusion rates in shoulder arthroplasty patients with preoperative anaemia. Shoulder Elbow. 2020;12:61-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Carbone A, Poeran J, Zubizarreta N, Chan J, Mazumdar M, Parsons BO, Galatz LM, Cagle PJ. Administration of tranexamic acid during total shoulder arthroplasty is not associated with increased risk of complications in patients with a history of thrombotic events. J Shoulder Elbow Surg. 2021;30:104-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |