Published online May 6, 2023. doi: 10.12998/wjcc.v11.i13.2874
Peer-review started: December 29, 2022
First decision: January 17, 2023
Revised: February 17, 2023
Accepted: April 4, 2023
Article in press: April 4, 2023
Published online: May 6, 2023
Processing time: 116 Days and 20.9 Hours
Intensive care units (ICU) for various reasons, including the increasing age of admitted patients, comorbidities, and increasingly complex surgical procedures (e.g., transplants), have become "the epicenter" of nosocomial infections, these are characterized by the presence of multidrug-resistant organisms (MDROs) as the cause of infection. Therefore, the perfect match of fragile patients and MDROs, as the cause of infection, makes ICU mortality very high. Furthermore, carbapenems were considered for years as last-resort antibiotics for the treatment of infections caused by MDROs; unfortunately, nowadays carbapenem resistance, mainly among Gram-negative pathogens, is a matter of the highest concern for world
Core Tip: Intensive care units for various reasons have become "the epicenter" of nosocomial infections due to multidrug-resistant organisms: a perfect combination of critically ill patients and multidrug-resistant organisms, as the cause of infection, makes these patients' mortality very high. This comprehensive review aims to outline the problem from the clinician's perspective, focusing on the new definition and epidemiology of the most common multidrug-resistant organisms that are Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacterales to emphasize the importance of the problem.
- Citation: Pace MC, Corrente A, Passavanti MB, Sansone P, Petrou S, Leone S, Fiore M. Burden of severe infections due to carbapenem-resistant pathogens in intensive care unit. World J Clin Cases 2023; 11(13): 2874-2889
- URL: https://www.wjgnet.com/2307-8960/full/v11/i13/2874.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i13.2874
Carbapenem resistance is such an important public health issue worldwide[1,2] that the 2017 World Health Organization (WHO) global priority list of pathogens ranks carbapenem-resistant Enterobacteriaceae (CRE), carbapenem-resistant Pseudomonas aeruginosa (CRPA), and carbapenem-resistant Acinetobacter baumannii (CRAB) in the highest priority category (i.e., Critical)[3]. Infections sustained by these bacteria lead to longer lengths of stay, increased healthcare costs, and higher mortality[4-6], especially in patients admitted to the intensive care unit (ICU)[7]. Many studies demonstrated the link between carbapenem use and carbapenem resistance[8-10]. This has even greater clinical relevance when we consider that the rise in the consumption rate of carbapenems was 45% worldwide[11]. Carbapenems are the third most widely used class of antibiotics worldwide for community-acquired infections in ICU (10.7%) and the first class for hospital-acquired infections (HAI) (21.5%)[12]. This comprehensive review aims to analyze from the perspective of worldwide epidemiology the global burden of severe infections supported by carbapenems-resistant germs in the ICU setting.
To review the published clinical data on the epidemiology of carbapenem resistance in the ICU setting, a systematic search of the biomedical literature was conducted. Medline (via PubMed) was searched, limited from 2012 to 2022, for articles using the following terms: [(carbapenem or imipenem or meropenem or doripenem or ertapenem) and (resistance or resistant or susceptible or susceptibility)] or (carbapenemase). The result of this search was combined with three separate searches for ‘‘Pseudomonas aeruginosa’’, ‘‘Acinetobacter baumannii’’ and “Enterobacteriales or Enterobacteriaceae”. The retrieved studies were scheduled from the geographical area of origin in the five continents: ‘‘Africa’’, ‘‘America’’, ‘‘Asia”, “Europe”, “and Australia”.
Carbapenem-resistant Gram-negative bacteria (GNBs), namely, CRE (e.g., Klebsiella pneumoniae, Escherichia coli), CRAB and CRPA, are a matter of national and international concern as they are an emerging cause of HAI that pose a significant threat to public health. The term ‘CROS’ is used as a generic term that refers to all of these GNBs[13]. Centers for disease control and prevention (CDC) define CRE as multidrug-resistant organisms that are resistant to at least one of the carbapenem antibiotics (ertapenem, meropenem, doripenem, or imipenem) or produce a carbapenemase. CRE is a phenotypic definition (i.e., based on the organism susceptibility pattern). A lot of different mechanisms (i.e., genotypes) can result in carbapenem resistance, for example, the production of enzymes that break down carbapenems and related antimicrobials making them ineffective: CRE that produce carbapenemases are called carbapenemase-producing CRE (CP-CRE); therefore, CP-CRE are a subset of all CRE (approximately 30% of CRE carry a carbapenemase), carbapenemase genes are often on mobile genetic elements, which can be easily shared between bacteria, leading to the rapid spread of resistance. Carbapenemases are classified by ambler into three classes - A, B and D (class C includes enzymes that hydrolyze primarily cephalosporins[14]) based on their central catalytic domain and substrate preference[15]. Class A [e.g., Klebsiella pneumoniae carbapenemase (KPC), imipenem-hydrolyzing β-lactamase and Serratia marcescens enzyme] and D [oxacillin carbapenemase/oxacillinase (OXA)] carbapenemases have serine residues in their active sites and hence are called serine-proteases, while Class B [New Delhi metallo-β-lactamase (NDM), Verona integron-encoded metallo-β-lactamase (VIM) and imipenemase metallo-β-lactamase (IMP)] enzymes are metallo-β-lactamases with zinc in the active site[16]. The five carbapenemases most frequently identified in CRE are KPC, which was the first carbapenemase identified in the United States (US) in 2001, the NDM, VIM, oxacillinase-48 (OXA-48-type), and IMP[17]. The European committee on antimicrobial susceptibility testing defined the meropenem breakpoints for Escherichia coli and Klebsiella pneumoniae as S ≤ 2 mg/L and R > 8 mg/L; the corresponding breakpoints for ertapenem are S ≤ 0.5 mg/L and R > 0.5 mg/L. Isolates with meropenem minimum inhibitory concentration (MIC) > 2 mg/L and/or ertapenem MIC > 0.5 mg/L are considered resistant and should be investigated for carbapenem resistance mechanisms. This approach will not identify all Escherichia coli and klebsiella pneumoniae isolates but will detect most isolates with clinically significant carbapenem non-susceptibility. As the CDC also the European CDC encourages proceeding with the detection of carbapenemase production in carbapenem non-susceptible isolates with MIC values above the susceptible breakpoint[18].
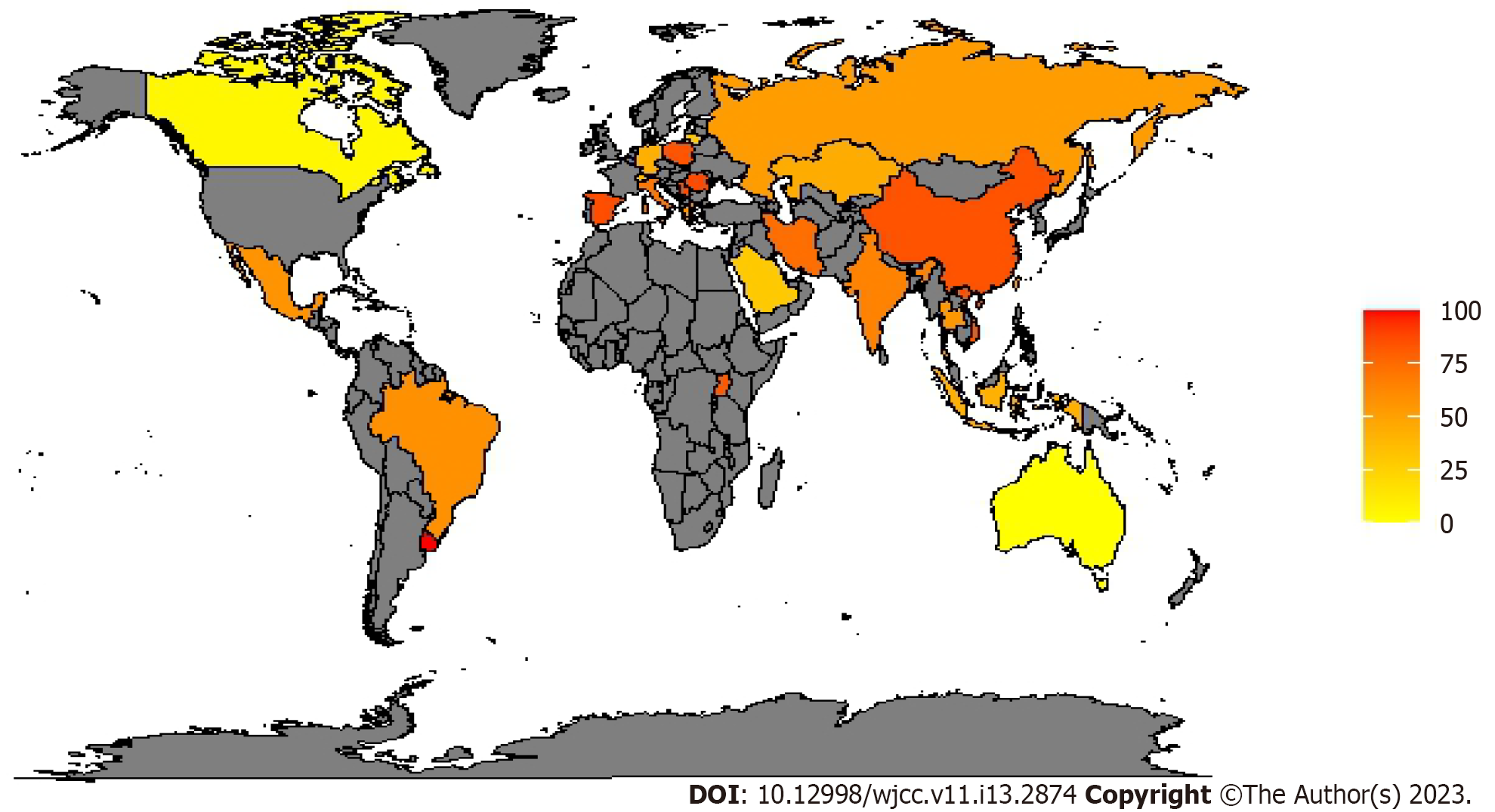
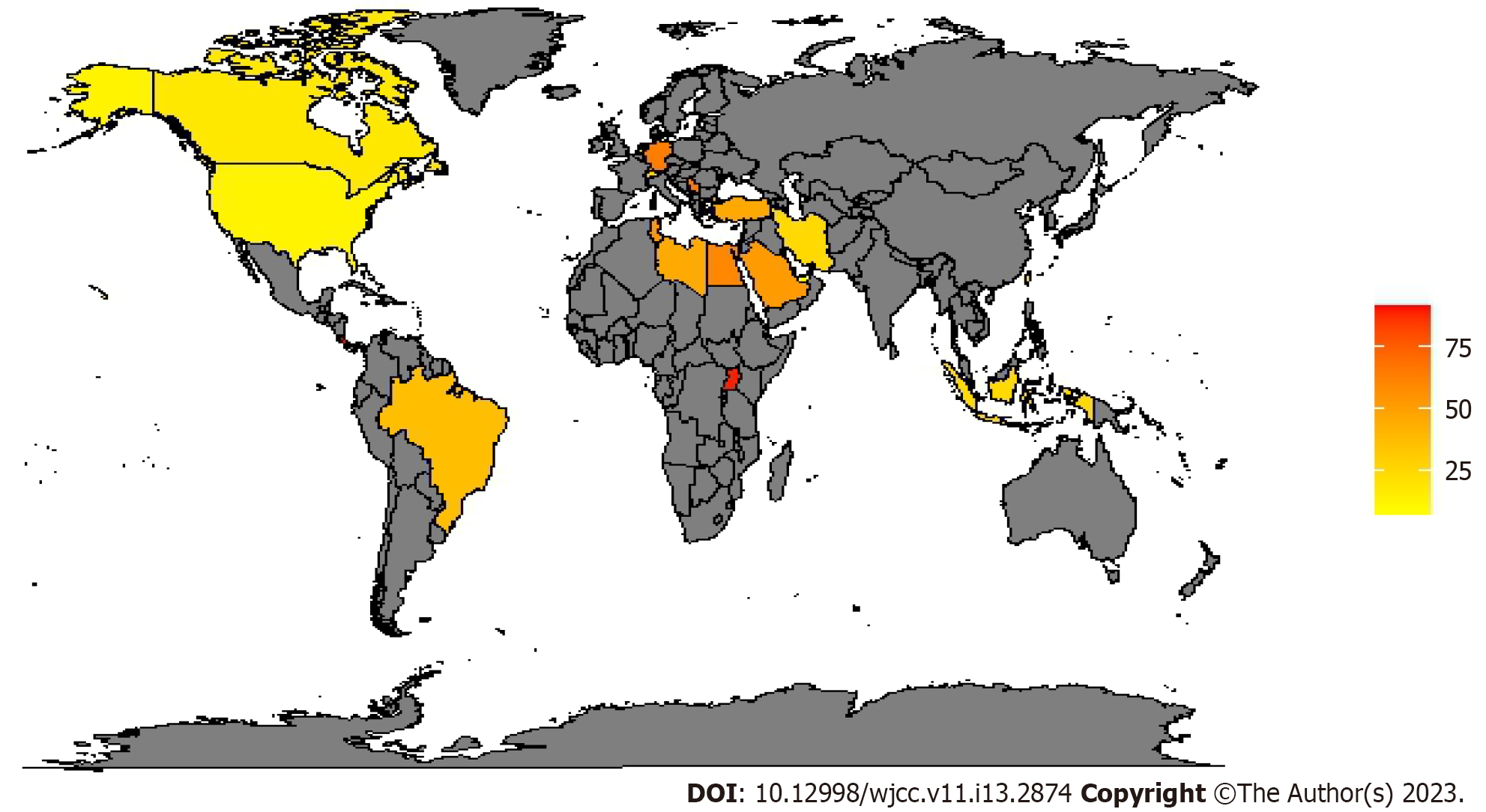
To monitor antibiotic resistance and plan contrast strategies, the different continents established epidemiological surveillance networks: European antimicrobial resistance surveillance network and central Asian and eastern European surveillance of antimicrobial resistance in Europe and Asia while the national healthcare safety network at the CDC in the US. They documented that multidrug-resistant organisms (MDROs) have become much more prevalent during the last decade[19-21]. CDC estimates that each year in the US, at least 2.8 million people get an antibiotic-resistant infection, and more than 35000 people die. The estimated national cost to treat infections caused by six MDROs identified in the last CDC report and frequently found in healthcare can be substantial—more than $4.6 billion annually[22]. In a report conducted for “the review on antimicrobial resistance (AMR)”, commissioned in July 2014 by the United Kingdom prime minister, it is predicted that the toll of global antimicrobial resistance will be 10 million deaths per year and up to $100 trillion lost to the global economy by 2050[23]. In a survey promoted by the European society of intensive care medicine, 12.4% of ICU physicians reported that they had, during the preceding six months, at least one patient with an infection caused by a bacterium resistant to all or almost all antibiotics available in their ICU[24]. An international multicenter study concluded that 19% of patients admitted to the ICU for more than 24 hours acquired an infection, with rates ranging between 2.3% and 49.2% depending on the hospital unit[25]. The most common ICU-acquired infections are pneumonia, surgical site infection, gastrointestinal infection, urinary tract infection (UTI) and bloodstream infection (BSI)[26]. In a large surveillance report from 183 US hospitals, 84% of BSI were related to the use of a central line catheter, 39% of pneumonia cases were ventilator-associated pneumonia and 68% of UTIs were related to urinary catheters[27]. According to the Gram staining results, bacteria can be classified into 2 categories: GNBs and Gram-positive bacteria (GPBs). Infections caused by multidrug-resistant GNBs are more frequent than multidrug-resistant GPBs, compared to the past. in a large prevalence study on infected ICU patients with isolates from 75 countries, 62% were GNBs, 47% were GPBs and 19% were fungal[28]. Many acronyms help clinicians remember the most prevalent germs: ESKAPE organisms identify a group of highly resistant germs that 'escape' to β-lactam antibiotics and consist of Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter spp., Pseudomonas aeruginosa, and Enterobacter spp.[29,30]. ESKAPE organisms represent the 6 most common MDROs of HAI[31]. However, since it was pointed out that this acronym excluded other enteric GNBs including Escherichia coli, it was modified into ESKAPE+C where “c” refers to Clostridium difficile, an important nosocomial pathogen that may easily acquire an MDROs phenotype and “e” refers Enterobacteriaceae covering all enteric GNBs including Escherichia coli, Klebsiella pneumoniae, Proteus spp. and Enterobacter spp.[32]. In Europe and other areas, of particular concern is the rapid spread of resistance mediated by extended-spectrum β-lactamases (ESBLs), especially in Klebsiella pneumoniae. ESBLs organisms are usually resistant to multiple antimicrobials, including third-generation and fourth-generation cephalosporins and aztreonam[33]. Sader and colleagues in their large cross-national research study reported that among Escherichia coli isolates from the ICUs, 13.7% were ESBLs producers while ESBLs-klebsiella spp. were 17.2%[34]. Another antibiotic class that over time increased the resistance of Escherichia coli is that of fluoroquinolones, usually considered active in this species[35,36]. Resistance of Pseudomonas aeruginosa to fluoroquinolones and imipenem has increased rapidly; above 10% of Pseudomonas aeruginosa are now resistant to multiple antibiotics classes such as cephalosporins, carbapenems, aminoglycosides and fluoroquinolones[33]. The increased use of carbapenems, which are among the most effective classes of antibiotics active against MDROs contributed to the emergence of CRE or CRAB[37,38]: Up to 25% of Acinetobacter baumannii isolates are CRAB[33]. The CRAB prevalence in Europe seems to be higher in south-eastern Europe, with the highest prevalence in Romania (86.5% meropenem 94.6% imipenem resistance)[39]. In the American continent, there seems to be a north-south gradient with all isolated Acinetobacter baumannii resistant to carbapenems in Uruguay[40], and practically absent in Canada[41]. More contained data come from Asia with China which seems to have the greatest number of CRAB. As for the African continent, there are few studies on the prevalence of carbapenem resistance[42,43]; in a study conducted in Uganda, the prevalence of CRAB is 81.25%[44]. Table 1 and Figure 1 report the worldwide prevalence of meropenem-resistant Acinetobacter baumannii; we decided to use meropenem as a benchmark to determine the occurrence of carbapenem resistance, to make tables and figures easier to read because in vitro studies involving isolates from ICU patients indicate that meropenem is more active against most GNBs than other comparators (including imipenem)[45]. More contained data concern the CRPA: In Europe, the data are more varied with very variable resistance, also between homogeneous nations in terms of geography, economy, and social progress; for example, in the Netherlands, the prevalence is 8.3%-17%[46] while in Germany it is 66.7%[47]. In North America the prevalence does not seem to exceed the two-fifths of the isolates, on the contrary, in a study conducted in Costa Rica, these exceeded four-fifths[48]. In Asia, the highest prevalence is in Korea with 92.9% of the BSI isolated from a burn ICU[49]. In Africa, the prevalence varies from about half of the isolates to almost all, as in Uganda with 88.8% of the CRPA[44] (Table 2). Figure 2 reports the worldwide prevalence of meropenem-resistant Pseudomonas aeruginosa.
| Continent | Country | Prevalence | Site of infection | Ref. |
| Africa | Uganda | 81.25 | Mix | [44] |
| America | Brazil | 22.8-94.2 | Mix | [42,59] |
| Canada | 4.4 | Mix | [41] | |
| French Guiana | 16.2 | Mix | [60] | |
| Mexico | 56.6 | Mix | [61] | |
| Uruguay | 100 | Mix | [40] | |
| United States | 61.2-74.2 | Mix | [26] | |
| Asia | China | 76.7-91.8 | Mix | [42,43] |
| India | 65.2 | VAP | [62] | |
| Indonesia | 16.7-68 | Mix | [50,63] | |
| Iran | 53.8-94.5 | Mix | [64,65] | |
| Jordan | 88.2 | Mix | [66] | |
| Kazakhstan | 44.4 | Mix | [67] | |
| Korea | 55.8-91.8 | Mix | [52,53] | |
| Saudi Arabia | 6.2-52.6 | Mix | [68,69] | |
| Taiwan | 50.7 | Mix | [70] | |
| Thailand | 40.5-69 | Mix | [71,72] | |
| Vietnam | 84 | VAP | [73] | |
| Europe | Germany | 431 | Mix | [74,75] |
| Greece | 58.9 | Mix | [76] | |
| Italy | 70 | VAP | [77] | |
| Lithuania | 30 | VAP | [78] | |
| Poland | 74.9-92.3 | Mix | [54,55] | |
| Romania | 86.5 | Mix | [39] | |
| Russia | 38-67.5 | Mix | [79,80] | |
| Serbia | 82 | HAC | [81] | |
| 85.3 | VAP | |||
| Spain | 86.05 | Mix | [82] | |
| Switzerland | 37 | Mix | [51] |
| Continent | Country | Prevalence | Site of infection | Ref. |
| Africa | Egypt | 41.82-78 | Mix | [83,84] |
| Lebanon | 42.9 | Mix | [83] | |
| Libya | 46 | Mix | [83] | |
| Tunisia | 53.71 | Mix | [83] | |
| Uganda | 88.8 | Mix | [83] | |
| America | Brazil | 22.9-51.8 | Mix | [85,86] |
| Canada | 18.3 | Mix | [41] | |
| Costa Rica | 91.3 | Ns | [48] | |
| United States | 12.9-43.3 | Mix | [87,88] | |
| Asia | Indonesia | 12.4-38.1 | Mix | [89,90] |
| Indonesia | 12.4-38.1 | Mix | [89,90] | |
| Iran | 25 | BSI | [91] | |
| Korea | 50-92.9 | BSI | [49,92] | |
| Qatar | 85.7 | Mix | [93] | |
| Saudi Arabia | 52.5 | Mix | [83] | |
| Taiwan | 22.5 | Mix | [94] | |
| United Arab Emirates | 7.7 | Mix | [83] | |
| Turkey | 46.7 | Mix | [95] | |
| Europe | Germany | 61-66.7 | Mix | [47,75] |
| Netherlands | 8.3-17 | Mix | [46] | |
| Serbia | 65.1 | HAC | [81] | |
| 70.2 | VAP | |||
| Switzerland | 27 | Mix | [51] |
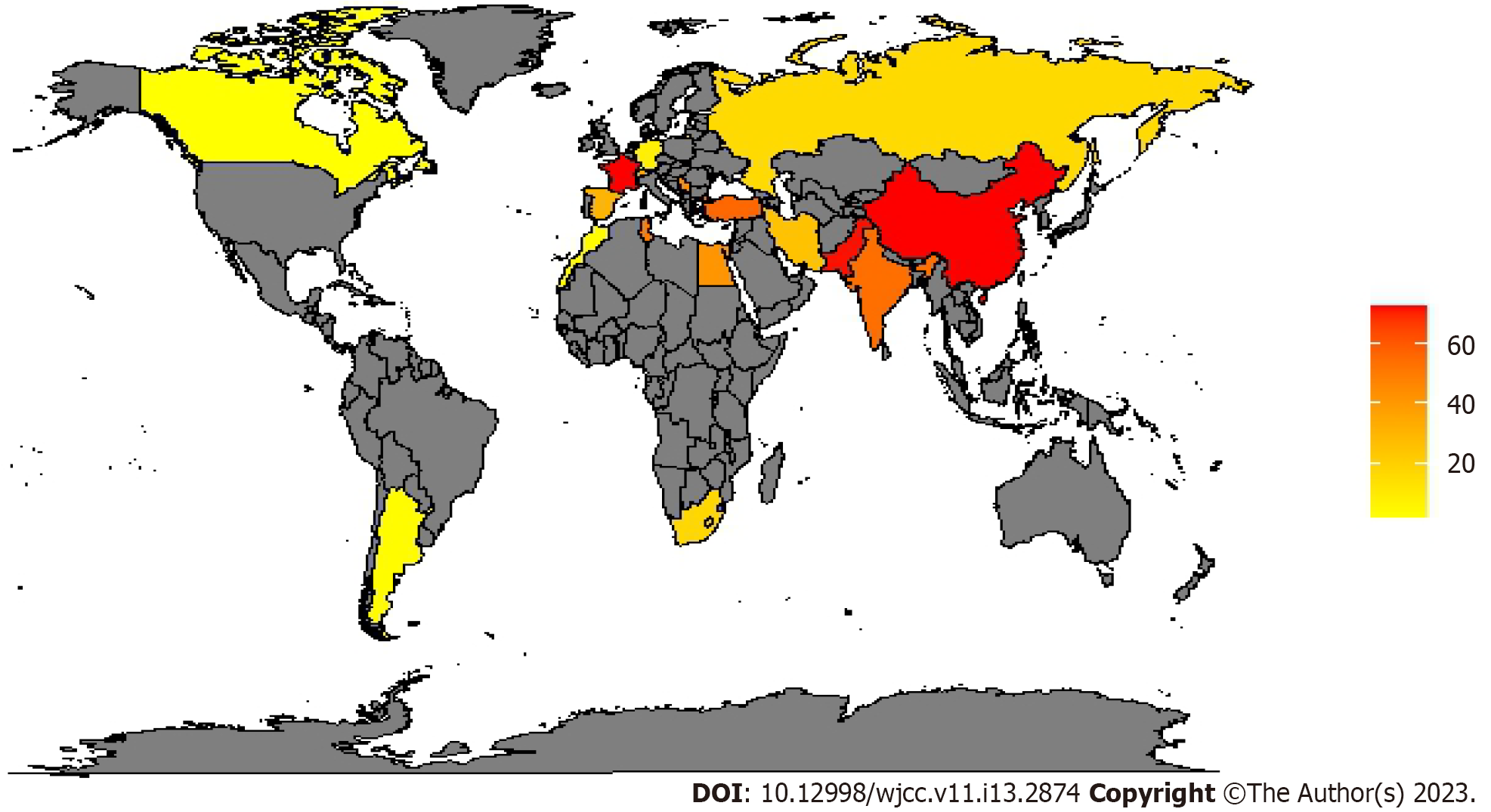
CRE account for approximately 20%-70% of Enterobacterales isolated in Europe[50,51], in North America, they remain almost non-existent in Canada[41], with a prevalence similar to the European one in the US[52-55]. In Asia data are very varied with a prevalence in China of 56.6%-76.7% of carbapenem-resistant Klebsiella pneumoniae (CRKP)[56,57]. From studies conducted in the African continent, Tunisia seems to be the country with the highest prevalence with a percentage of 85.2% of CRKP[58]. In Table 3 we reported the worldwide prevalence of meropenem-resistant Enterobacteriales, and in Figure 3 is shown the worldwide prevalence of CRKP which is the most common CRE.
| Continent | Country | Pathogen | Prevalence | Site of infection | Ref. |
| Africa | Egypt | Enterobacter cloacae; Escherichia coli; Klebsiella pneumoniae | 43.5; 27.1; 53.7 | Mix | [96] |
| Morocco | Enterobacteriales | 2.6 | Mix | [97] | |
| South Africa | Enterobacter spp. Other; Klebsiella spp. | 18; 6; 18 | Mix | [98] | |
| Tunisia | Enterobacter aerogenes; Enterobacter cloacae; Escherichia coli; K. Pneumonia; Providencia Stuartii | 0.9; 9.8; 2.9; 85.2; 0.9 | Mix | [58] | |
| America | Argentina | Enterobacteriales | 2.8 | BSI | [99] |
| Canada | Enterobacter cloacae; Escherichia coli; K. pmeumoniae; S marcescens | 0.8; 0.1; 0.2;0.5 | Mix | [41] | |
| United States | Citrobacter spp.; Enterobacter aerogenes; Enterobacter cloacae; Escherichia coli; Klebsiella oxytoca; Klebsiella pneumonia | 4; 6; 42; 14; 4; 30 | Mix | [87,88,100,101] | |
| Asia | China | Escherichia coli; Klebsiella Pneumoniae | 11.9; 57-76.7 | Mix | [56,57,102] |
| India | Klebsiella spp. | 54 | Mix | [103] | |
| Iran | Klebsiella Pneumoniae | 25.3 | Mix | [104] | |
| Korea | Enterobacteriales | 31.1 | BSI | [49] | |
| Pakistan | Klebsiella Pneumoniae | 72 | Mix | [97] | |
| Turkey | Klebsiella Pneumoniae | 44.7-67.47 | Mix | [105] | |
| Europe | France | Enterobacteriales | 72.8 | Mix | [106] |
| Germany | Escherichia coli; Klebsiella Pneumoniae | 3; 13 | Mix | [75] | |
| Greece | Klebsiella pneumoniae | 74 | NS | [107] | |
| Russia | Escherichia coli; Klebsiella spp.; Proteus spp. | 3; 16; 29 | NS | [108] | |
| Serbia | Enterobacter spp. | 36.4/35.9 | HAC/VAP | [81] | |
| Klebsiella pneumoniae | 50/56.8 | ||||
| Proteus mirabilis | 40/39.5 | ||||
| Spain | Enterobacteriales | 30.3 | NS | [84] | |
| Switzerland | Enterobacter spp.; Escherichia coli; Klebsiella pneumoniae | 77; 8; 11 | Mix | [50] |
Many risk factors can contribute to the genesis of antimicrobial resistance. They can be categorized as host, environmental, human, and protective barrier integrity factors[109]. Host risk factors include advanced age, organ and bone marrow transplant, end-stage renal disease in dialysis, intra-abdominal surgical procedures, cancer chemotherapy, immunosuppressive disease or therapy[26,110-112]. Prior use of antibiotics (90 days), prolonged antimicrobial usage and hospitalization (more than 5 days), use of indwelling catheters, long mechanical ventilation and residence in nursing homes and long-term care facilities are other important risk factors[110,112,113]. Numerous drugs used in ICU can be a risk factor predisposing patients to infections such as pneumonia (e.g., sedatives and muscle relaxants because they can reduce the cough and swallow reflexes) or gastrointestinal infections (e.g., proton pump inhibitors for stress ulcer prophylaxis because they disrupt the normal non-pathogenic bacterial flora)[110]. In this category, an important independent risk factor is previous MDROs infection or MDROs colonization. If the latter case occurs the probability of developing an infection is high[113]. Considering that some microorganisms can survive on surfaces, environmental is a category of risk factors, very dangerous for the genesis of antimicrobial resistance. It includes poor cleaning and disinfection of environmental surfaces as well as medical devices used for patient care (e.g., stethoscopes, thermometers, suction apparatus) that so became a source or reservoir to disseminate germs to other patients[114]. Among environmental risk factors, colonization pressure is of great importance. First described by Bonten for vancomycin-resistant Enterococci[115], and later for other bacteria as well[116-118], it is a critical parameter in the epidemiology of MDROs defined as the proportion of patients colonized with a microorganism in a given geographic area for a specified period[119]. It can be used to estimate the probability of cross-contamination[118], which is in turn an important indicator of poor hygiene especially when there is a clonal relationship of isolates[120]. In their study, Arvaniti et al[121] found that out of the total number of patients admitted to their ICU, 5.7% were already colonized at the hospitalization and of these 15.7% acquired Acinetobacter spp. during their ICU stay.
The main physical barriers of our body are the skin and mucosa membranes. They represent the first defensive bulwark against infections in general and therefore also for those supported by MDROs. Damage or interruption of their integrity using invasive devices in the ICU increases the risk of infections. In a recent meta-analysis by Hui Ang and Xuan, it was found that male gender (OR 1.40, 95%CI: 1.09, 1.80), having an operative procedure (OR 1.31, 95%CI: 1.10, 1.56), a central venous catheter (OR 1.22, 95%CI: 1.01, 1.48), mechanical ventilation (OR 1.25, 95%CI: 1.07, 1.46), previous antibiotic therapy (OR 1.66, 95%CI: 1.41, 1.96), length of ICU stay (weighted mean difference 8.18, 95%CI: 0.27, 16.10) were the identified risk factors associated with MDROs infections in ICU[122].
Infection prevention strategies can be divided into vertical or horizontal approaches[123,125]. Both go to integrate themselves into complex and various strategies to prevent MDROs infections. Vertical approaches involve the reduction of the risk of colonization, infection and transmission from high-risk pathogens or a specific group of them (e.g., Clostridium difficile, multidrug-resistant GNBs, and others)[124]. For this reason, they are valuable tools in controlling and managing an outbreak[123,124]. Vertical approaches are centered on the use of active surveillance testing to detect patients who are MDROs carriers (i.e., asymptomatic colonizers) and separate them from patients who are not colonized with that specific pathogen. This is because asymptomatic colonizers can spread the microorganism contaminating the environment and devices and favoring transmission through direct and indirect contact[124]. Examples of active surveillance testing are a rectal culture for CRE. Vertical strategies include also contact precaution and targeted decolonization (TD) for specific pathogens. TD has some limitations: the different decolonization strategies reduce the diffusion of a single specific target organism and not all-important organisms, such as multidrug-resistant GNBs and VRE, have options for decolonization[126]. Horizontal infection prevention strategies aim to reduce the risk of infections sustained by a broad spectrum of pathogens[124]. They include standard precautions (such as hand hygiene and use of personal protective equipment) and antimicrobial stewardship (AS). It should be noted that some interventions falling within the vertical approach, such as the use of gloves with or without gowns or the decolonization of the skin, can be applied to all patients (i.e., in a horizontal approach), not just those with a specific pathogen. According to the CDC and the WHO, hand hygiene remains the simplest and most important practice in infection control. In May 2009 the WHO drew up a simple and precise infographic (called "The 5 moments of hand hygiene") for hand hygiene or the transition from one patient to the next, to prevent cross-transmission[127]. Despite the evidence showing the effectiveness of hand hygiene in preventing infections and efforts to increase compliance rate, it remains low at between 40% and 60%[128,129]. AS is a set of strategies used to improve the use of antibiotics and limit the onset of resistance. It is centred on a systematic approach in multidisciplinary teams[130,131].
An AS programme should provide for: (1) The systematic search for causal agents by carrying out targeted crop surveys; the use of molecular biology tests can also enable important data to be obtained quickly; (2) Limiting the use of broad-spectrum drugs and reducing the duration of empirical therapy through de-escalation strategies[132], with timely replacement of these drugs with other narrow-spectrum drugs; (3) Base therapies on pharmacokinetic and pharmacodynamic criteria adapted to the conditions of critical patients and any changes in the volume of distribution, metabolism, and elimination of drugs; and (4) Optimization of therapy (i.e., adequate dosage, optimal mode of administration for the shortest possible time).
About AS it is important to note that data suggest that 30% to 60% of antibiotics prescribed in ICU are unnecessary, inappropriate, or suboptimal[133]. One of the possible reasons for this is the widespread belief that once the diagnosis of infection is made it is necessary to immediately start the antibiotic therapy with broad-spectrum drugs as each delay is associated with a worsening of the patient's outcome. This is true in infections with a rapid evolution (e.g., Meningitis) or for patients hemodynamically unstable. However, data suggest that in patients with infection but stable, a limited delay in the start of antibiotic therapy allowing the execution of targeted cultures would allow a more appropriate treatment and an improvement of the outcome[134]. It seems to be essential to identify protocols for the quickest identification of the germ causing the infection[135], in order not to use combination therapies whose efficacy on MDROs is not always the most effective[136,137]. A paradigmatic case seems to be the use of colistin in combination, which is the most common use in clinical practice[138], but randomized studies have not shown any benefits even in strains resistant to retrospectively identified as colistin-resistant[139]. Environmental cleaning and disinfection are other essential horizontal strategies for the control of infections and especially the prevention of cross-contamination[109]. It is important that in every hospital there is a systematic protocol for environmental cleaning and disinfection. It should address regular daily high-touch areas frequently exposed to human contact and emphasize adequate disinfection of the discharged patient’s room as a terminal cleaning practice[140].
Currently, antibiotics are still the first therapeutic weapon for patients with MDROs infection in ICU[141]. Despite government efforts and incentives for pharmacological research of new molecules, few antimicrobial agents remain effective against MDROs that are available in clinical practice. New antimicrobial agents recently approved or in advanced phases of clinical development including the new beta-lactam and beta-lactamase inhibitor combinations (ceftolozane/tazobactam, ceftazidime/avibactam, meropenem/vaborbactam, imipenem/cilastatin/relebactam, aztreonam/avibactam), siderophore cephalosporins (cefiderocol), aminoglycosides (plazomicin) and tetracyclines (eravacycline)[142]. Numerous incentives have been provided to encourage researchers to work on alternative strategies to reverse the resistance trend. There are numerous alternative therapeutic weapons to antimicrobials in the study that could be used in the future[141]. Our microbiota remains an important ally in the battle against MDROs infections. Therefore, it must remain unaltered. Two therapeutic options are currently being investigated to remove the antibiotic residues active in the colonic space where the highest concentrations of intestinal bacteria are found. The first is the use of an engineered, broad-spectrum beta-lactamase that aims at decaying any beta-lactamase in the gut. The second is colon-delivered active charcoal, which aims to adsorb free colonic compounds[141]. Phage therapy is another therapeutic alternative with an interest in the future. A serious advantage of phages over antibiotics is that is highly specific. For this, they can be a perfect weapon to decontaminate MDROs from the gastrointestinal tract, as only MDROs strains would be targeted while commensal strains would be spared[141]. Like phage another specific future possibility against MDROs infection is antibodies. To overcome the issue of immune reaction against monoclonal antibodies, they are now humanized. Examples of antibodies that are being developed in this context target virulence factors: Alpha-toxin of Staphylococcus aureus, the type III secretion system of Pseudomonas aeruginosa, and the toxin B of Clostridium difficile[141]. In addition, a vaccine against multidrug-resistant Acinetobacter baumannii is also under investigation at the preclinical stage[141].
A Specific carbapenem-resistant and carbapenemase-producing Organism Prevention Program for Public Health and Healthcare is recently uploaded by the California Department of Public Health; it is clearly articulated ten different points: (1) Laboratory Identification (implement the updated laboratory breakpoints for carbapenems and Enterobacterales); (2) Surveillance (ensure that the laboratory rapidly notifies infection prevention and clinical staff when a patient with carbapenem resistance is identified); (3) Colonization Testing (perform CRE colonization testing upon ICU admission of high-risk patients); (4) Infection Control Measures (place patients infected or colonized with CRE in a single room whenever possible, and implement Standard and Contact precautions); (5) Adherence Monitoring (use infection control assessment and adherence monitoring tools); (6) Environmental Cleaning (Ensure thorough daily and terminal environmental cleaning. Focus on high-touch surfaces or any shared reusable medical equipment); (7) Interfacility Communication (Communicate CRE status to the receiving facility ahead of time to ensure appropriate care is maintained when transferring a patient); (8) AS (Implement strategies to limit the use of broad-spectrum antimicrobial agents and an antimicrobial stewardship program); (9) Regional Prevention (Participate in regional efforts to prevent the spread of drug-resistant infections); and (10) Reporting (Report CPO cases through CalREDIE electronic laboratory reporting[143].
Antimicrobial resistance remains a huge public health problem on a global scale whose weight has a huge cost in terms of health expenditure and human lives. At present, antimicrobial agents remain the only causal therapeutic strategy available. Thanks to the efforts of research, in the future, we could use new therapeutic weapons as alternatives or even superior to antimicrobial agents[141]. At present, it is important to preserve the effectiveness of the last molecules put on the market, through a systematic implementation of strategies to minimize or prevent risk factors (first the pressure selection) and the spread of MDROs. For this purpose, in primis, the knowledge of local epidemiology and the creation of antimicrobial programs and diagnostic stewardship are mandatory to ensure the appropriateness of antimicrobial therapies. The WHO Global Action Plan on antimicrobial resistance gives strategic objectives, one of which is to strengthen knowledge through surveillance to cover the gaps in knowledge on the incidence, prevalence, and range of antimicrobial resistance across different geo
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Hu H, China; Jain N S-Editor: Ma YJ L-Editor: A P-Editor: Zhao S
| 1. | Hussein K, Raz-Pasteur A, Finkelstein R, Neuberger A, Shachor-Meyouhas Y, Oren I, Kassis I. Impact of carbapenem resistance on the outcome of patients' hospital-acquired bacteraemia caused by Klebsiella pneumoniae. J Hosp Infect. 2013;83:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 2. | Centers for Disease Control and Prevention (CDC). Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morb Mortal Wkly Rep. 2013;62:165-170. [PubMed] |
| 3. | World health Organization. WHO publishes list of bacteria for which new antibiotics are urgently needed. Available from: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed. |
| 4. | Bartsch SM, McKinnell JA, Mueller LE, Miller LG, Gohil SK, Huang SS, Lee BY. Potential economic burden of carbapenem-resistant Enterobacteriaceae (CRE) in the United States. Clin Microbiol Infect. 2017;23:48.e9-48.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 152] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 5. | Ben-David D, Kordevani R, Keller N, Tal I, Marzel A, Gal-Mor O, Maor Y, Rahav G. Outcome of carbapenem resistant Klebsiella pneumoniae bloodstream infections. Clin Microbiol Infect. 2012;18:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 250] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 6. | Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol. 2008;29:1099-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 655] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 7. | van Loon K, Voor In 't Holt AF, Vos MC. A Systematic Review and Meta-analyses of the Clinical Epidemiology of Carbapenem-Resistant Enterobacteriaceae. Antimicrob Agents Chemother. 2018;62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 172] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 8. | Coppry M, Jeanne-Leroyer C, Noize P, Dumartin C, Boyer A, Bertrand X, Dubois V, Rogues AM. Antibiotics associated with acquisition of carbapenem-resistant Pseudomonas aeruginosa in ICUs: a multicentre nested case-case-control study. J Antimicrob Chemother. 2019;74:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Woerther PL, Lepeule R, Burdet C, Decousser JW, Ruppé É, Barbier F. Carbapenems and alternative β-lactams for the treatment of infections due to extended-spectrum β-lactamase-producing Enterobacteriaceae: What impact on intestinal colonisation resistance? Int J Antimicrob Agents. 2018;52:762-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Raman G, Avendano EE, Chan J, Merchant S, Puzniak L. Risk factors for hospitalized patients with resistant or multidrug-resistant Pseudomonas aeruginosa infections: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2018;7:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 159] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 11. | Van Boeckel TP, Gandra S, Ashok A, Caudron Q, Grenfell BT, Levin SA, Laxminarayan R. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis. 2014;14:742-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1295] [Cited by in RCA: 1386] [Article Influence: 126.0] [Reference Citation Analysis (0)] |
| 12. | Versporten A, Zarb P, Caniaux I, Gros MF, Drapier N, Miller M, Jarlier V, Nathwani D, Goossens H, Global-PPS network. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: results of an internet-based global point prevalence survey. Lancet Glob Health. 2018;6:e619-e629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 429] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 13. | World Health Organization. Implementation manual to prevent and control the spread of carbapenem-resistant organisms at the national and health care facility level. Geneva, 201. Accessed September 30, 2022. Available from: https://www.who.int/publications/i/item/WHO-UHC-SDS-2019-6. |
| 14. | Bush K, Jacoby GA. Updated functional classification of beta-lactamases. Antimicrob Agents Chemother. 2010;54:969-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1377] [Cited by in RCA: 1546] [Article Influence: 96.6] [Reference Citation Analysis (0)] |
| 15. | Ambler RP. The structure of beta-lactamases. Philos Trans R Soc Lond B Biol Sci. 1980;289:321-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1155] [Cited by in RCA: 1256] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 16. | Nordmann P, Poirel L. Epidemiology and Diagnostics of Carbapenem Resistance in Gram-negative Bacteria. Clin Infect Dis. 2019;69:S521-S528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 444] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 17. | CRE Technical Information. Healthcare-Associated Infections (HAIs). Centres for Disease Control and Prevention (CDC). Accessed September 30, 2022. Available from: https://www.cdc.gov/hai/organisms/cre/technical-info.html. |
| 18. | European Centre for Disease Prevention and Control. Expert consensus protocol on carbapenem resistance detection and characterisation for the survey of carbapenem- and/or colistin-resistant Enterobacteriaceae – Version 3.0. Stockholm: ECDC; 2019. Accessed September 30, 2022. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/expert-consensus-protocol-carbapenem-resistance.pdf. |
| 19. | European Centre for Disease Prevention and Control. Antimicrobial resistance in the EU/EEA (EARS-Net) - Annual Epidemiological Report 2019. Accessed September 30, 2022. Available from: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2019. |
| 20. | World Health Organization. Regional Office for E. Central Asian and European surveillance of antimicrobial resistance: annual report 2020. Accessed September 30, 2022. Available from: https://apps.who.int/iris/handle/10665/345873. |
| 21. | Weiner-Lastinger LM, Abner S, Edwards JR, Kallen AJ, Karlsson M, Magill SS, Pollock D, See I, Soe MM, Walters MS, Dudeck MA. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network, 2015-2017. Infect Control Hosp Epidemiol. 2020;41:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 385] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 22. | Centers for Disease Control and Prevention. About Antimicrobial Resistance. Accessed September 30, 2022. Available from: https://www.cdc.gov/drugresistance/about.html. |
| 23. | Review on Antimicrobial Resistance. Tackling drug-resistant infections globally. Accessed September 30, 2022. Available from: https://amr-review.org/. |
| 24. | Lepape A, Jean A, De Waele J, Friggeri A, Savey A, Vanhems P, Gustin MP, Monnet DL, Garnacho-Montero J, Kohlenberg A. European intensive care physicians' experience of infections due to antibiotic-resistant bacteria. Antimicrob Resist Infect Control. 2020;9:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 25. | Alberti C, Brun-Buisson C, Burchardi H, Martin C, Goodman S, Artigas A, Sicignano A, Palazzo M, Moreno R, Boulmé R, Lepage E, Le Gall R. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive Care Med. 2002;28:108-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 571] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 26. | MacVane SH. Antimicrobial Resistance in the Intensive Care Unit: A Focus on Gram-Negative Bacterial Infections. J Intensive Care Med. 2017;32:25-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 27. | Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, Ray SM, Thompson DL, Wilson LE, Fridkin SK; Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370:1198-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2799] [Cited by in RCA: 2786] [Article Influence: 253.3] [Reference Citation Analysis (0)] |
| 28. | Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, Reinhart K; EPIC II Group of Investigators. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323-2329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2205] [Cited by in RCA: 2338] [Article Influence: 146.1] [Reference Citation Analysis (0)] |
| 29. | Guervil DJ, Chau T. Trends in multidrug-resistant gram-negative bacilli and the role of prolonged β-lactam infusion in the intensive care unit. Crit Care Nurs Q. 2013;36:345-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Iovene MR, Pota V, Galdiero M, Corvino G, Di Lella FM, Stelitano D, Passavanti MB, Pace MC, Alfieri A, Di Franco S, Aurilio C, Sansone P, Niyas VKM, Fiore M. First Italian outbreak of VIM-producing Serratia marcescens in an adult polyvalent intensive care unit, August-October 2018: A case report and literature review. World J Clin Cases. 2019;7:3535-3548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Rice LB. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis. 2008;197:1079-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1195] [Cited by in RCA: 1504] [Article Influence: 88.5] [Reference Citation Analysis (0)] |
| 32. | Peterson LR. Bad bugs, no drugs: no ESCAPE revisited. Clin Infect Dis. 2009;49:992-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 33. | Fish DN, Ohlinger MJ. Antimicrobial resistance: factors and outcomes. Crit Care Clin. 2006;22:291-311, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Sader HS, Farrell DJ, Flamm RK, Jones RN. Antimicrobial susceptibility of Gram-negative organisms isolated from patients hospitalised with pneumonia in US and European hospitals: results from the SENTRY Antimicrobial Surveillance Program, 2009-2012. Int J Antimicrob Agents. 2014;43:328-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 172] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 35. | Robicsek A, Jacoby GA, Hooper DC. The worldwide emergence of plasmid-mediated quinolone resistance. Lancet Infect Dis. 2006;6:629-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 637] [Cited by in RCA: 636] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 36. | Jacoby GA. Mechanisms of resistance to quinolones. Clin Infect Dis. 2005;41 Suppl 2:S120-S126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 581] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 37. | Fiore M, Di Franco S, Alfieri A, Passavanti MB, Pace MC, Petrou S, Martora F, Leone S. Spontaneous bacterial peritonitis due to carbapenemase-producing Enterobacteriaceae: Etiology and antibiotic treatment. World J Hepatol. 2020;12:1136-1147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Baditoiu L, Axente C, Lungeanu D, Muntean D, Horhat F, Moldovan R, Hogea E, Bedreag O, Sandesc D, Licker M. Intensive care antibiotic consumption and resistance patterns: a cross-correlation analysis. Ann Clin Microbiol Antimicrob. 2017;16:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 39. | Lăzureanu V, Poroșnicu M, Gândac C, Moisil T, Bădițoiu L, Laza R, Musta V, Crișan A, Marinescu AR. Infection with Acinetobacter baumannii in an intensive care unit in the Western part of Romania. BMC Infect Dis. 2016;16 Suppl 1:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 40. | Bado I, Papa-Ezdra R, Delgado-Blas JF, Gaudio M, Gutiérrez C, Cordeiro NF, García-Fulgueiras V, Araújo Pirez L, Seija V, Medina JC, Rieppi G, Gonzalez-Zorn B, Vignoli R. Molecular Characterization of Carbapenem-Resistant Acinetobacter baumannii in the Intensive Care Unit of Uruguay's University Hospital Identifies the First rmtC Gene in the Species. Microb Drug Resist. 2018;24:1012-1019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Denisuik AJ, Garbutt LA, Golden AR, Adam HJ, Baxter M, Nichol KA, Lagacé-Wiens P, Walkty AJ, Karlowsky JA, Hoban DJ, Mulvey MR, Zhanel GG. Antimicrobial-resistant pathogens in Canadian ICUs: results of the CANWARD 2007 to 2016 study. J Antimicrob Chemother. 2019;74:645-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 42. | Fiorentin Vandresen D, Carolina Lucio L, Shigueyasu Yamada R, Paula Vieira A, Ani Caovilla Follador F, Pitt Benedetti V, Pereira Bento Casaril K, Viviane Buzanello Martins C, Welter Wendt G, Elize Defante Ferreto L. Associated factors of Acinetobacter baumannii complex in hospitalized patients: A case-control study. J Infect Dev Ctries. 2021;15:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 43. | Chen Y, Yang Y, Liu L, Qiu G, Han X, Tian S, Zhao J, Chen F, Grundmann H, Li H, Sun J, Han L. High prevalence and clonal dissemination of OXA-72-producing Acinetobacter baumannii in a Chinese hospital: a cross sectional study. BMC Infect Dis. 2018;18:491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 44. | Aruhomukama D, Najjuka CF, Kajumbula H, Okee M, Mboowa G, Sserwadda I, Mayanja R, Joloba ML, Kateete DP. bla(VIM)- and bla(OXA)-mediated carbapenem resistance among Acinetobacter baumannii and Pseudomonas aeruginosa isolates from the Mulago hospital intensive care unit in Kampala, Uganda. BMC Infect Dis. 2019;19:853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 45. | Hurst M, Lamb HM. Meropenem: a review of its use in patients in intensive care. Drugs. 2000;59:653-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 53] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Croughs PD, Klaassen CHW, van Rosmalen J, Maghdid DM, Boers SA, Hays JP, Goessens WHF; Dutch Antibiotic Resistance Surveillance Group. Unexpected mechanisms of resistance in Dutch Pseudomonas aeruginosa isolates collected during 14 years of surveillance. Int J Antimicrob Agents. 2018;52:407-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 47. | Schäfer E, Malecki M, Tellez-Castillo CJ, Pfennigwerth N, Marlinghaus L, Higgins PG, Mattner F, Wendel AF. Molecular surveillance of carbapenemase-producing Pseudomonas aeruginosa at three medical centres in Cologne, Germany. Antimicrob Resist Infect Control. 2019;8:208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 48. | Toval F, Guzmán-Marte A, Madriz V, Somogyi T, Rodríguez C, García F. Predominance of carbapenem-resistant Pseudomonas aeruginosa isolates carrying blaIMP and blaVIM metallo-β-lactamases in a major hospital in Costa Rica. J Med Microbiol. 2015;64:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 49. | Park JJ, Seo YB, Choi YK, Kym D, Lee J. Changes in the prevalence of causative pathogens isolated from severe burn patients from 2012 to 2017. Burns. 2020;46:695-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 50. | Anggraini D, Kemal RA, Hadi U, Kuntaman K. The susceptibility pattern and distribution of blaOXA-23 genes of clinical isolate Acinetobacter baumannii in a tertiary hospital, Indonesia. J Infect Dev Ctries. 2022;16:821-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 51. | Barnsteiner S, Baty F, Albrich WC, Babouee Flury B, Gasser M, Plüss-Suard C, Schlegel M, Kronenberg A, Kohler P; Swiss Centre for Antibiotic Resistance (ANRESIS). Antimicrobial resistance and antibiotic consumption in intensive care units, Switzerland, 2009 to 2018. Euro Surveill. 2021;26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 52. | Liu Q, Li W, Du X, Zhong T, Tang Y, Feng Y, Tao C, Xie Y. Risk and Prognostic Factors for Multidrug-Resistant Acinetobacter Baumannii Complex Bacteremia: A Retrospective Study in a Tertiary Hospital of West China. PLoS One. 2015;10:e0130701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 53. | Chiang CH, Pan SC, Yang TS, Matsuda K, Kim HB, Choi YH, Hori S, Wang JT, Sheng WH, Chen YC, Chang FY, Chang SC. Healthcare-associated infections in intensive care units in Taiwan, South Korea, and Japan: recent trends based on national surveillance reports. Antimicrob Resist Infect Control. 2018;7:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 54. | Ziółkowski G, Pawłowska I, Krawczyk L, Wojkowska-Mach J. Antibiotic consumption versus the prevalence of multidrug-resistant Acinetobacter baumannii and Clostridium difficile infections at an ICU from 2014-2015. J Infect Public Health. 2018;11:626-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 55. | Litwin A, Fedorowicz O, Duszynska W. Characteristics of Microbial Factors of Healthcare-Associated Infections Including Multidrug-Resistant Pathogens and Antibiotic Consumption at the University Intensive Care Unit in Poland in the Years 2011-2018. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 56. | Chang H, Wei J, Zhou W, Yan X, Cao X, Zuo L, Chen S, Yao K, Huang R, Chen Y, Wu C. Risk factors and mortality for patients with Bloodstream infections of Klebsiella pneumoniae during 2014-2018: Clinical impact of carbapenem resistance in a large tertiary hospital of China. J Infect Public Health. 2020;13:784-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 57. | Zhang WX, Chen HY, Chen C, Chen JH, Wan FS, Li LX, Chen M, Zhang J. Resistance Phenotype and Molecular Epidemiology of Carbapenem-Resistant Klebsiella pneumoniae Isolates in Shanghai. Microb Drug Resist. 2021;27:1312-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 58. | Ben Helal R, Dziri R, Chedly M, Klibi N, Barguellil F, El Asli MS, Ben Moussa M. Occurrence and Characterization of Carbapenemase-Producing Enterobacteriaceae in a Tunisian Hospital. Microb Drug Resist. 2018;24:1361-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 59. | Kurihara MNL, Sales RO, Silva KED, Silva GD, Mansano MCT, Mahmoud FF, Simionatto S. High lethality rate of carbapenem-resistant Acinetobacter baumannii in Intensive Care Units of a Brazilian hospital: An epidemiologic surveillance study. Rev Soc Bras Med Trop. 2022;55:e05292021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 60. | Mahamat A, Bertrand X, Moreau B, Hommel D, Couppie P, Simonnet C, Kallel H, Demar M, Djossou F, Nacher M. Clinical epidemiology and resistance mechanisms of carbapenem-resistant Acinetobacter baumannii, French Guiana, 2008-2014. Int J Antimicrob Agents. 2016;48:51-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 61. | Uc-Cachón AH, Gracida-Osorno C, Luna-Chi IG, Jiménez-Guillermo JG, Molina-Salinas GM. High Prevalence of Antimicrobial Resistance Among Gram-Negative Isolated Bacilli in Intensive Care Units at a Tertiary-Care Hospital in Yucatán Mexico. Medicina (Kaunas). 2019;55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 62. | Kumari M, Verma S, Venkatesh V, Gupta P, Tripathi P, Agarwal A, Siddiqui SS, Arshad Z, Prakash V. Emergence of blaNDM-1 and blaVIM producing Gram-negative bacilli in ventilator-associated pneumonia at AMR Surveillance Regional Reference Laboratory in India. PLoS One. 2021;16:e0256308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 63. | Saharman YR, Karuniawati A, Sedono R, Aditianingsih D, Sudarmono P, Goessens WHF, Klaassen CHW, Verbrugh HA, Severin JA. Endemic carbapenem-nonsusceptible Acinetobacter baumannii-calcoaceticus complex in intensive care units of the national referral hospital in Jakarta, Indonesia. Antimicrob Resist Infect Control. 2018;7:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 64. | Pourajam S, Kalantari E, Talebzadeh H, Mellali H, Sami R, Soltaninejad F, Amra B, Sajadi M, Alenaseri M, Kalantari F, Solgi H. Secondary Bacterial Infection and Clinical Characteristics in Patients With COVID-19 Admitted to Two Intensive Care Units of an Academic Hospital in Iran During the First Wave of the Pandemic. Front Cell Infect Microbiol. 2022;12:784130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 65. | Ranjbar R, Farahani A. Study of genetic diversity, biofilm formation, and detection of Carbapenemase, MBL, ESBL, and tetracycline resistance genes in multidrug-resistant Acinetobacter baumannii isolated from burn wound infections in Iran. Antimicrob Resist Infect Control. 2019;8:172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 66. | Nazer LH, Kharabsheh A, Rimawi D, Mubarak S, Hawari F. Characteristics and Outcomes of Acinetobacter baumannii Infections in Critically Ill Patients with Cancer: A Matched Case-Control Study. Microb Drug Resist. 2015;21:556-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 67. | Viderman D, Brotfain E, Khamzina Y, Kapanova G, Zhumadilov A, Poddighe D. Bacterial resistance in the intensive care unit of developing countries: Report from a tertiary hospital in Kazakhstan. J Glob Antimicrob Resist. 2019;17:35-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 68. | Aljindan R, Bukharie H, Alomar A, Abdalhamid B. Prevalence of digestive tract colonization of carbapenem-resistant Acinetobacter baumannii in hospitals in Saudi Arabia. J Med Microbiol. 2015;64:400-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 69. | Al-Hamad A, Pal T, Leskafi H, Abbas H, Hejles H, Alsubikhy F, Darwish D, Ghazawi A, Sonnevend A. Molecular characterization of clinical and environmental carbapenem resistant Acinetobacter baumannii isolates in a hospital of the Eastern Region of Saudi Arabia. J Infect Public Health. 2020;13:632-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 70. | Chen YP, Liang CC, Chang R, Kuo CM, Hung CH, Liao TN, Liao CS. Detection and Colonization of Multidrug Resistant Organisms in a Regional Teaching Hospital of Taiwan. Int J Environ Res Public Health. 2019;16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 71. | Chusri S, Chongsuvivatwong V, Rivera JI, Silpapojakul K, Singkhamanan K, McNeil E, Doi Y. Molecular epidemiology and spatiotemporal analysis of hospital-acquired Acinetobacter baumannii infection in a tertiary care hospital in southern Thailand. J Hosp Infect. 2017;95:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 72. | Kiddee A, Assawatheptawee K, Na-Udom A, Treebupachatsakul P, Wangteeraprasert A, Walsh TR, Niumsup PR. Risk Factors for Gastrointestinal Colonization and Acquisition of Carbapenem-Resistant Gram-Negative Bacteria among Patients in Intensive Care Units in Thailand. Antimicrob Agents Chemother. 2018;62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 73. | Le Minh V, Thi Khanh Nhu N, Vinh Phat V, Thompson C, Huong Lan NP, Thieu Nga TV, Thanh Tam PT, Tuyen HT, Hoang Nhu TD, Van Hao N, Thi Loan H, Minh Yen L, Parry CM, Trung Nghia HD, Campbell JI, Hien TT, Thwaites L, Thwaites G, Van Vinh Chau N, Baker S. In vitro activity of colistin in antimicrobial combination against carbapenem-resistant Acinetobacter baumannii isolated from patients with ventilator-associated pneumonia in Vietnam. J Med Microbiol. 2015;64:1162-1169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 74. | Wendel AF, Malecki M, Otchwemah R, Tellez-Castillo CJ, Sakka SG, Mattner F. One-year molecular surveillance of carbapenem-susceptible A. baumannii on a German intensive care unit: diversity or clonality. Antimicrob Resist Infect Control. 2018;7:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 75. | Maechler F, Peña Diaz LA, Schröder C, Geffers C, Behnke M, Gastmeier P. Prevalence of carbapenem-resistant organisms and other Gram-negative MDRO in German ICUs: first results from the national nosocomial infection surveillance system (KISS). Infection. 2015;43:163-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 76. | Karampatakis T, Tsergouli K, Politi L, Diamantopoulou G, Iosifidis E, Antachopoulos C, Karyoti A, Mouloudi E, Tsakris A, Roilides E. Molecular Epidemiology of Endemic Carbapenem-Resistant Gram-Negative Bacteria in an Intensive Care Unit. Microb Drug Resist. 2019;25:712-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 77. | Delle Rose D, Pezzotti P, Fortunato E, Sordillo P, Gini S, Boros S, Meledandri M, Gallo MT, Prignano G, Caccese R, D'Ambrosio M, Citterio G, Rocco M, Leonardis F, Natoli S, Fontana C, Favaro M, Celeste MG, Franci T, Testore GP, Andreoni M, Sarmati L. Clinical predictors and microbiology of ventilator-associated pneumonia in the intensive care unit: a retrospective analysis in six Italian hospitals. Eur J Clin Microbiol Infect Dis. 2016;35:1531-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 78. | Čiginskienė A, Dambrauskienė A, Rello J, Adukauskienė D. Ventilator-Associated Pneumonia due to Drug-Resistant Acinetobacter baumannii: Risk Factors and Mortality Relation with Resistance Profiles, and Independent Predictors of In-Hospital Mortality. Medicina (Kaunas). 2019;55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 79. | Mayanskiy N, Chebotar I, Alyabieva N, Kryzhanovskaya O, Savinova T, Turenok A, Bocharova Y, Lazareva A, Polikarpova S, Karaseva O. Emergence of the Uncommon Clone ST944/ST78 Carrying bla(OXA-40-like) and bla(CTX-M-like) Genes Among Carbapenem-Nonsusceptible Acinetobacter baumannii in Moscow, Russia. Microb Drug Resist. 2017;23:864-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 80. | Ershova K, Savin I, Kurdyumova N, Wong D, Danilov G, Shifrin M, Alexandrova I, Sokolova E, Fursova N, Zelman V, Ershova O. Implementing an infection control and prevention program decreases the incidence of healthcare-associated infections and antibiotic resistance in a Russian neuro-ICU. Antimicrob Resist Infect Control. 2018;7:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 81. | Djordjevic ZM, Folic MM, Jankovic SM. Distribution and antibiotic susceptibility of pathogens isolated from adults with hospital-acquired and ventilator-associated pneumonia in intensive care unit. J Infect Public Health. 2017;10:740-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 82. | Rodríguez-Lucas C, Rodicio MR, Vázquez X, Escudero D, Quindós B, Alaguero M, Fernández J. Extensively drug-resistant Acinetobacter baumannii carrying bla(OXA-23-like) and armA in a hospital after an intervention in the intensive care unit which ended a long-standing endemicity. Eur J Clin Microbiol Infect Dis. 2021;40:385-389. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 83. | Al-Orphaly M, Hadi HA, Eltayeb FK, Al-Hail H, Samuel BG, Sultan AA, Skariah S. Epidemiology of Multidrug-Resistant Pseudomonas aeruginosa in the Middle East and North Africa Region. mSphere. 2021;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 84. | Elbaradei A, Sayedahmed MS, El-Sawaf G, Shawky SM. Screening of mcr-1 among Gram-Negative Bacteria from Different Clinical Samples from ICU Patients in Alexandria, Egypt: One-Year Study. Pol J Microbiol. 2022;71:83-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 85. | de Almeida Silva KCF, Calomino MA, Deutsch G, de Castilho SR, de Paula GR, Esper LMR, Teixeira LA. Molecular characterization of multidrug-resistant (MDR) Pseudomonas aeruginosa isolated in a burn center. Burns. 2017;43:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 86. | Rodrigues YC, Furlaneto IP, Maciel AHP, Quaresma AJPG, de Matos ECO, Conceição ML, Vieira MCDS, Brabo GLDC, Sarges EDSNF, Lima LNGC, Lima KVB. High prevalence of atypical virulotype and genetically diverse background among Pseudomonas aeruginosa isolates from a referral hospital in the Brazilian Amazon. PLoS One. 2020;15:e0238741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 87. | Jarrell AS, Kruer RM, Berescu LD, Pronovost PJ, Trivedi JB. Factors associated with in-hospital mortality among critically ill surgical patients with multidrug-resistant Gram-negative infections. J Crit Care. 2018;43:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 88. | Sader HS, Mendes RE, Streit JM, Carvalhaes CG, Castanheira M. Antimicrobial susceptibility of Gram-negative bacteria from intensive care unit and non-intensive care unit patients from United States hospitals (2018-2020). Diagn Microbiol Infect Dis. 2022;102:115557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 89. | Pelegrin AC, Saharman YR, Griffon A, Palmieri M, Mirande C, Karuniawati A, Sedono R, Aditianingsih D, Goessens WHF, van Belkum A, Verbrugh HA, Klaassen CHW, Severin JA. High-Risk International Clones of Carbapenem-Nonsusceptible Pseudomonas aeruginosa Endemic to Indonesian Intensive Care Units: Impact of a Multifaceted Infection Control Intervention Analyzed at the Genomic Level. mBio. 2019;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 90. | Saharman YR, Pelegrin AC, Karuniawati A, Sedono R, Aditianingsih D, Goessens WHF, Klaassen CHW, van Belkum A, Mirande C, Verbrugh HA, Severin JA. Epidemiology and characterisation of carbapenem-non-susceptible Pseudomonas aeruginosa in a large intensive care unit in Jakarta, Indonesia. Int J Antimicrob Agents. 2019;54:655-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 91. | Emami A, Pirbonyeh N, Keshavarzi A, Bazargani A, Hassanpour S, Javanmardi F. Evaluating the Saliva of Burn ICU Patients for Resistant Infections Harbor Metallo-β-Lactamase Genes. J Burn Care Res. 2020;41:647-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 92. | Kang JS, Moon C, Mun SJ, Lee JE, Lee SO, Lee S, Lee SH. Antimicrobial Susceptibility Trends and Risk Factors for Antimicrobial Resistance in Pseudomonas aeruginosa Bacteremia: 12-Year Experience in a Tertiary Hospital in Korea. J Korean Med Sci. 2021;36:e273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 93. | Baiou A, Elbuzidi AA, Bakdach D, Zaqout A, Alarbi KM, Bintaher AA, Ali MMB, Elarabi AM, Ali GAM, Daghfal J, Almaslamani MA, Ibrahim ASS, Alkhal A, Omrani AS. Clinical characteristics and risk factors for the isolation of multi-drug-resistant Gram-negative bacteria from critically ill patients with COVID-19. J Hosp Infect. 2021;110:165-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 94. | Lin KY, Lauderdale TL, Wang JT, Chang SC. Carbapenem-resistant Pseudomonas aeruginosa in Taiwan: Prevalence, risk factors, and impact on outcome of infections. J Microbiol Immunol Infect. 2016;49:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 95. | Acar A, Karaahmetoğlu G, Akalın H, Altay AF. Pooled prevalence and trends of antimicrobial resistance in Pseudomonas aeruginosa clinical isolates over the past 10 years in Turkey: A meta-analysis. J Glob Antimicrob Resist. 2019;18:64-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 96. | Kotb S, Lyman M, Ismail G, Abd El Fattah M, Girgis SA, Etman A, Hafez S, El-Kholy J, Zaki MES, Rashed HG, Khalil GM, Sayyouh O, Talaat M. Epidemiology of Carbapenem-resistant Enterobacteriaceae in Egyptian intensive care units using National Healthcare-associated Infections Surveillance Data, 2011-2017. Antimicrob Resist Infect Control. 2020;9:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 97. | Chabah M, Chemsi M, Zerouali K, Alloula O, Lehlimi M, Habzi A, Benomar S. Healthcare-associated infections due to carbapenemase-producing Enterobacteriaceae: Bacteriological profile and risk factors. Med Mal Infect. 2016;46:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 98. | Law T, Chibabhai V, Nana T. Analysis and comparison of cumulative antibiograms for the Charlotte Maxeke Johannesburg Academic Hospital adult intensive care and high-care units, 2013 and 2017. S Afr Med J. 2019;110:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 99. | Lipari FG, Hernández D, Vilaró M, Caeiro JP, Saka HA. [Clinical, epidemiological and microbiological characterization of bacteremia produced by carbapenem-resistant enterobacteria in a university hospital in Córdoba, Argentina]. Rev Chilena Infectol. 2020;37:362-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 100. | Johnson JK, Robinson GL, Pineles LL, Ajao AO, Zhao L, Albrecht JS, Harris AD, Thom KA, Furuno JP. Carbapenem MICs in Escherichia coli and Klebsiella Species Producing Extended-Spectrum β-Lactamases in Critical Care Patients from 2001 to 2009. Antimicrob Agents Chemother. 2017;61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 101. | Tamma PD, Kazmi A, Bergman Y, Goodman KE, Ekunseitan E, Amoah J, Simner PJ. The Likelihood of Developing a Carbapenem-Resistant Enterobacteriaceae Infection during a Hospital Stay. Antimicrob Agents Chemother. 2019;63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 102. | Li Y, Sun QL, Shen Y, Zhang Y, Yang JW, Shu LB, Zhou HW, Wang Y, Wang B, Zhang R, Wang S, Shen Z. Rapid Increase in Prevalence of Carbapenem-Resistant Enterobacteriaceae (CRE) and Emergence of Colistin Resistance Gene mcr-1 in CRE in a Hospital in Henan, China. J Clin Microbiol. 2018;56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 103. | Sodhi K, Mittal V, Arya M, Kumar M, Phillips A, Kajla B. Pattern of colistin resistance in Klebsiella isolates in an Intensive Care Unit of a tertiary care hospital in India. J Infect Public Health. 2020;13:1018-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 104. | Darabi N, Motazakker M, Khalkhali HR, Yousefi S. A multicenter study of β-lactamase-producing Klebsiella pneumoniae isolated from university teaching hospitals of Urmia, Iran. J Infect Dev Ctries. 2019;13:690-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 105. | Candevir Ulu A, Güven Gökmen T, Kibar F, Kurtaran B, Önlen C, Kuşçu F, İnal AS, Kömür S, Yaman A, Aksu HSZ, Taşova Y. Molecular epidemiology of carbapenem-resistant Klebsiella pneumoniae at a Turkish centre: Is the increase of resistance a threat for Europe? J Glob Antimicrob Resist. 2017;11:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 106. | Davido B, Moussiegt A, Dinh A, Bouchand F, Matt M, Senard O, Deconinck L, Espinasse F, Lawrence C, Fortineau N, Saleh-Mghir A, Caballero S, Escaut L, Salomon J. Germs of thrones - spontaneous decolonization of Carbapenem-Resistant Enterobacteriaceae (CRE) and Vancomycin-Resistant Enterococci (VRE) in Western Europe: is this myth or reality? Antimicrob Resist Infect Control. 2018;7:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 107. | Karampatakis T, Antachopoulos C, Iosifidis E, Tsakris A, Roilides E. Molecular epidemiology of carbapenem-resistant Klebsiella pneumoniae in Greece. Future Microbiol. 2016;11:809-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 108. | Partina I, Kalinogorskaya O, Kojima S, Gostev V, Volkova M, Ageevets V, Lobzin Y, Sidorenko S. Surveillance of antimicrobial susceptibility of Enterobacteriaceae pathogens isolated from intensive care units and surgical units in Russia. Jpn J Antibiot. 2016;69:41-51. [PubMed] |
| 109. | Riley MM. The Rising Problem of Multidrug-Resistant Organisms in Intensive Care Units. Crit Care Nurse. 2019;39:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 110. | Brusselaers N, Vogelaers D, Blot S. The rising problem of antimicrobial resistance in the intensive care unit. Ann Intensive Care. 2011;1:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 209] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 111. | Umscheid CA, Agarwal RK, Brennan PJ; Healthcare Infection Control Practices Advisory Committee. Updating the guideline development methodology of the Healthcare Infection Control Practices Advisory Committee (HICPAC). Am J Infect Control. 2010;38:264-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 112. | Djibré M, Fedun S, Le Guen P, Vimont S, Hafiani M, Fulgencio JP, Parrot A, Denis M, Fartoukh M. Universal versus targeted additional contact precautions for multidrug-resistant organism carriage for patients admitted to an intensive care unit. Am J Infect Control. 2017;45:728-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 113. | Martín-Loeches I, Diaz E, Vallés J. Risks for multidrug-resistant pathogens in the ICU. Curr Opin Crit Care. 2014;20:516-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 114. | Kanamori H, Parobek CM, Juliano JJ, van Duin D, Cairns BA, Weber DJ, Rutala WA. A Prolonged Outbreak of KPC-3-Producing Enterobacter cloacae and Klebsiella pneumoniae Driven by Multiple Mechanisms of Resistance Transmission at a Large Academic Burn Center. Antimicrob Agents Chemother. 2017;61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 115. | Bonten MJ, Slaughter S, Ambergen AW, Hayden MK, van Voorhis J, Nathan C, Weinstein RA. The role of "colonization pressure" in the spread of vancomycin-resistant enterococci: an important infection control variable. Arch Intern Med. 1998;158:1127-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 315] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 116. | Merrer J, Santoli F, Appéré de Vecchi C, Tran B, De Jonghe B, Outin H. "Colonization pressure" and risk of acquisition of methicillin-resistant Staphylococcus aureus in a medical intensive care unit. Infect Control Hosp Epidemiol. 2000;21:718-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 206] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 117. | Harris AD, Johnson JK, Thom KA, Morgan DJ, McGregor JC, Ajao AO, Moore AC, Comer AC, Furuno JP. Risk factors for development of intestinal colonization with imipenem-resistant Pseudomonas aeruginosa in the intensive care unit setting. Infect Control Hosp Epidemiol. 2011;32:719-722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 118. | Ajao AO, Harris AD, Roghmann MC, Johnson JK, Zhan M, McGregor JC, Furuno JP. Systematic review of measurement and adjustment for colonization pressure in studies of methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, and clostridium difficile acquisition. Infect Control Hosp Epidemiol. 2011;32:481-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 119. | Bonten MJ, Gaillard CA, Johanson WG Jr, van Tiel FH, Smeets HG, van der Geest S, Stobberingh EE. Colonization in patients receiving and not receiving topical antimicrobial prophylaxis. Am J Respir Crit Care Med. 1994;150:1332-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 120. | Bonten MJ. Colonization pressure: a critical parameter in the epidemiology of antibiotic-resistant bacteria. Crit Care. 2012;16:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 121. | Arvaniti K, Lathyris D, Ruimy R, Haidich AB, Koulourida V, Nikolaidis P, Matamis D, Miyakis S. The importance of colonization pressure in multiresistant Acinetobacter baumannii acquisition in a Greek intensive care unit. Crit Care. 2012;16:R102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 122. | Ang H, Sun X. Risk factors for multidrug-resistant Gram-negative bacteria infection in intensive care units: A meta-analysis. Int J Nurs Pract. 2018;24:e12644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 123. | Popovich KJ. Another success story for horizontal infection control strategies--which one? Crit Care Med. 2014;42:2292-2293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 124. | Septimus E, Weinstein RA, Perl TM, Goldmann DA, Yokoe DS. Approaches for preventing healthcare-associated infections: go long or go wide? Infect Control Hosp Epidemiol. 2014;35:797-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 125. | Wenzel RP, Edmond MB. Infection control: the case for horizontal rather than vertical interventional programs. Int J Infect Dis. 2010;14 Suppl 4:S3-S5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 126. | Calfee DP, Salgado CD, Milstone AM, Harris AD, Kuhar DT, Moody J, Aureden K, Huang SS, Maragakis LL, Yokoe DS; Society for Healthcare Epidemiology of America. Strategies to prevent methicillin-resistant Staphylococcus aureus transmission and infection in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35:772-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 127. | World Health Organization. WHO guidelines on hand hygiene in health care. Accessed September 30, 2022. Available from: https://www.who.int/publications/i/item/9789241597906. |
| 128. | Erasmus V, Daha TJ, Brug H, Richardus JH, Behrendt MD, Vos MC, van Beeck EF. Systematic review of studies on compliance with hand hygiene guidelines in hospital care. Infect Control Hosp Epidemiol. 2010;31:283-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 807] [Cited by in RCA: 666] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 129. | Kowitt B, Jefferson J, Mermel LA. Factors associated with hand hygiene compliance at a tertiary care teaching hospital. Infect Control Hosp Epidemiol. 2013;34:1146-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 130. | Luyt CE, Bréchot N, Trouillet JL, Chastre J. Antibiotic stewardship in the intensive care unit. Crit Care. 2014;18:480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 219] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 131. | Onorato L, Macera M, Calò F, Monari C, Russo F, Iovene MR, Signoriello G, Annibale R, Pace MC, Aurilio C, Gaeta GB, Coppola N. The effect of an antimicrobial stewardship programme in two intensive care units of a teaching hospital: an interrupted time series analysis. Clin Microbiol Infect. 2020;26:782.e1-782.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 132. | Tabah A, Bassetti M, Kollef MH, Zahar JR, Paiva JA, Timsit JF, Roberts JA, Schouten J, Giamarellou H, Rello J, De Waele J, Shorr AF, Leone M, Poulakou G, Depuydt P, Garnacho-Montero J. Antimicrobial de-escalation in critically ill patients: a position statement from a task force of the European Society of Intensive Care Medicine (ESICM) and European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Critically Ill Patients Study Group (ESGCIP). Intensive Care Med. 2020;46:245-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 133. | Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P T. 2015;40:277-283. [PubMed] |
| 134. | Hranjec T, Rosenberger LH, Swenson B, Metzger R, Flohr TR, Politano AD, Riccio LM, Popovsky KA, Sawyer RG. Aggressive versus conservative initiation of antimicrobial treatment in critically ill surgical patients with suspected intensive-care-unit-acquired infection: a quasi-experimental, before and after observational cohort study. Lancet Infect Dis. 2012;12:774-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 135. | Fiore M, Maraolo AE, Leone S, Gentile I, Cuomo A, Schiavone V, Bimonte S, Pace MC, Cascella M. Spontaneous peritonitis in critically ill cirrhotic patients: a diagnostic algorithm for clinicians and future perspectives. Ther Clin Risk Manag. 2017;13:1409-1414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 136. | Fiore M, Alfieri A, Di Franco S, Pace MC, Simeon V, Ingoglia G, Cortegiani A. Ceftazidime-Avibactam Combination Therapy Compared to Ceftazidime-Avibactam Monotherapy for the Treatment of Severe Infections Due to Carbapenem-Resistant Pathogens: A Systematic Review and Network Meta-Analysis. Antibiotics (Basel). 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 137. | Fiore M, Corrente A, Pace MC, Alfieri A, Simeon V, Ippolito M, Giarratano A, Cortegiani A. Ceftolozane-Tazobactam Combination Therapy Compared to Ceftolozane-Tazobactam Monotherapy for the Treatment of Severe Infections: A Systematic Review and Meta-Analysis. Antibiotics (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 138. | Giacobbe DR, Saffioti C, Losito AR, Rinaldi M, Aurilio C, Bolla C, Boni S, Borgia G, Carannante N, Cassola G, Ceccarelli G, Corcione S, Dalla Gasperina D, De Rosa FG, Dentone C, Di Bella S, Di Lauria N, Feasi M, Fiore M, Fossati S, Franceschini E, Gori A, Granata G, Grignolo S, Grossi PA, Guadagnino G, Lagi F, Maraolo AE, Marinò V, Mazzitelli M, Mularoni A, Oliva A, Pace MC, Parisini A, Patti F, Petrosillo N, Pota V, Raffaelli F, Rossi M, Santoro A, Tascini C, Torti C, Trecarichi EM, Venditti M, Viale P, Signori A, Bassetti M, Del Bono V, Giannella M, Mikulska M, Tumbarello M, Viscoli C; SITA GIOVANI (Young Investigators Group of the Società Italiana Terapia Antinfettiva) and the COLI-CROSS Study Group. Use of colistin in adult patients: A cross-sectional study. J Glob Antimicrob Resist. 2020;20:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 139. | Dickstein Y, Lellouche J, Ben Dalak Amar M, Schwartz D, Nutman A, Daitch V, Yahav D, Leibovici L, Skiada A, Antoniadou A, Daikos GL, Andini R, Zampino R, Durante-Mangoni E, Mouton JW, Friberg LE, Dishon Benattar Y, Bitterman R, Neuberger A, Carmeli Y, Paul M; AIDA Study Group. Treatment Outcomes of Colistin- and Carbapenem-resistant Acinetobacter baumannii Infections: An Exploratory Subgroup Analysis of a Randomized Clinical Trial. Clin Infect Dis. 2019;69: 769-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 140. | Gupta R, Hannon E, Huprikar S, Bassily-Marcus A, Manasia A, Oropello J, Kohli-Seth R. Getting to zero: Reduction in the incidence of multidrug-resistant organism infections using an integrated infection control protocol in an intensive care unit. Am J Infect Control. 2016;44: 1695-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 141. | Bassetti M, Poulakou G, Ruppe E, Bouza E, Van Hal SJ, Brink A. Antimicrobial resistance in the next 30 years, humankind, bugs and drugs: a visionary approach. Intensive Care Med. 2017;43: 1464-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 172] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 142. | Leone S, Damiani G, Pezone I, Kelly ME, Cascella M, Alfieri A, Pace MC, Fiore M. New antimicrobial options for the management of complicated intra-abdominal infections. Eur J Clin Microbiol Infect Dis. 2019;38: 819-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 143. | Healthcare-Associated Infections (Hai) Program. Carbapenem-resistant and Carbapenemase-producing Organisms for Public Health and Healthcare Providers. Accessed September 30, 2022. Available from: https://www.cdph.ca.gov/Programs/CHCQ/HAI/Pages/CRE_InfectionPreventionStrategies.aspx. |
| 144. | World Health Organization. Global action plan on antimicrobial resistance. Accessed February 1, 2023. Available from: https://www.who.int/publications/i/item/9789241509763. |
| 145. | Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399:629-655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8908] [Cited by in RCA: 7340] [Article Influence: 2446.7] [Reference Citation Analysis (0)] |