Published online May 6, 2023. doi: 10.12998/wjcc.v11.i13.2925
Peer-review started: January 17, 2023
First decision: February 17, 2023
Revised: February 17, 2023
Accepted: March 30, 2023
Article in press: March 30, 2023
Published online: May 6, 2023
Processing time: 97 Days and 12.1 Hours
Obesity is characterized by excessive adipose tissue accumulation, which impacts physiological, metabolic, and immune functions. Several respiratory infections, including bacterial pneumonia, influenza, and coronavirus disease 2019, appear to be linked to unfavorable results in individuals with obesity. These may be attributed to the direct mechanical/physiological effects of excess body fat on the lungs’ function. Notably, adipose tissue dysfunction is associated with a low-grade chronic inflammatory status and hyperleptinemia, among other characteristics. These have all been linked to immune system dysfunction and weakened immune responses to these infections. A better understanding and clinical awareness of these risk factors are necessary for better disease outcomes.
Core Tip: Obesity influences the development and outcome of various respiratory infections. This is mediated in various ways, including through direct physiological impacts on the lungs and airways and via the dysfunctional adipose tissue, inducing a low-grade inflammatory status that potentially affects the immune response to certain pathogens. These include, notably, influenza and coronavirus disease 2019. Clinicians should be aware of these unique challenges in this subset of patients and take preventive and aggressive therapeutic measures as needed.
- Citation: Lempesis IG, Georgakopoulou VE. Implications of obesity and adiposopathy on respiratory infections; focus on emerging challenges. World J Clin Cases 2023; 11(13): 2925-2933
- URL: https://www.wjgnet.com/2307-8960/full/v11/i13/2925.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i13.2925
Obesity is a complex chronic disease linked to increased risk of nearly every chronic condition, including insulin resistance states, diabetes, cardiometabolic diseases, and various types of cancer, overall resulting in poor quality of life and reduced life expectancy[1-3]. Furthermore, obesity has a significant impact on respiratory health, and this has become even more apparent during the coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory coronavirus 2 (SARS-CoV-2)[4,5], as obesity has been associated with an increased risk of infection and unfavorable clinical outcomes in people with obesity that encounter COVID-19[6,7]. However, the burden of obesity on various infectious diseases was already recorded before and independently of the COVID-19 pandemic[8,9].
Obesity has been linked to an increased risk of pulmonary infections, including pneumonia, bronchitis, chronic obstructive pulmonary disease (COPD) exacerbations, and various other viral infections that we will further describe here[10]. Some of the mechanisms by which obesity increases the risk of respiratory infections include changes in pulmonary function, e.g., decreased lung volumes, impaired gas exchange, and secretion mobilization[10-13]. Moreover, mechanical blockage can also be caused by fat deposition in the upper airways[10,13-15]. Notable obesity appears to affect the immune system through a chronic low-grade inflammatory status[2,16]. Adipose tissue, which increases with obesity, is an active endocrine organ that secretes adipocytokines and other inflammatory mediators[2]. Such substances sustain a low-grade systemic inflammation, potentially impairing the immune system's capacity to combat infections[2,8,16,17].
In this review, we presented the numerous pathophysiological implications of obesity and dysfunctional adipose tissue on pulmonary function, the immune system, and pulmonary infections. We summarized the overall impact of obesity on disease outcome and highlighted various emerging and ongoing challenging infections, including, tuberculosis, COVID-19, influenza, and other bacterial or viral infections. Finally, we explored the impact of obesity on various vaccines and suggest strategies to prevent and treat lung infections in individuals with obesity.
Obesity has been associated with a variety of respiratory disorders, notably COPD, asthma, obstructive sleep apnea (OSA), pulmonary embolic disease, and aspiration pneumonia[18]. Several epidemiological studies have reported a relationship between respiratory tract infections and obesity, in particular higher prevalence, disease duration, and mortality[17]. Individuals with overweight or obesity had a higher rate of outpatient visits for acute respiratory infections during influenza season than individuals with normal weight, according to a large cohort study from Canada that examined a variety of acute upper (nasopharyngitis, sinusitis, tonsillitis) and lower (bronchitis, pneumonia, influenza, and other viral infections)[19]. In the United States, comparable results were obtained for the risk of community-acquired pneumonia[20] and chronic bronchitis in children, adolescents, and adults[21,22]. More recently, obesity and diabetes were recorded among the high-risk factors for severe COVID-19[23]. Notably, even though obesity is a significant risk factor for the occurrence of acute respiratory distress syndrome (ARDS) and acute lung injury (ALI), in a recent meta-analysis it was recorded that, as opposed to those with a normal body mass index (BMI), ARDS/ALI outcomes were more favorable in the individuals in the obesity group[24]. Overall, individuals with obesity are at increased risk of severe infections, delayed recovery, and complications like ARDS or ALI[10,25].
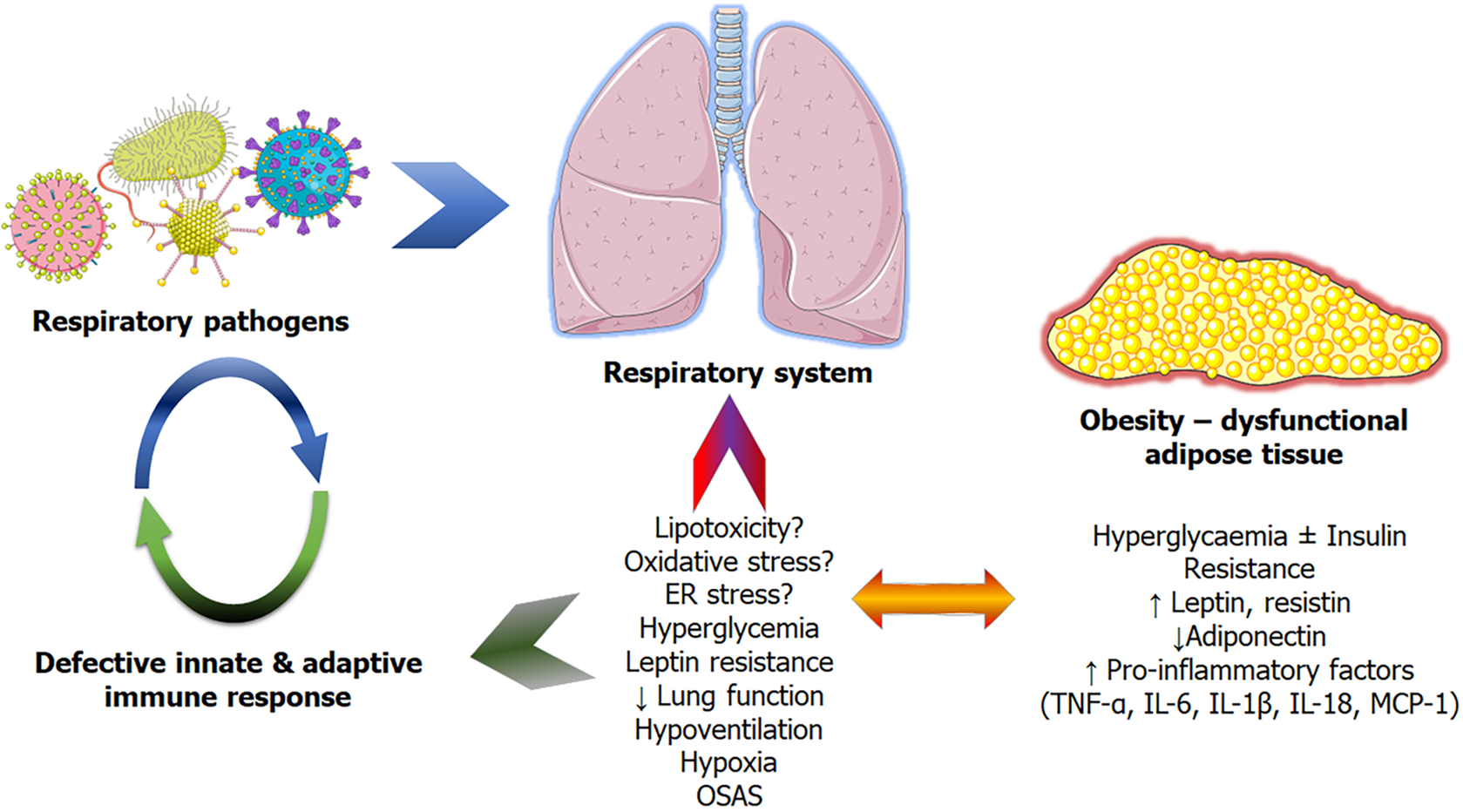
Although the precise mechanisms linking obesity and an increased risk of pulmonary infections remain unknown, several potential factors have been hypothesized and proposed[10,11,15,26]. These are divided into two categories: First, anatomical-functional changes caused by the mechanical impediment of excess adipose tissue, which blunts respiratory processes and contributes to respiratory diseases[11,15]. And secondly, due to the obesity-related adipose tissue dysfunction/adiposopathy resulting in low-grade inflammation, hyperinsulinemia, hyperglycemia, and hyperleptinemia, all of which contribute to a weakening of both innate and adaptive immunity[17,26] (Figure 1).
Obesity alters the mechanical characteristics of both the lung and thorax substantially, owing mostly to fat accumulation in the mediastinum and abdominal cavities[10]. These result in a decrease in the compliance of the lungs, thorax, and the respiratory system as a whole[10,27]. Moreover, as adipose tissue accumulates in the thoracic and abdominal cavities, the diaphragm's downward movement and the thoracic wall's outward mobility are restricted[10,28]. This affects the breathing pattern, resulting in a significant decrease in both the expiratory reserve volume and the lung's resting volume, known as functional residual capacity (FRC). The decrease in FRC is related to the degree of obesity[10,29]. Importantly, body fat distribution plays an important role, with abdominal and upper body accumulation being independent of the BMI in relation to the worsening of these parameters[12,30,31]. Obesity frequently causes increased respiratory system resistance, as well as airway restriction and closure, and airway hyperresponsiveness, resulting in unfavorable peripheral airway compression/closure results. This interferes with proper ventilation and may result in hypoxemia as a result of mismatch and trapping of airway contents such as mucus and germs, predisposing to infections[10,32-34]. Hypoxia caused by lung impairments, as discussed in the following section, may exacerbate adipose tissue dysfunction[2]. Finally, other obesity-related lung diseases, including COPD, asthma, hypoventilation syndrome, OSA, and obesity and gastroesophageal reflux disease, may further predispose to infections[26,35].
Excessive fat accumulation, adipose tissue (AT) malfunction (distinguished by low-grade inflammation), and ectopic fat deposition, particularly visceral, all play important roles in the pathophysiology of obesity and its comorbidities[36-40]. Adipocyte hypertrophy is characteristic of dysfunctional AT, which itself is linked to persistent low-grade inflammation. AT inflammation is partially caused by adipocytes which are secreting pro-inflammatory cytokines [including tumour necrosis factor alpha (TNF-α), and monocyte chemoattractant protein-1, and various interleukins (IL) notably IL-1β, -6], proinflammatory adipokines (leptin and resistin), and decreased levels of anti-inflammatory adipokines such as adiponectin, but also by the influx of numerous types of specialised, pro-inflammatory immune cells, such as macrophages[2,35,41,42]. Obesity may also have an imbalance in the pro- and anti-inflammatory immune cell ratio, favoring pro-inflammatory immune cell infiltration or activation and thus favoring an inflammatory state[2,16]. Moreover, adiposopathy is characterized by adipocytokine dysregulation, hormonal (insulin, catecholamines) resistance, impaired metabolism, reactive oxygen species (ROS)-induced stress and mitochondrial dysfunction, and anomalous oxygen levels, all of which pertain to ectopic fat accumulation and associated comorbidities[2,38,43,44]. Notably, in the presence of comorbidities such as OSA with hypoxic episodes of severe oxygen deprivation and acute duration, they may act negatively on the dysfunctional adipose tissue, leading to a vicious circle, as many adipocytokines appear to be oxygen-dependent, particularly in individuals with obesity[2,16]. As a result of these events, there is systemic inflammation, which may eventually compromise innate and adaptive immune function[35,45]. Confounding factors that could potentially affect immune response and infection risk independently of BMI could be comorbidities (cardiovascular disease, type 2 diabetes mellitus), altered nutrition (specific low-quality diets), and physical inactivity[9].
Increased TNF-α, IL-1, and IL-6 Levels in adipocytokine dysregulation may result in a weakened immune response[17,45,46]. Additionally, increased circulating leptin levels (a hallmark of obesity, directly proportional to AT mass) could contribute to altered immune responses as many cell types of the innate immune system express leptin receptors[17,47,48]. For instance, monocytes appear to exert a more pronounced pro-inflammatory response, and neutrophils are even more reactive to ROS once they are treated with leptin in vitro[17,49,50]. Leptin appears to affect various stages of B and T cell maturation and functions[17,51,52]. Moreover, hyperleptinemia was shown to impact the host defense in humans and murine models via effects on neutrophils[17,53]. Metabolic dysfunction associated with hyperinsulinemia may also contribute to immune system dysregulation[17]. It is crucial to highlight that these mechanisms are not necessarily exclusive and that they most likely interact to increase the overall incidence of lung infections in individuals with obesity. Furthermore, the mechanisms may differ based on the individual's underlying health problems and the kind of infection, as will be highlighted in the following sections.
Obesity is associated with an increased risk of several respiratory infections, including tuberculosis, influenza, pneumococcal, staphylococcal, and more recently COVID-19-associated pneumonia[6,35,54]. Obesity and coexisting diabetes raise morbidity from pneumococcal pneumonia and influenza, and notably, diabetes influences tuberculosis control and increases drug resistance as well as mortality[35,55].
As the innate immune response, which is the first line of defense against pathogenic bacteria, is likely suppressed because of the persistent low-grade inflammatory status[26], obesity has been shown to have an impact on the outcome of severe bacterial infections[56]. Obesity appears to influence and increase the risk of Streptococcus pneumoniae in a variety of populations, particularly the elderly[57]. It has also been proposed that hyperleptinemia, which is commonly associated with obesity, affects host defense against S. pneumoniae in humans[58]. Obesity is associated with unfavorable clinical outcomes in adults with community-acquired pneumonia of various etiologies[59]. Moreover, a link between BMI and mortality in hospitalized patients with community-acquired pneumonia has been recorded[60]. Finally, diet-induced models of obesity have shown that excess adiposity affects the in vivo host defense against Klebsiella pneumonia[61].
Obesity has been linked to an increased risk of several viral respiratory infections, including the notably recurrent influenza and ongoing COVID-19 pandemics, but also respiratory syncytial virus infection in children[6,62-64].
When it comes to influenza, especially influenza A viral infections, obesity appears to negatively impact humoral immunity[65] and the combined innate and adaptive responses already at the respiratory epithelium level[66-68]. Adiposity may also have a negative impact on influenza virus-related critical illnesses[69]. Immunomodulatory approaches to T cell metabolism have been explored to improve host immunity against influenza-related infections[70].
The potential negative impacts of obesity on COVID-19 have been largely described already[71,72]. Several studies have shown a direct link between obesity and COVID-19’s severity and mortality[73,74]. Among other cardiometabolic risk factors, obesity appears to be a significant independent factor[75]. This appears to be the case for unfavorable outcomes in critically ill patients with COVID-19[76].
The COVID-19-associated pathophysiological response is associated with the expression of the angiotensin converting enzyme 2 (ACE2) receptors in target tissues[5,77-79]. Many organ systems, including the lungs, adipose tissue, and blood vessels, express ACE2 receptors[80]. Notably, higher levels of ACE2 have been hypothesized and demonstrated in the adipose tissue of individuals with obesity, suggesting that adipose tissue may play a role in acting as a "reservoir" for SARS-CoV-2[81,82]. The S protein of SARS-CoV-2 is responsible for significant immune response induction in the host and, via binding to ACE2 receptors on the target cells, mediates cellular invasion[83]. Likely to influenza viral infections, the role of hyperleptinemia in obesity has been speculated for COVID-19[84]. Finally, obesity-related low-grade chronic inflammation may be directly related to higher expression of ACE2 and pathway-associated components, as well as decreased vitamin D bioavailability, and gut microbiome dysbiosis[85-87].
As demonstrated, obesity has a negative impact on the immune system, and these implications raise concerns about the absence of vaccine-induced immunity in these patients, necessitating a consideration of how this subpopulation might be better protected[88,89]. Cohort studies have shown that, particularly for influenza vaccination, individuals with obesity may have a lower immune response than those of normal weight[90,91]. Several degrees of evidence also suggest the importance of vaccination against COVID-19 and obesity, also from real-world data[92]. However, overall, the vaccination of individuals with obesity is of paramount importance and should not be avoided, even if reduced responsiveness is suspected.
In conclusion, obesity is a major risk factor for several respiratory infections and their severity. Changes in lung function, adipose tissue accumulation and dysfunction, and immune system dysfunction all contribute to the higher risk. It is important for individuals with overweight or obesity to undertake preventive steps to maintain their weight. These include dietary and habitual patterns that can lead and maintain weight loss and if necessary, following failure of these steps to implement medicinal avenues[93-95]. Preventive measures to lower the risk of lung infections including face covering and meticulous vaccinations against respiratory pathogens and frequent medical evaluations. Furthermore, healthcare practitioners should be aware of the increased risk of lung infections in these individuals and act in preventive ways and escalate treatment measures if necessary.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Belančić A, Croatia; Said MA, Saudi Arabia S-Editor: Li L L-Editor: A P-Editor: Chen YX
| 1. | Lempesis IG, Tsilingiris D, Liu J, Dalamaga M. Of mice and men: Considerations on adipose tissue physiology in animal models of obesity and human studies. Metabol Open. 2022;15:100208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 2. | Lempesis IG, van Meijel RLJ, Manolopoulos KN, Goossens GH. Oxygenation of adipose tissue: A human perspective. Acta Physiol (Oxf). 2020;228:e13298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 3. | Hruby A, Hu FB. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics. 2015;33:673-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1738] [Cited by in RCA: 1718] [Article Influence: 171.8] [Reference Citation Analysis (0)] |
| 4. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14766] [Article Influence: 2953.2] [Reference Citation Analysis (0)] |
| 5. | Georgakopoulou VE, Makrodimitri S, Triantafyllou M, Samara S, Voutsinas PM, Anastasopoulou A, Papageorgiou CV, Spandidos DA, Gkoufa A, Papalexis P, Xenou E, Chelidonis G, Sklapani P, Trakas N, Sipsas NV. Immature granulocytes: Innovative biomarker for SARSCoV2 infection. Mol Med Rep. 2022;26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Goossens GH, Dicker D, Farpour-Lambert NJ, Frühbeck G, Mullerova D, Woodward E, Holm JC. Obesity and COVID-19: A Perspective from the European Association for the Study of Obesity on Immunological Perturbations, Therapeutic Challenges, and Opportunities in Obesity. Obes Facts. 2020;13:439-452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 7. | Frühbeck G, Baker JL, Busetto L, Dicker D, Goossens GH, Halford JCG, Handjieva-Darlenska T, Hassapidou M, Holm JC, Lehtinen-Jacks S, Mullerova D, O'Malley G, Sagen JV, Rutter H, Salas XR, Woodward E, Yumuk V, Farpour-Lambert NJ. European Association for the Study of Obesity Position Statement on the Global COVID-19 Pandemic. Obes Facts. 2020;13:292-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 8. | Karlsson EA, Beck MA. The burden of obesity on infectious disease. Exp Biol Med (Maywood). 2010;235:1412-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 9. | Dobner J, Kaser S. Body mass index and the risk of infection - from underweight to obesity. Clin Microbiol Infect. 2018;24:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 237] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 10. | Dixon AE, Peters U. The effect of obesity on lung function. Expert Rev Respir Med. 2018;12:755-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 485] [Article Influence: 69.3] [Reference Citation Analysis (2)] |
| 11. | Hegewald MJ. Impact of obesity on pulmonary function: current understanding and knowledge gaps. Curr Opin Pulm Med. 2021;27:132-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Molani Gol R, Rafraf M. Association between abdominal obesity and pulmonary function in apparently healthy adults: A systematic review. Obes Res Clin Pract. 2021;15:415-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Brock JM, Billeter A, Müller-Stich BP, Herth F. Obesity and the Lung: What We Know Today. Respiration. 2020;99:856-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 14. | Schwartz AR, Patil SP, Squier S, Schneider H, Kirkness JP, Smith PL. Obesity and upper airway control during sleep. J Appl Physiol (1985). 2010;108:430-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Kaw R, Wong J, Mokhlesi B. Obesity and Obesity Hypoventilation, Sleep Hypoventilation, and Postoperative Respiratory Failure. Anesth Analg. 2021;132:1265-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Lempesis IG, Hoebers N, Essers Y, Jocken JWE, Rouschop KMA, Blaak EE, Manolopoulos KN, Goossens GH. Physiological Oxygen Levels Differentially Regulate Adipokine Production in Abdominal and Femoral Adipocytes from Individuals with Obesity Versus Normal Weight. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Pugliese G, Liccardi A, Graziadio C, Barrea L, Muscogiuri G, Colao A. Obesity and infectious diseases: pathophysiology and epidemiology of a double pandemic condition. Int J Obes (Lond). 2022;46:449-465. [PubMed] [DOI] [Full Text] |
| 18. | McClean KM, Kee F, Young IS, Elborn JS. Obesity and the lung: 1. Epidemiology. Thorax. 2008;63:649-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 214] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 19. | Campitelli MA, Rosella LC, Kwong JC. The association between obesity and outpatient visits for acute respiratory infections in Ontario, Canada. Int J Obes (Lond). 2014;38:113-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Baik I, Curhan GC, Rimm EB, Bendich A, Willett WC, Fawzi WW. A prospective study of age and lifestyle factors in relation to community-acquired pneumonia in US men and women. Arch Intern Med. 2000;160:3082-3088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 249] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 21. | Lee YL, Chen YC, Chen YA. Obesity and the occurrence of bronchitis in adolescents. Obesity (Silver Spring). 2013;21:E149-E153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Maccioni L, Weber S, Elgizouli M, Stoehlker AS, Geist I, Peter HH, Vach W, Nieters A. Obesity and risk of respiratory tract infections: results of an infection-diary based cohort study. BMC Public Health. 2018;18:271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Zhou Y, Chi J, Lv W, Wang Y. Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (Covid-19). Diabetes Metab Res Rev. 2021;37:e3377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 358] [Cited by in RCA: 315] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 24. | Zhi G, Xin W, Ying W, Guohong X, Shuying L. "Obesity Paradox" in Acute Respiratory Distress Syndrome: Asystematic Review and Meta-Analysis. PLoS One. 2016;11:e0163677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 131] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 25. | Huttunen R, Syrjänen J. Obesity and the risk and outcome of infection. Int J Obes (Lond). 2013;37:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 398] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 26. | Muscogiuri G, Pugliese G, Laudisio D, Castellucci B, Barrea L, Savastano S, Colao A. The impact of obesity on immune response to infection: Plausible mechanisms and outcomes. Obes Rev. 2021;22:e13216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (1)] |
| 27. | Pelosi P, Croci M, Ravagnan I, Tredici S, Pedoto A, Lissoni A, Gattinoni L. The effects of body mass on lung volumes, respiratory mechanics, and gas exchange during general anesthesia. Anesth Analg. 1998;87:654-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 135] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Sugerman H, Windsor A, Bessos M, Wolfe L. Intra-abdominal pressure, sagittal abdominal diameter and obesity comorbidity. J Intern Med. 1997;241:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 325] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 29. | Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest. 2006;130:827-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 606] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 30. | Ochs-Balcom HM, Grant BJ, Muti P, Sempos CT, Freudenheim JL, Trevisan M, Cassano PA, Iacoviello L, Schünemann HJ. Pulmonary function and abdominal adiposity in the general population. Chest. 2006;129:853-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 31. | Leone N, Courbon D, Thomas F, Bean K, Jégo B, Leynaert B, Guize L, Zureik M. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med. 2009;179:509-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 374] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 32. | Chapman DG, Berend N, King GG, Salome CM. Increased airway closure is a determinant of airway hyperresponsiveness. Eur Respir J. 2008;32:1563-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 33. | Pellegrino R, Gobbi A, Antonelli A, Torchio R, Gulotta C, Pellegrino GM, Dellacà R, Hyatt RE, Brusasco V. Ventilation heterogeneity in obesity. J Appl Physiol (1985). 2014;116:1175-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Hedenstierna G, Santesson J, Norlander O. Airway closure and distribution of inspired gas in the extremely obese, breathing spontaneously and during anaesthesia with intermittent positive pressure ventilation. Acta Anaesthesiol Scand. 1976;20:334-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 61] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Mancuso P. Obesity and respiratory infections: does excess adiposity weigh down host defense? Pulm Pharmacol Ther. 2013;26:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 36. | Goossens GH. The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol Behav. 2008;94:206-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 376] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 37. | Blüher M. Adipose tissue dysfunction contributes to obesity related metabolic diseases. Best Pract Res Clin Endocrinol Metab. 2013;27:163-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 274] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 38. | Goossens GH, Blaak EE. Adipose tissue dysfunction and impaired metabolic health in human obesity: a matter of oxygen? Front Endocrinol (Lausanne). 2015;6:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 39. | Goossens GH. The Metabolic Phenotype in Obesity: Fat Mass, Body Fat Distribution, and Adipose Tissue Function. Obes Facts. 2017;10:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 465] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 40. | Frühbeck G, Busetto L, Dicker D, Yumuk V, Goossens GH, Hebebrand J, Halford JGC, Farpour-Lambert NJ, Blaak EE, Woodward E, Toplak H. The ABCD of Obesity: An EASO Position Statement on a Diagnostic Term with Clinical and Scientific Implications. Obes Facts. 2019;12:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 173] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 41. | Lee BC, Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim Biophys Acta. 2014;1842:446-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 498] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 42. | Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3548] [Cited by in RCA: 4381] [Article Influence: 257.7] [Reference Citation Analysis (0)] |
| 43. | Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371:1131-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 741] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 44. | Frayn KN, Karpe F. Regulation of human subcutaneous adipose tissue blood flow. Int J Obes (Lond). 2014;38:1019-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 45. | Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2065] [Cited by in RCA: 2251] [Article Influence: 118.5] [Reference Citation Analysis (0)] |
| 46. | Hornung F, Rogal J, Loskill P, Löffler B, Deinhardt-Emmer S. The Inflammatory Profile of Obesity and the Role on Pulmonary Bacterial and Viral Infections. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 47. | Kalaitzopoulos DR, Lempesis IG, Samartzis N, Kolovos G, Dedes I, Daniilidis A, Nirgianakis K, Leeners B, Goulis DG, Samartzis EP. Leptin concentrations in endometriosis: A systematic review and meta-analysis. J Reprod Immunol. 2021;146:103338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 48. | La Cava A, Matarese G. The weight of leptin in immunity. Nat Rev Immunol. 2004;4:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 637] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 49. | Papathanassoglou E, El-Haschimi K, Li XC, Matarese G, Strom T, Mantzoros C. Leptin receptor expression and signaling in lymphocytes: kinetics during lymphocyte activation, role in lymphocyte survival, and response to high fat diet in mice. J Immunol. 2006;176:7745-7752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 183] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 50. | Caldefie-Chezet F, Poulin A, Tridon A, Sion B, Vasson MP. Leptin: a potential regulator of polymorphonuclear neutrophil bactericidal action? J Leukoc Biol. 2001;69:414-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 51. | Claycombe K, King LE, Fraker PJ. A role for leptin in sustaining lymphopoiesis and myelopoiesis. Proc Natl Acad Sci U S A. 2008;105:2017-2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 52. | Martín-Romero C, Santos-Alvarez J, Goberna R, Sánchez-Margalet V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol. 2000;199:15-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 383] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 53. | Ubags ND, Stapleton RD, Vernooy JH, Burg E, Bement J, Hayes CM, Ventrone S, Zabeau L, Tavernier J, Poynter ME, Parsons PE, Dixon AE, Wargo MJ, Littenberg B, Wouters EF, Suratt BT. Hyperleptinemia is associated with impaired pulmonary host defense. JCI Insight. 2016;1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 54. | Fisher-Hoch SP, Mathews CE, McCormick JB. Obesity, diabetes and pneumonia: the menacing interface of non-communicable and infectious diseases. Trop Med Int Health. 2013;18:1510-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 55. | Badawi A, Gregg B, Vasileva D. Systematic analysis for the relationship between obesity and tuberculosis. Public Health. 2020;186:246-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 56. | Alsiö Å, Nasic S, Ljungström L, Jacobsson G. Impact of obesity on outcome of severe bacterial infections. PLoS One. 2021;16:e0251887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 57. | Frasca D, McElhaney J. Influence of Obesity on Pneumococcus Infection Risk in the Elderly. Front Endocrinol (Lausanne). 2019;10:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 58. | Hales C, Burnet L, Coombs M, Collins AM, Ferreira DM. Obesity, leptin and host defence of Streptococcus pneumoniae: the case for more human research. Eur Respir Rev. 2022;31. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 59. | Viasus D, Pérez-Vergara V, Carratalà J. Effect of Undernutrition and Obesity on Clinical Outcomes in Adults with Community-Acquired Pneumonia. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (1)] |
| 60. | Kim RY, Glick C, Furmanek S, Ramirez JA, Cavallazzi R. Association between body mass index and mortality in hospitalised patients with community-acquired pneumonia. ERJ Open Res. 2021;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 61. | Mancuso P, Curtis JL, Weitzel AM, Griffin CA, Bouchard B, Freeman CM, Bridges D, Singer K. Diet-induced obesity in mice impairs host defense against Klebsiella pneumonia in vivo and glucose transport and bactericidal functions in neutrophils in vitro. Am J Physiol Lung Cell Mol Physiol. 2022;322:L116-L128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 62. | Akiyama N, Segawa T, Ida H, Mezawa H, Noya M, Tamez S, Urashima M. Bimodal effects of obesity ratio on disease duration of respiratory syncytial virus infection in children. Allergol Int. 2011;60:305-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 63. | Moser JS, Galindo-Fraga A, Ortiz-Hernández AA, Gu W, Hunsberger S, Galán-Herrera JF, Guerrero ML, Ruiz-Palacios GM, Beigel JH; La Red ILI 002 Study Group. Underweight, overweight, and obesity as independent risk factors for hospitalization in adults and children from influenza and other respiratory viruses. Influenza Other Respir Viruses. 2019;13:3-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 64. | Bhattacharya I, Ghayor C, Pérez Dominguez A, Weber FE. From Influenza Virus to Novel Corona Virus (SARS-CoV-2)-The Contribution of Obesity. Front Endocrinol (Lausanne). 2020;11:556962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 65. | Rojas-Osornio SA, Cruz-Hernández TR, Drago-Serrano ME, Campos-Rodríguez R. Immunity to influenza: Impact of obesity. Obes Res Clin Pract. 2019;13:419-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 66. | Honce R, Schultz-Cherry S. Impact of Obesity on Influenza A Virus Pathogenesis, Immune Response, and Evolution. Front Immunol. 2019;10:1071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 313] [Cited by in RCA: 304] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 67. | Green WD, Beck MA. Obesity Impairs the Adaptive Immune Response to Influenza Virus. Ann Am Thorac Soc. 2017;14:S406-S409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 181] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 68. | Paich HA, Sheridan PA, Handy J, Karlsson EA, Schultz-Cherry S, Hudgens MG, Noah TL, Weir SS, Beck MA. Overweight and obese adult humans have a defective cellular immune response to pandemic H1N1 influenza A virus. Obesity (Silver Spring). 2013;21:2377-2386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 145] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 69. | Kalil AC, Thomas PG. Influenza virus-related critical illness: pathophysiology and epidemiology. Crit Care. 2019;23:258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 316] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 70. | Greene E, MacIver NJ. Targeting T cell (oxidative) metabolism to improve immunity to viral infection in the context of obesity. Front Immunol. 2022;13:1025495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 71. | Albashir AAD. The potential impacts of obesity on COVID-19. Clin Med (Lond). 2020;20:e109-e113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 72. | Yu W, Rohli KE, Yang S, Jia P. Impact of obesity on COVID-19 patients. J Diabetes Complications. 2021;35:107817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 73. | Abumweis S, Alrefai W, Alzoughool F. Association of obesity with COVID-19 diseases severity and mortality: A meta-analysis of studies. Obes Med. 2022;33:100431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 74. | Sjögren L, Stenberg E, Thuccani M, Martikainen J, Rylander C, Wallenius V, Olbers T, Kindblom JM. Impact of obesity on intensive care outcomes in patients with COVID-19 in Sweden-A cohort study. PLoS One. 2021;16:e0257891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 75. | Fayol A, Livrozet M, Pereira H, Diehl JL, Lebeaux D, Arlet JB, Cholley B, Carette C, Carves JB, Czernichow S, Hauw C, Hamada SR, Jannot AS, Volle G, Masurkar N, Mirault T, Planquette B, Sanchez O, Châtellier G, Azizi M, Hulot JS. Cardiometabolic Disorders and the Risk of Critical COVID-19 as Compared to Influenza Pneumonia. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 76. | Rossi AP, Gottin L, Donadello K, Schweiger V, Nocini R, Taiana M, Zamboni M, Polati E. Obesity as a risk factor for unfavourable outcomes in critically ill patients affected by Covid 19. Nutr Metab Cardiovasc Dis. 2021;31:762-768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 77. | Georgakopoulou VE, Gkoufa A, Damaskos C, Papalexis P, Pierrakou A, Makrodimitri S, Sypsa G, Apostolou A, Asimakopoulou S, Chlapoutakis S, Sklapani P, Trakas N, Spandidos DA. COVID-19-associated acute appendicitis in adults. A report of five cases and a review of the literature. Exp Ther Med. 2022;24:482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 78. | Georgakopoulou VE, Avramopoulos P, Papalexis P, Bitsani A, Damaskos C, Garmpi A, Venetikou MS, Paramythiotis D, Karlafti E, Sklapani P, Trakas N, Spandidos DA. COVID-19 induced hypoparathyroidism: A case report. Exp Ther Med. 2022;23:346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 79. | Georgakopoulou VE, Lembessis P, Skarlis C, Gkoufa A, Sipsas NV, Mavragani CP. Hematological Abnormalities in COVID-19 Disease: Association With Type I Interferon Pathway Activation and Disease Outcomes. Front Med (Lausanne). 2022;9:850472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 80. | Ozkurt Z, Çınar Tanrıverdi E. COVID-19: Gastrointestinal manifestations, liver injury and recommendations. World J Clin Cases. 2022;10:1140-1163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (7)] |
| 81. | Herta T, Berg T. COVID-19 and the liver - Lessons learned. Liver Int. 2021;41 Suppl 1:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 82. | Pasquarelli-do-Nascimento G, Braz-de-Melo HA, Faria SS, Santos IO, Kobinger GP, Magalhães KG. Hypercoagulopathy and Adipose Tissue Exacerbated Inflammation May Explain Higher Mortality in COVID-19 Patients With Obesity. Front Endocrinol (Lausanne). 2020;11:530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 83. | Georgakopoulou VE, Gkoufa A, Garmpis N, Makrodimitri S, Papageorgiou CV, Barlampa D, Garmpi A, Chiapoutakis S, Sklapani P, Trakas N, Damaskos C. COVID-19 and Acute Pancreatitis: A Systematic Review of Case Reports and Case Series. Ann Saudi Med. 2022;42:276-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 84. | Guglielmi V, Colangeli L, D'Adamo M, Sbraccia P. Susceptibility and Severity of Viral Infections in Obesity: Lessons from Influenza to COVID-19. Does Leptin Play a Role? Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 85. | Belančić A, Kresović A, Rački V. Potential pathophysiological mechanisms leading to increased COVID-19 susceptibility and severity in obesity. Obes Med. 2020;19:100259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 86. | Belančić A. Gut microbiome dysbiosis and endotoxemia - Additional pathophysiological explanation for increased COVID-19 severity in obesity. Obes Med. 2020;20:100302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 87. | Lempesis IG, Karlafti E, Papalexis P, Fotakopoulos G, Tarantinos K, Lekakis V, Papadakos SP, Cholongitas E, Georgakopoulou VE. COVID-19 and liver injury in individuals with obesity. World J Gastroenterol. 2023;29:908-916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 88. | Painter SD, Ovsyannikova IG, Poland GA. The weight of obesity on the human immune response to vaccination. Vaccine. 2015;33:4422-4429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 150] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 89. | Tagliabue C, Principi N, Giavoli C, Esposito S. Obesity: impact of infections and response to vaccines. Eur J Clin Microbiol Infect Dis. 2016;35:325-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 90. | Clarke M, Mathew SM, Giles LC, Pena AS, Barr IG, Richmond PC, Marshall HS. A Prospective Study Investigating the Impact of Obesity on the Immune Response to the Quadrivalent Influenza Vaccine in Children and Adolescents. Vaccines (Basel). 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 91. | Jagielska AM, Brydak LB, Nitsch-Osuch AS. Immunogenicity of Split Inactivated Quadrivalent Influenza Vaccine in Adults with Obesity in the 2017/2018 Season. Med Sci Monit. 2021;27:e929572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 92. | Piernas C, Patone M, Astbury NM, Gao M, Sheikh A, Khunti K, Shankar-Hari M, Dixon S, Coupland C, Aveyard P, Hippisley-Cox J, Jebb SA. Associations of BMI with COVID-19 vaccine uptake, vaccine effectiveness, and risk of severe COVID-19 outcomes after vaccination in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2022;10:571-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 93. | Sagris M, Kokkinidis DG, Lempesis IG, Giannopoulos S, Rallidis L, Mena-Hurtado C, Bakoyiannis C. Nutrition, dietary habits, and weight management to prevent and treat patients with peripheral artery disease. Rev Cardiovasc Med. 2020;21:565-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 94. | Lempesis IG, Liu J, Dalamaga M. The catcher in the gut: Tirzepatide, a dual incretin analog for the treatment of type 2 diabetes mellitus and obesity. Metabol Open. 2022;16:100220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 95. | Vallianou NG, Tsilingiris D, Kounatidis D, Lempesis IG, Karampela I, Dalamaga M. Sodiumglucose cotransporter2 inhibitors in obesity and associated cardiometabolic disorders: where do we stand? Pol Arch Intern Med. 2022;132. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |