Published online Apr 26, 2023. doi: 10.12998/wjcc.v11.i12.2817
Peer-review started: December 21, 2022
First decision: January 30, 2023
Revised: March 13, 2023
Accepted: March 29, 2023
Article in press: March 29, 2023
Published online: April 26, 2023
Processing time: 125 Days and 17 Hours
Bell’s palsy is an idiopathic facial palsy with an unknown cause, and 75% of patients heal spontaneously. However, the other 25% of patients continue experiencing mild or severe disabilities, resulting in a reduced quality of life. Currently, various treatment methods have been developed to treat this disease. However, there is controversy regarding their effectiveness, and new alternative treatments are needed.
The patient suffered from left-sided facial paralysis due to Bell’s palsy for 7 years. The patient received an uncultured umbilical cord-derived mesenchymal stem cell transplant eight times for treatment. After follow-up for 32 mo, the paralysis was cured, and there was no recurrence.
Uncultured umbilical cord-derived mesenchymal stem cell transplantation may be a potential treatment for patients with Bell’s palsy who do not spontaneously recover.
Core Tip: The effectiveness of the current treatment methods for Bell’s palsy is debated. Therefore, alternative treatments are needed. In this study, we treated a patient with Bell’s palsy classified as moderately severe dysfunction using uncultured umbilical cord-derived mesenchymal stem cells. After follow-up for 32 mo, the paralysis was cured, and there was no recurrence. This method could be a new treatment option to replace existing treatments for Bell’s palsy.
- Citation: Ahn H, Jung WJ, Lee SY, Lee KH. Recovery from Bell’s palsy after treatment using uncultured umbilical cord-derived mesenchymal stem cells: A case report. World J Clin Cases 2023; 11(12): 2817-2824
- URL: https://www.wjgnet.com/2307-8960/full/v11/i12/2817.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i12.2817
Facial paralysis is a disease in which one side of the facial muscles become suddenly or gradually paralyzed. While facial paralysis can be caused by a number of factors, Bell’s palsy-defined idiopathic facial paralysis is the cause of 60%-75% of all cases[1], and the cause of Bell’s palsy is unknown. Although approximately 75% of the patients will heal spontaneously, it can lead to severe temporary oral insufficiency and potentially cause permanent eye damage because the eyelid on the affected side is unable to close[2,3]. Moderate to severe facial asymmetry persists in approximately 25% of patients with Bell’s palsy, often compromising the patient’s quality of life[2,3]. This long-term side effect of Bell’s palsy can be devastating for patients[2,3].
Although Bell’s palsy is considered idiopathic, herpes virus-specific immune response and ischemic or hereditary factors are closely related to its etiology[1,4,5]. In the early stages of the disorder, steroid therapy or antiviral administration has been shown to improve symptoms[6-8]. Nevertheless, in patients with long-term facial muscle dysfunction, symptoms may improve with facial exercises, acupuncture, and occupational and speech therapy[1,9-11]. In severe cases, symptoms can be relieved by nerve decompression and plastic surgery procedures[1,12]. Various treatment methods for patients with insufficient recovery from Bell’s palsy have been developed, but the effectiveness of these methods is debated. Despite receiving various treatments, some patients still have symptoms of paralysis. Therefore, alternative treatments for these patients are still needed[1,6,13].
Based on the results of previous studies, mesenchymal stem cell (MSC) transplantation treatment may be effective for treating Bell’s palsy. MSCs play an effective role in suppressing the function of the herpes virus and eliminating inflammation[14-16]. In addition, MSCs secrete cytokines that protect and regenerate neuronal cells and have the potential to differentiate into neuronal cells, which aids the regeneration of the damaged lesion site[17,18]. Therefore, we hypothesized that MSCs may be an effective treatment for Bell’s palsy in patients with insufficient recovery. In this study, we used uncultured umbilical cord-derived (UC)-MSCs to treat a patient with Bell’s palsy who experienced insufficient recovery. The patient had suffered from Bell’s palsy for 7 years. We report the treatment of this case using UC-MSCs as evidence that it may be a potential effective treatment of Bell’s palsy.
On March 5, 2013, a 49-year-old female, who suffered from Bell’s palsy, visited the 97.7 B&H Clinic. The patient had paralysis on the left side of the face.
The patient was diagnosed with Bell’s palsy in 2006. At first, the patient experienced only pain on the face, but tremors and paralysis gradually appeared on the left side of her face. As inferred from the patient’s comments, at the time of diagnosis, the patient had grade 4 (moderately severe dysfunction) facial paralysis according to the House-Brackmann facial nerve grading system (Table 1)[1]. She was treated with steroids when symptoms first appeared, but the treatment was ineffective. She was further treated with acupuncture, meridian massage, and herbal medicine, but the paralysis remained.
| Grade | Description | Characteristics |
| 1 | Normal | Normal facial function in all areas |
| 2 | Mild dysfunction | Slight weakness noticeable upon close inspection; may have very slight synkinesis |
| 3 | Moderate dysfunction | Obvious, but not disfiguring, difference between two sides; noticeable but not severe synkinesis, contracture, or hemifacial spasm; complete eye closure with effort |
| 4 | Moderately severe dysfunction | Obvious weakness or disfiguring asymmetry; normal symmetry and tone at rest; incomplete eye closure |
| 5 | Severe dysfunction | Barely perceptible motion; asymmetry at rest |
| 6 | Total paralysis | No movement |
The patient had no specific diseases or disorders.
The patient’s father suffered from a brain hemorrhage. However, the patient had no history of brain hemorrhage or other related diseases.
At the time of the first visit, the patient participated in a brief question-and-answer session to confirm the history of the present illness and symptoms. The patient had left-side facial paralysis with the following symptoms: muscle tremors, disfiguring asymmetry, and incomplete left eye closure. Based on the House-Brackmann facial nerve grading system, we classified the patient as a grade 4, moderately severe dysfunction (Table 1)[1].
Bell’s palsy does not require blood tests for diagnosis or treatment. However, a complete blood count, basic metabolic panel, comprehensive metabolic panel, lipid panel, thyroid panel, and cardiac biomarkers were performed to check the patient’s health. Upon examination, everything was normal.
Imaging examinations were not performed.
The patient was diagnosed, in our clinic, with Bell’s palsy with insufficient recovery. In addition, the House-Brackmann facial nerve grading system evaluation through question-and-answer with the patient determined that the patient’s symptoms had not improved over the 7 years after the first diagnosis.
UCs were donated by the Obstetrics and Gynecology Department at Lynn Woman’s Hospital (Seoul, South Korea). The donors’ mothers consented to the donation of the UCs. The safety of the donated UCs was confirmed through the mothers’ medical histories and blood and urine tests.
UC-MSCs were isolated from the donated UCs as described previously[17,19,20]. The UC was first disinfected with 70% ethanol and then washed with 1 × phosphate-buffered saline. Then, three vessels and the amniotic membrane of the UC were removed, and the UC was cut into 2-3 cm pieces with surgical scissors. The cut tissues were placed in a 50-mL conical tube containing a mixture solution of collagenase and hyaluronidase, further minced with surgical scissors and ground with a disposable tissue grinder, and incubated in a 37 °C, 50 mL/L CO2 incubator for 1 h. The mixture solution was filtered (100 μm) and then centrifuged to collect the flow-through containing the purified UC-MSCs. The UC-MSC samples were resuspended in CryoStor® CS10 (Stemcell Technologies, Cambridge, MA, United States), frozen at -80 °C for 1 d, and transferred to a liquid nitrogen tank for storage until clinical application.
The isolated UC-MSCs were suitable for treatment after confirmation of negative microbiological tests and of the expression level of MSC-specific proteins (CD73 ≥ 70%, CD90 ≥ 90%, and CD105 ≥ 90%) (data not shown). The expression level of MSC-specific proteins was measured using CyFlow® Cube 6 (Sysmex, Lincolnshire, IL, United States) and FCS Express 5 software (De Novo Software, Glendale, CA, United States).
We prepared a stock 4 mL injection solution consisting of uncultured UC-MSCs and 0.9% sodium chloride, USP with a concentration of 1 × 106 cells/mL. Before injection, the prepared 4 mL injection solution was divided into four 1 mL Ultra-FineTM II Insulin Syringes (BD Biosciences, Franklin Lakes, NJ, United States) containing 1 mL each.
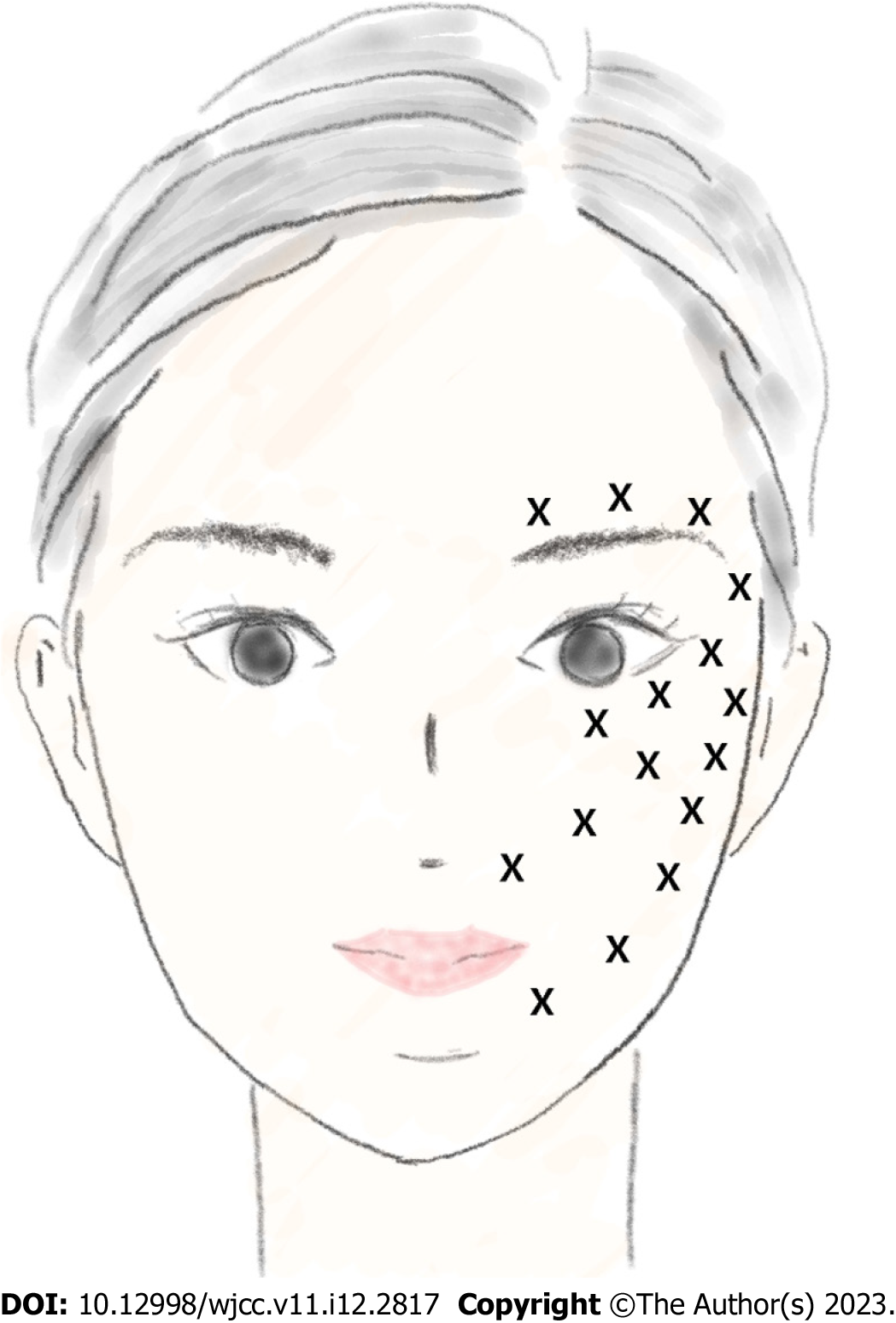
We injected the injection solution evenly over the left-side of the patient’s face. Each site was injected with 0.25 mL at a depth of 0.4-0.6 cm (a total of 16 injections were performed). Each injection site is marked with an ‘X’ in Figure 1.
The patient received a total of eight treatments at 2-mo intervals for 14 mo, and was followed up 18 mo after the end of treatment. The Bell’s palsy did not recur during this period.
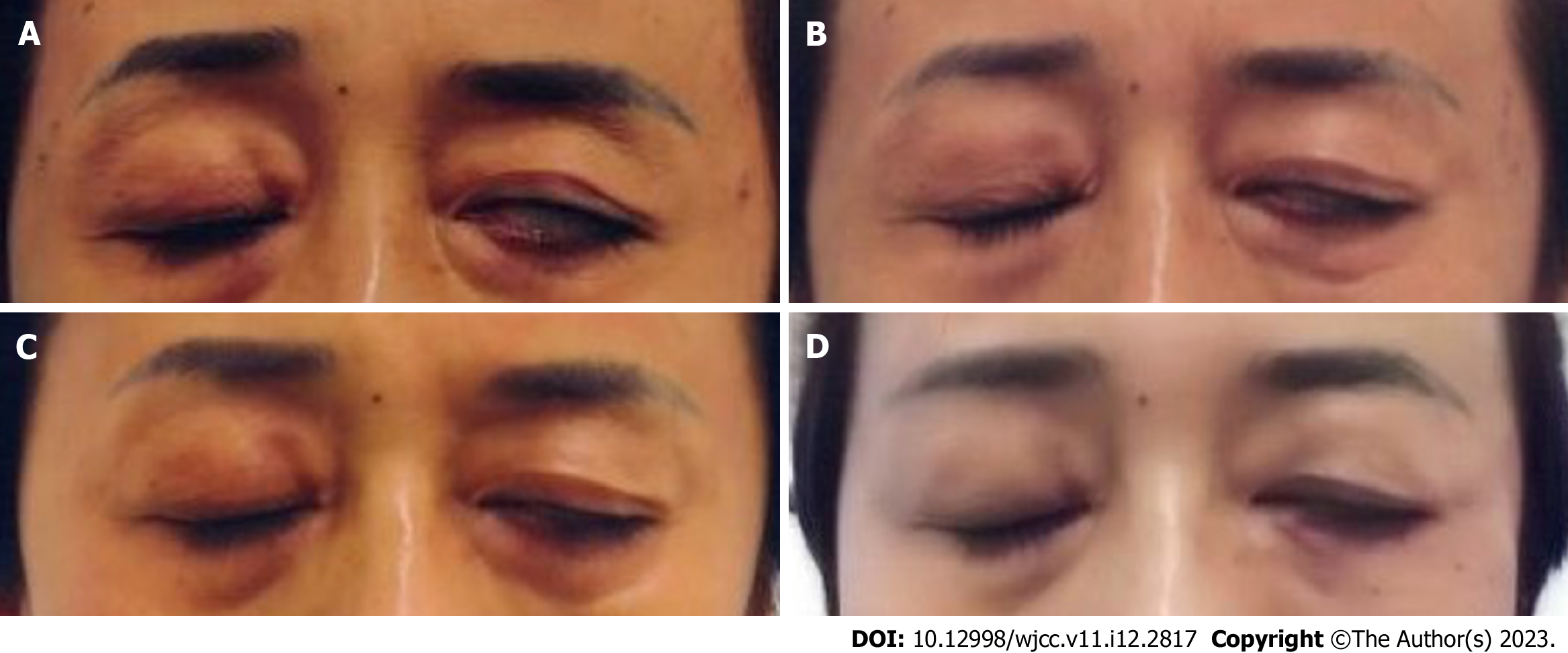
After UC-MSC transplantation, the patient experienced rapid improvement in the closure of her left eye. Before UC-MSC transplantation, the patient could only close her left eye about 50%. Three months after the first treatment, the patient was able to achieve left eye closure to 70%. This symptom continued to improve, and by 22 mo after the first treatment, the patient was able to completely close her left eye (Figure 1). In addition, before treatment, the patient’s left eyebrow was located lower than the right eyebrow. During the follow-up period, the muscles around the eyebrow normalized, and the eyebrows were even (Figure 2).
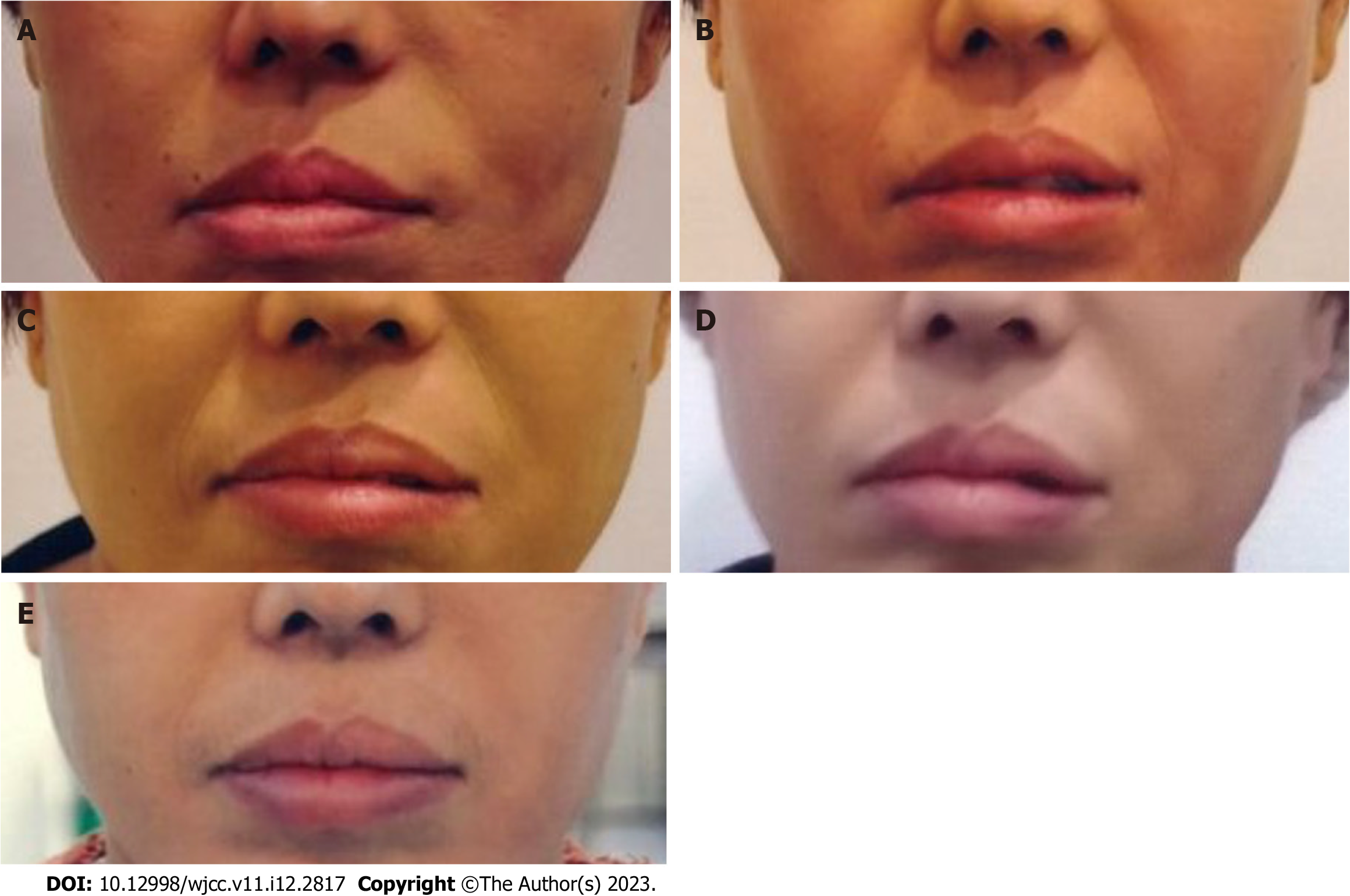
The patient also experienced relief of asymmetrical lips after UC-MSC transplantation. Before treatment, the patient had difficulty speaking because her lips were slightly tilted to the right. Three months after the first treatment, the patient reported that the muscles around the lips had softened, and her speech became easier. The patient’s lips were gradually improved and normalized over 28 mo (Figure 3).
Immediately after each treatment, the patient showed no specific local facial nor systemic abnormalities as reactions. Also, during the treatment and follow-up period, the patient did not report any experience of abnormalities nor of side effects.
The patient had suffered from Bell’s palsy for 7 years and remained disabled despite various treatments (including steroid drug therapy, acupuncture, meridian massage, and herbal medicine). The patient lived with facial asymmetry and discomfort due to stiffness in the affected region; these problems caused the patient to have psychological stress due to loss of self-confidence and lack of sleep. Eventually, her quality of life was greatly reduced due to related social avoidance.
Bell’s palsy is an idiopathic disease of unknown cause[1]. Researchers have hypothesized that Bell’s palsy develops as a result of damage to the facial nerve system due to various factors, including an immune response, inflammation, ischemia, and hereditary factors[1,4,5]. Although 75% of patients with Bell’s palsy recover spontaneously, the remaining 25% experience mild or severe disability, which reduces their quality of life[2,3]. To increase the cure rate of Bell’s palsy, steroids or antiviral drugs are given in the early course of the disorder, but their effectiveness is debated[6,7,21]. Patients with Bell’s palsy who experience insufficient recovery can receive various treatments such as meridian massage, acupuncture, exercise therapy, etc. In severe cases, nerve decompression and plastic surgery procedures can be performed[1,9-11]. Even though new treatments have been developed for patients with Bell’s palsy experiencing insufficient recovery, they are controversial, and new alternatives are needed. As mentioned in the previous section, the cause of Bell’s palsy is not clearly known, but there are several suspected causes, such as a viral infection or damage to the facial neuron by an assortment of proposed factors[1,4,5]. According to various recent studies, MSCs have antiviral, anti-inflammatory, neuronal protective, and regeneration functions[14,16-18]. Based on these findings, we hypothesized that MSC transplantation could treat patients with Bell’s palsy experiencing insufficient recovery. Although the cause of Bell’s palsy is unknown, transplantation of MSCs has the potential to overcome the presumed causes of Bell’s palsy.
Based on these findings, we hypothesized that MSC transplantation could successfully treat patients with Bell’s palsy experiencing insufficient recovery.
Although UC-MSCs are allogeneic cells, they are typically not rejected by the recipient and can be used universally[22]. In general, culturing is performed to obtain the number of MSCs required for treatment, but previous studies have indicated that MSCs undergo changes in their properties during culture, such as loss of their differentiation potential and change in their ability to secrete various cytokines and proteins due to the cell aging that occurs in the culturing period[23-25]. For these reasons, we used uncultured UC-MSCs, which are the youngest and most universally available, for treatment. Three months after the first treatment, the patient reported improvement in the closure of her left eye and stiff muscles around the lips. Over the 32-mo follow-up period, the patient reported that the symptoms gradually improved and normalized. There was no recurrence of Bell’s palsy symptoms during the follow-up.
A limitation of this report is the lack of pre-treatment images due to the refusal of the patient to have “before” images taken. However, 3 mo after treatment the patient consented to have images taken due to the improvement of symptoms. Although this is a case report of only 1 patient, we showed that uncultured UC-MSCs were effective in treating Bell’s palsy. A well-controlled and large-scale clinical study is required to provide further evidence that uncultured UC-MSC transplantation is an effective treatment for Bell’s palsy.
In this case study, a patient suffering from Bell’s palsy for 7 years was treated with uncultured UC-MSC transplantation. Although this is a case report of 1 patient, we expect that a randomized controlled trial will provide evidence that using uncultured UC-MSC transplantation to treat patients with Bell’s palsy with insufficient recovery is an effective new treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neurosciences
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Li X, China; Parrino D, Italy; Soni M, United States; Sun P, China; Zhang Q, China S-Editor: Gong ZM L-Editor: A P-Editor: Zhang XD
| 1. | Singh A, Deshmukh P. Bell's Palsy: A Review. Cureus. 2022;14:e30186. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Prud'hon S, Kubis N. [Bell's palsy]. Rev Med Interne. 2019;40:28-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Zhang W, Xu L, Luo T, Wu F, Zhao B, Li X. The etiology of Bell's palsy: a review. J Neurol. 2020;267:1896-1905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 167] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 4. | Zandian A, Osiro S, Hudson R, Ali IM, Matusz P, Tubbs SR, Loukas M. The neurologist's dilemma: a comprehensive clinical review of Bell's palsy, with emphasis on current management trends. Med Sci Monit. 2014;20:83-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | Eviston TJ, Croxson GR, Kennedy PG, Hadlock T, Krishnan AV. Bell's palsy: aetiology, clinical features and multidisciplinary care. J Neurol Neurosurg Psychiatry. 2015;86:1356-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 160] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 6. | Salinas RA, Alvarez G, Daly F, Ferreira J. Corticosteroids for Bell's palsy (idiopathic facial paralysis). Cochrane Database Syst Rev. 2010;CD001942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Cao J, Zhang X, Wang Z. Effectiveness comparisons of antiviral treatments for Bell palsy: a systematic review and network meta-analysis. J Neurol. 2022;269:1353-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Gagyor I, Madhok VB, Daly F, Sullivan F. Antiviral treatment for Bell's palsy (idiopathic facial paralysis). Cochrane Database Syst Rev. 2019;9:CD001869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Chen N, Zhou M, He L, Zhou D, Li N. Acupuncture for Bell's palsy. Cochrane Database Syst Rev. 2010;2010:CD002914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Law D, McDonough S, Bleakley C, Baxter GD, Tumilty S. Laser acupuncture for treating musculoskeletal pain: a systematic review with meta-analysis. J Acupunct Meridian Stud. 2015;8:2-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Teixeira LJ, Soares BG, Vieira VP, Prado GF. Physical therapy for Bell s palsy (idiopathic facial paralysis). Cochrane Database Syst Rev. 2008;CD006283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Wang Z, Chai Y, Chen Z, Wu H, Wang Z. Endoscopic transcanal facial nerve decompression in Bell's palsy: A pilot study. Am J Otolaryngol. 2022;43:103167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Murthy JM, Saxena AB. Bell's palsy: Treatment guidelines. Ann Indian Acad Neurol. 2011;14:S70-S72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Klimova RR, Demidova NA, Masalova OV, Kushch AA. Preventive Vaccination with Mesenchymal Stem Cells Protects Mice from Lethal Infection Caused by Herpes Simplex Virus 1. Mol Biol. 2021;55:413-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 15. | Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13:392-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 903] [Cited by in RCA: 1056] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 16. | Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012;20:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 633] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 17. | Ahn H, Lee SY, Jung WJ, Lee KH. Treatment of acute ischemic stroke by minimally manipulated umbilical cord-derived mesenchymal stem cells transplantation: A case report. World J Stem Cells. 2021;13:1151-1159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Doeppner TR, Herz J, Görgens A, Schlechter J, Ludwig AK, Radtke S, de Miroschedji K, Horn PA, Giebel B, Hermann DM. Extracellular Vesicles Improve Post-Stroke Neuroregeneration and Prevent Postischemic Immunosuppression. Stem Cells Transl Med. 2015;4:1131-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 582] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 19. | Ahn H, Lee SY, Jung WJ, Lee KH. Treatment of syringomyelia using uncultured umbilical cord mesenchymal stem cells: A case report and review of literature. World J Stem Cells. 2022;14:303-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Ahn H, Lee SY, Jung WJ, Pi J, Lee KH. Psoriasis treatment using minimally manipulated umbilical cord-derived mesenchymal stem cells: A case report. World J Clin Cases. 2021;9:6798-6803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Pitaro J, Waissbluth S, Daniel SJ. Do children with Bell's palsy benefit from steroid treatment? Int J Pediatr Otorhinolaryngol. 2012;76:921-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Carrade DD, Affolter VK, Outerbridge CA, Watson JL, Galuppo LD, Buerchler S, Kumar V, Walker NJ, Borjesson DL. Intradermal injections of equine allogeneic umbilical cord-derived mesenchymal stem cells are well tolerated and do not elicit immediate or delayed hypersensitivity reactions. Cytotherapy. 2011;13:1180-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Jones E, Schäfer R. Biological differences between native and cultured mesenchymal stem cells: implications for therapies. Methods Mol Biol. 2015;1235:105-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Javazon EH, Beggs KJ, Flake AW. Mesenchymal stem cells: paradoxes of passaging. Exp Hematol. 2004;32:414-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 355] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 25. | Gu Y, Li T, Ding Y, Sun L, Tu T, Zhu W, Hu J, Sun X. Changes in mesenchymal stem cells following long-term culture in vitro. Mol Med Rep. 2016;13:5207-5215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |