Published online Apr 26, 2023. doi: 10.12998/wjcc.v11.i12.2740
Peer-review started: December 28, 2022
First decision: February 15, 2023
Revised: February 20, 2023
Accepted: March 23, 2023
Article in press: March 23, 2023
Published online: April 26, 2023
Processing time: 118 Days and 5.4 Hours
Although the gastrointestinal tract is the most affected by Crohn’s disease (CD), the condition triggers other consequent manifestations, and iron deficiency anemia (IDA) is one of the most common. Intravenous (IV) iron replacement is currently available through several drugs, such as ferric hydroxide sucrose and ferric carboxymaltose (FCM). However, the clinical management of these conditions can be challenging.
To elucidate the drug’s effectiveness, the present study analyzed, through medical records, the clinical and epidemiological data of a cohort of patients with active CD who received IV FCM for the IDA treatment.
This retrospective observational study included 25 patients with active CD, severe anemia, and refractory to previous conventional treatments. Patients were evaluated two times: During the last treatment with ferric hydroxide sucrose and treatment with FCM.
After treatment with FCM, parameters of IDA assessment significantly improved, serum hemoglobin (Hb) levels increased in 93% of patients (P < 0.0001), and in 44%, there was an increase of ≥ 2 g/dL in a single application. In addition, 86% of the patients showed an increase in serum iron (P < 0.0001) and ferritin (P = 0.0008) and 50% in transferrin saturation (P = 0.01). The serum iron levels at baseline showed a negative association with the ileal and colonic CD and use of biologics and a positive association with patients who developed CD later in life after the age of 40 (A3) and with a stenosing (B2) and fistulizing (B3) phenotype. The values of Hb and hematocrit after ferric hydroxide sucrose treatment remained similar to those found before treatment.
This study demonstrated that FCM is an important therapeutic strategy for treating IDA in CD patients, achieving satisfactory results in refractory cases.
Core Tip: In this observational cohort study, treatment with a single dose of ferric carboxymaltose demonstrated a significant improvement in the hematological parameters evaluated for the treatment of iron deficiency anemia in Crohn’s disease patients at a tertiary center of a developing country. These results may contribute to guiding clinical treatment of this condition, mainly in cases of refractoriness to ferric hydroxide sucrose.
- Citation: Siqueira NSN, Pascoal LB, Rodrigues BL, de Castro MM, Martins ASC, Araújo DOS, Gomes LEM, Camargo MG, Ayrizono MLS, Leal RF. Ferric carboxymaltose for anemia in Crohn’s disease patients at a tertiary center: A retrospective observational cohort study. World J Clin Cases 2023; 11(12): 2740-2752
- URL: https://www.wjgnet.com/2307-8960/full/v11/i12/2740.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i12.2740
Crohn’s disease (CD) is an inflammatory bowel disease (IBD) characterized by a chronic inflammatory disorder of the gastrointestinal tract[1,2]. Although the gastrointestinal tract is the most affected section in CD, the affection may occur under other manifestations[3,4], such as anemia, among the most common in IBD[5-7]. This condition is commonly defined by the World Health Organization (WHO) as when hemoglobin (Hb) is below average values (less than 12 g∕dL in women and 13 g∕dL in men)[8]. Its prevalence varies widely, depending on the evaluated CD patients (hospitalized or outpatients).
Iron deficiency anemia (IDA) is usually determined by a negative balance in serum iron levels, where more iron is lost than is consumed in the diet[9]. In CD patients, chronic blood loss from the ulcerated intestinal mucosa associated with diarrhea, decreased iron intake due to dietary restriction, and deficiency in transluminal iron absorption during disease activity plays an important role in causing iron deficiency[10]. Erythropoiesis and iron metabolism may also be affected by increased serum hepcidin levels in high systemic inflammation, impairing dietary iron absorption and resulting in low serum iron levels[11].
Treatment of IDA aims to increase levels of Hb, serum ferritin (s-ferritin), and transferrin saturation (TSAT) levels above the lower threshold of normal to restore iron stocks to prevent recurrent anemia and not just to restore the short-term hematopoietic state[10]. Oral iron supplementation is usually the first choice for treatment, but intestinal absorption in CD patients can be compromised by the activity of the inflammatory process, which limits the effectiveness of treatment[3,12]. In addition, unabsorbed iron results in increased clinical activity in IBD patients due to gastrointestinal side effects, decreasing treatment adherence[13,14]. On the other hand, administering intravenous (IV) iron has helped in the correction of IDA of these patients and the maintenance of iron stocks in a faster and more prolonged response to treatment. Moreover, IV iron administration avoids gastrointestinal side effects, positively influencing treatment adherence and consequently improving quality of life[15,16].
Currently, IV iron replacement for treating IDA is available through several drugs distinguished by their complex chemistry, such as iron hydroxide sucrose and iron dextran[17]. Recent pharmacology progress has led ferric carboxymaltose (FCM) to correct the IDA through its pharmacokinetic characteristics[18,19]. FCM has a robust molecular structure containing stable iron in the form of a non-dextran iron complex with an iron hydroxide core (III) with a carbohydrate ligand. The structure is similar to ferritin’s, allowing for iron absorption without releasing free iron in the body. Therefore, administration can be performed in high doses (with a maximum dose of up to 1000 mg) in a safe and clinically well-tolerated manner[20,21]. Given the above, this study was designed to evaluate the effectiveness of FCM in treating IDA in CD patients in a tertiary center in Brazil, where IBD has become increasingly frequent, mainly in the last two decades[22].
In this observational retrospective cohort study, 25 patients with active CD who were followed at the IBD Unit of the Clinical Hospital of the University of Campinas (Unicamp) were included sequentially from October 2014 to November 2021. The patient’s clinical information was evaluated through outpatient database electronic records. Disease activity was determined by the CD Activity Index (CDAI), CD Endoscopic Index of Severity (CDEIS), magnetic resonance imaging enterography, and clinical and laboratory exams.
All patients presented severe anemia (Hb ≤ 10 g/dL and hematocrit < 41% for men and < 36% for women), according to the WHO[8], and they were refractory to previous conventional treatments. Iron deficiency was determined by TSAT < 20%, ferritin ≤ 100 ng/mL, and serum iron < 60 µg/dL.
Clinical characteristics and laboratory results were collected twice from the patient’s past clinical history: During the previous treatment with ferric hydroxide sucrose (a subgroup of the total cohort) and the therapy with FCM.
All laboratory parameters were evaluated before and after treatment with a single IV administration of FCM (500 mg) and after treatment with IV ferric hydroxide sucrose to elucidate the effectiveness of medication in this group of patients. The primary analysis parameters for anemia correction were Hb ≥ 12 g/dL in women and ≥ 13 g/dL in men or an increase of Hb ≥ 1 g/dL. Secondary analyses included increased serum levels of iron, s-ferritin, and TSAT.
All results are reported as the median ± SEM. The D'Agostino & Pearson and Shapiro-Wilk tests were used to investigate whether the data followed a normal Gaussian distribution (P > 0.1). The data were analyzed using Person’s χ2 and paired t-test, Wilcoxon matched pair test or Mann-Whitney Test. Data were analyzed using GraphPad Prism® 8, with a critical P-value of 5%. Finally, univariate and multiple regression analyses using generalized linear models were performed to investigate the association of the hematological parameters with the clinical and demographic characteristics of the patients. For this performance, the significant variables or the ones that adjusted other variables < 0.20 were maintained in multiple models, and data were analyzed using Stata® 14, with a critical value of 5%.
The clinical and demographic characteristics of the entire cohort are reported in Table 1. A total of 25 CD patients were included in the study, 12 males (48%) and 13 females (52%), with a median age of 37 (20-67) years. The median duration of CD was 144 (6-312) mo. According to the Montreal classification, one patient was diagnosed before the age of 16 years (A1, 4%), 22 patients between 17 and 40 years (A2, 88%), and 2 patients over 40 years old (A3, 8%). Five patients had terminal ileal location (L1, 20%), 4 colonic location (L2, 16%), and 16 ileal and colonic locations (L3, 64%). Regarding the behavior of CD, 8 patients had a non-penetrating pattern (B1, 32%), 13 had stenosing disease (B2, 52%), and the remaining 4 patients had a penetrating disease (B3,16%). Eleven patients had the concomitant perianal disease (44%), and 20 underwent previous surgeries because of CD complications, such as abscesses, stenosis, and fistula.
| Variables | Ferric carboxymaltose | Iron sucrose | P value |
| Sex (M/F) | 12/13 | 9/7 | 0.606a |
| Age (yr) | 37 (20-67) | 37 (25-67) | 0.989b |
| Disease duration (mo) | 144 (6-312) | 144 (24-312) | 0.718b |
| Age at the diagnostic A1/A2/A31 | 1/22/2 | 1/14/1 | 0.931a |
| Disease location L1/L2/L3/L41 | 5/4/16/0 | 4/3/9/0 | 0.882a |
| Behavior B1/B2/B31 | 8/13/4 | 4/8/4 | 0.750a |
| Perianal disease (yes/no) | 11/14 | 8/8 | 0.707a |
| Prior Surgery (yes/no) | 20/05 | 15/1 | 0.224a |
| Biologic therapy (yes/no) | 23/2 | 15/1 | 0.962a |
| Immunosuppressive therapy (yes/no) | 14/11 | 13/3 | 0.242a |
| Previous blood transfusion (yes/no) | 10/15 | 8/8 | 0.529a |
Almost all recruited patients were under biological therapy (23 patients, 92%), and 14 were using immunosuppressive therapy (56%). Regarding the treatment of IDA, all patients had previously used other medications, and 10 (40%) had a history of blood transfusion because of severe anemia.
Of the total patients, a subgroup of 16 used ferric hydroxide sucrose previously for the treatment of IDA; 9 males (56.3%) and 7 females (43.7%), with a median age of 37 (25-67) years. The median duration of CD was 144 (24-312) mo and, according to the Montreal classification, one patient was diagnosed before the age of 16 years (A1, 6.25%), 14 patients between 17 and 40 years (A2, 87.5%), and one patient over 40 years old (A3, 6.25%). Four patients had ileal location (L1, 25%), 3 colonic location (L2, 18.7%), and 9 ileal and colonic location (L3, 56.3%). Regarding the CD behavior, 4 patients had a non-penetrating pattern (B1, 25%), 8 stenotic disease (B2, 50%), and the remaining 4 patients had penetrating disease (B3, 25%). Eight patients had concomitant perianal disease (50%), and 15 had undergone previous surgeries for CD complications.
All recruited patients with a history of ferric hydroxide sucrose injection for the ADF treatment were under treatment for CD: 15 patients were under biological therapy (93.7%), and 13 were under immunosuppressive therapy (81.3%). Eight patients (50%) of this subgroup needed a blood transfusion because of severe anemia.
Patients with CD who received iron replacement therapy with FCM had their disease activity determined by nuclear magnetic resonance (presence of ulcers, mucosal enhancement of contrast, or alteration of mesentery associated with the affected intestinal area), colonoscopy with median CDEIS of 16.9 (5.6–26) and/or fecal calprotectin 1000 (104–1000) µg/g at baseline.
The median CDAI at baseline was 303.5 (128–537.6), and at the end of treatment, it decreased in most patients (61%), 235.8 (13.5–470), but without statistical significance (P = 0.21). Regarding other inflammatory biomarkers, most patients included in the study (64%) had serum C-reactive protein (CRP) levels > 3 mg/L (reference value lower than 3 mg/L), with a median of 5.91 (0.16-114) mg/L; and the ERS median was 30 (3-120) mm/h.
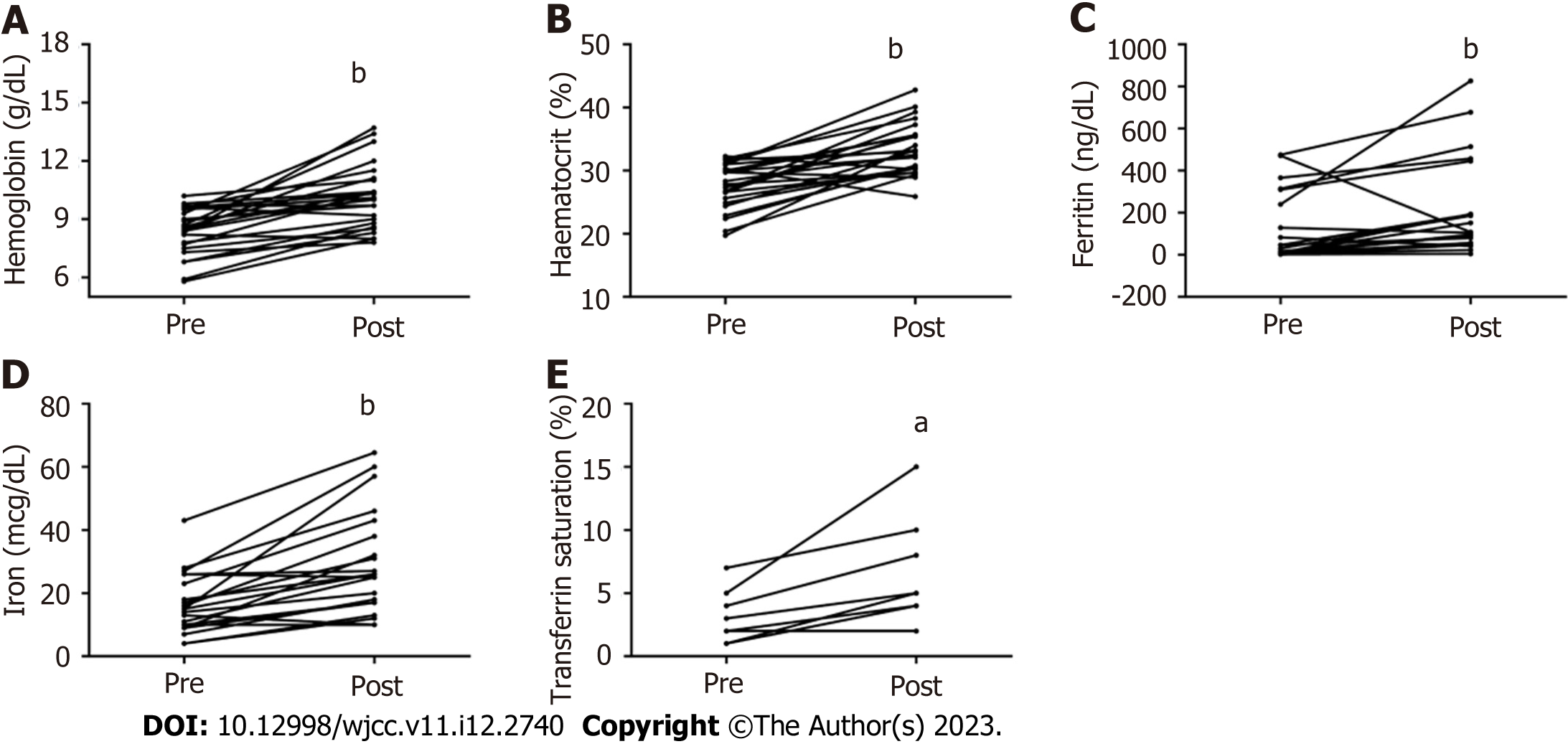
Hb levels increased in 93% of patients after treatment with FCM. The median Hb concentration increased from 8.5 g/dL (5.8–10) to 10.1g/dL (7.8–13.7) (P < 0.0001) (Figure 1A). In addition, correction of anemia and/or Hb increase ≥ 1 g/dL was achieved in 84% of patients with just one dose of medication. Eleven patients (44%) had an increase in Hb ≥ 2 g/dL. Hematocrit values were within normal parameters in 16% of patients after treatment with FCM, and there was a significant increase in 88% of patients with a median concentration from 27.8% (19.7–32.29) to 33% (25.9–42.8) (P < 0.0001) (Figure 1B, Table 2).
| Variables | Pre-therapy | Post-therapy | P value |
| Hemoglobin (mg/dL) | 8.5 (5.8-10) | 10.1 (7.8-13.7) | < 0.0001 |
| Haematocrit (%) | 27.8 (19.7-32.29) | 33 (25.9-42.8) | < 0.0001 |
| Ferritin (ng/dL) | 23.79 (0.5-475.4) | 100.38 (4.26-826.1) | 0.0008 |
| Iron (mcg/dL) | 15 (4-43) | 26 (10-64.52) | < 0.0001 |
| STAT (mcg/dL) | 3.5 (1-21) | 9 (2-26.3) | 0.01 |
| MCV (fL) | 74.6 (57.8-92.9) | 80.8 (64.4-97.8) | 0.05 |
| HCM (pg) | 22.3 (14.4-37.2) | 23.6 (18.5-30.7) | 0.15 |
| MCHC (g/dL) | 30.1 (24.9-38.8) | 30.65 (26.4-33.9) | 0.48 |
| Platelets (× 10³/µL) | 410 (200-888) | 321 (201-708) | 0.19 |
Moreover, serum iron levels increased in 18 patients (86%) after FCM injection. The median serum iron improved from 15 µg/dL (4–43) up to 26 µg/dL (10–64.52), demonstrating the satisfactory effect of the medication in only one application (P < 0.0001) (Figure 1C). Ferritin increased in 86% of patients after FCM: 23.79 ng/mL (0.5–475.4) at baseline and 100.38 ng/mL (4.26–826.1) after treatment, 77% of patients showed normalization of this parameter after treatment (P = 0.0008) (Figure 1D). Concerning the TAST, it increased under treatment with FCM, from 3.5 at the beginning of treatment (1–21) up to 9 (2–26.3) after injection (P = 0.01) (Figure 1E, Table 2).
After a single application with iron carboxymaltose, all patients had an increase in mean corpuscular volume levels, and most reached normalization in the parameter (59%) after treatment (P = 0.05) (Table 2). No statistical significance was found regarding the levels of Mean Corpuscular Hemoglobin, Mean Corpuscular Hemoglobin Concentration, and platelets analyzed after treatment (Table 2).
Concerning the hematimetric parameters and profile of iron, ferritin, and STAT, we analyzed the associations with the clinical variables under investigation. As observed in Table 3, although all patients included in the study had baseline serum iron levels below the normal range, the baseline serum iron levels were negatively associated with patients with an ileal and colonic CD (L3) who took biologics, but positively associated with patients who developed CD later in life after the age of 40 (A3) and with a stenosing (B2) and fistulizing (B3) phenotype. The highest level of ferritin after the application of FCM occurred in patients who presented colonic location (L2) and fistulizing phenotype (B3). The baseline serum STAT levels were associated with patients with stenosing (B2) and fistulizing (B3) phenotypes and perianal disease (Table 3).
| Variables | β value | 95%CI | P value |
| Serum iron levels at baseline | |||
| Age at the diagnostic1 | |||
| A3 | 50.119 | (15.778, 84.459) | 0.004 |
| Disease location1 | |||
| L3 | -16.328 | (-30.601, -2.055) | 0.025 |
| Behavior1 | |||
| B2 | 18.357 | (4.711, 32.002) | 0.008 |
| B3 | 21.961 | (2.931, 40.992) | 0.024 |
| Biologic therapy | |||
| Yes | -27.271 | (-53.623, -0.919) | 0.043 |
| Serum ferritin levels after FCM injection | |||
| Disease location1 | |||
| L2 | 348.460 | (56.087, 640.833) | 0.019 |
| Behavior1 | |||
| B3 | 335.922 | (57.389,614.454) | 0.018 |
| STAT at baseline | |||
| Behavior1 | |||
| B2 | 8.033 | (1.974, 14.091) | 0.009 |
| B3 | 13.866 | (5.762, 21.971) | 0.001 |
| Perianal disease | |||
| Yes | 10.5 | (3.907, 17.092) | 0.002 |
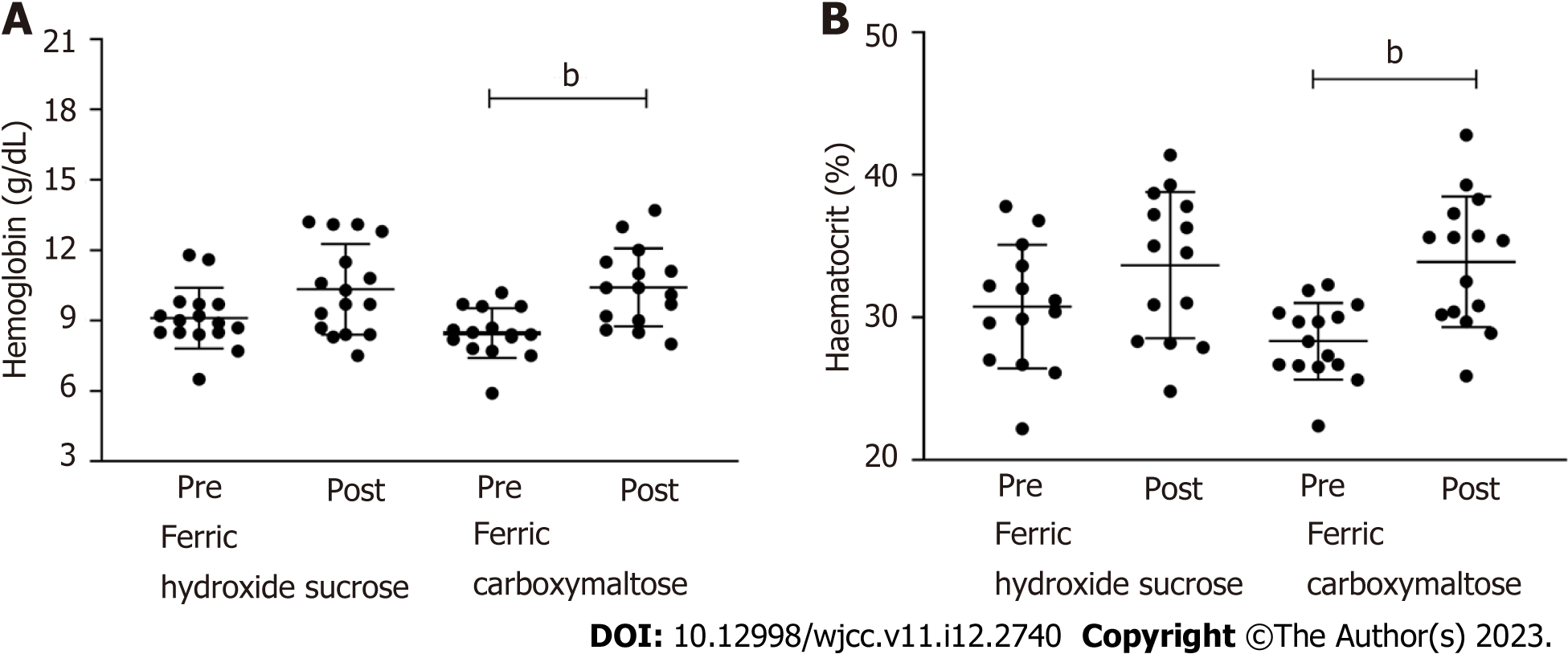
Finally, the study aimed to assess the effects of FCM in CD patients, taking into account the previous treatment of ferric hydroxide sucrose in these patients. For this analysis, a subgroup of patients from the total cohort was included, and Hb and hematocrit levels were obtained from the medical charts. The median duration of the treatment with ferric hydroxide sucrose was 5.5 mo (1-30), and the number of applications was 12 (4-32).
The median Hb concentration increased from 8.9 g/dL (6.5-11.8) to 9.7 g/dL (7.5-13.2), and the median hematocrit levels increased from 30.4 (22.2-37.8) to 34.54 (24.8-39.29) after treatment with ferric hydroxide sucrose. However, with no statistically significant difference (P < 0.05), this cohort comprises refractory patients to traditional iron replacement as expected. When these patients were treated with FCM, we observed increased Hb (P = 0.0005) and hematocrit (P = 0.0001) compared to the values obtained before treatment. (Figure 2A and B)
IDA is the most frequent clinical condition in CD patients. Usually, it is accompanied by the clinical and endoscopic activity of the disease, but at other times it can be the first manifestation that precedes the intestinal and abdominal symptoms and is one of the main causes of fatigue and poor quality of life. Our data demonstrated a significant improvement in Hb, iron, ferritin, and TAST levels after treatment with FCM. Although these findings have been previously proven in a few studies[10,20], our data showed significant improvement in the hematological parameters with a single dose of the medication.
Our results from the clinical practice confirmed the findings obtained by Sobrado et al[23], who evaluated FCM for the treatment of anemia in CD patients at a Brazilian center[23]. Although they performed a quite similar study, we included a larger number of patients treated at a tertiary center in selective and strictly defined disease activity criteria (endoscopic and/or radiological imaging). Furthermore, we compared the effects of FCM with previous ferric hydroxide sucrose treatment.
CD patients are at greater risk of developing anemia, especially with active disease[24,25]. To analyze the safety and efficacy of FCM for the anemia treatment of IBD patients, Stein et al[10] performed a prospective study. However, not only patients with severe CD activity were included, but also patients in remission, and the disease activity was based on rather unspecific criteria such as serum CRP, CDAI for patients diagnosed with CD, and Colitis Activity Index for patients with ulcerative colitis[11]. All patients in our study had severe disease activity determined by more specific criteria. 40% of our patients had severe activity determined by magnetic resonance enterography. The other 60% had CD activity determined by colonoscopy and fecal calprotectin. Furthermore, the median baseline CD activity assessed by the CDAI was 303.5, and CRP levels had a median of 5.91 before treatment with FCM.
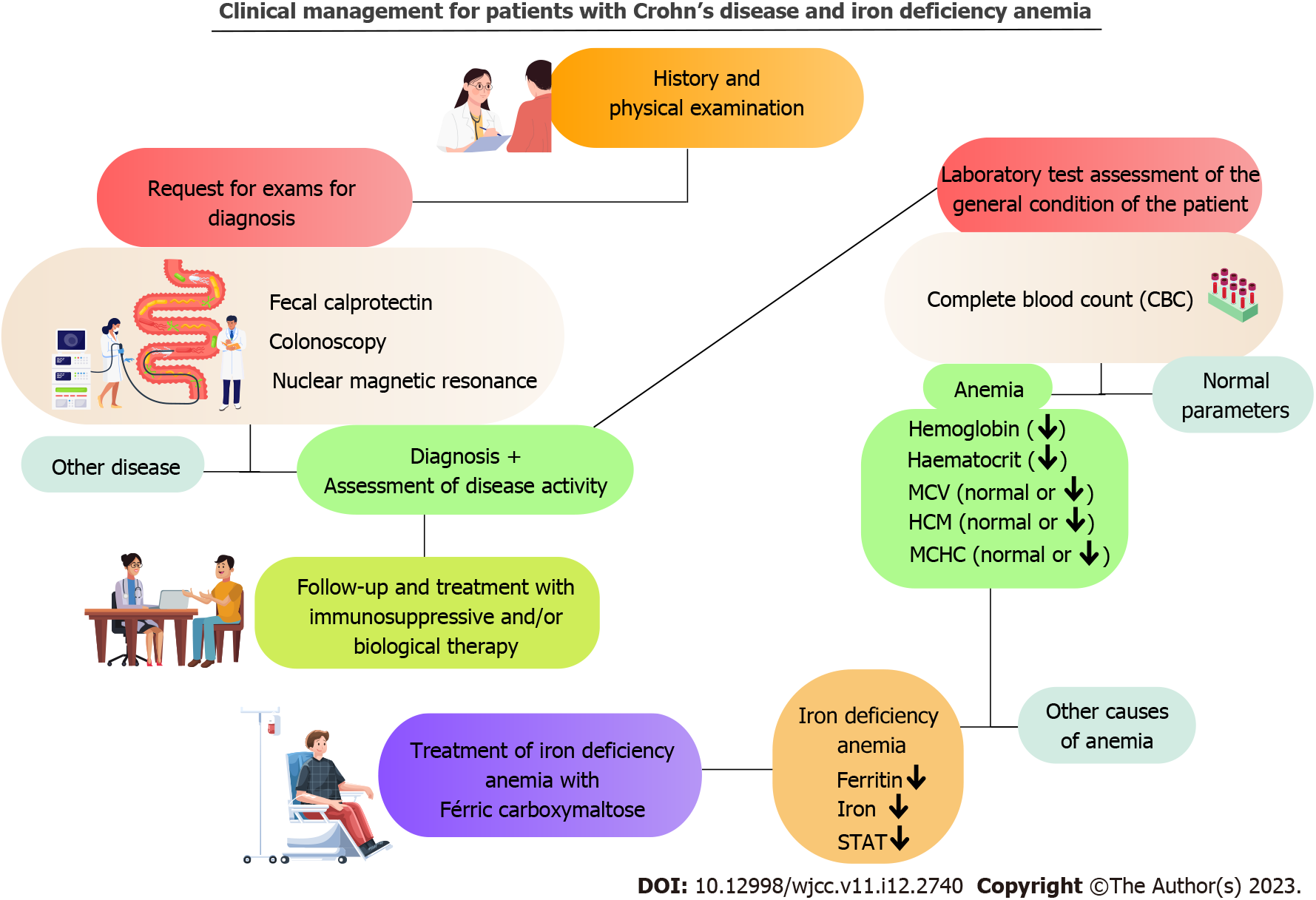
Usually, assistant physicians who take care of these chronic patients neglect the importance of IDA and accept it as a consequence of CD, so they proceed without having an established protocol based on gathering information and assessing alternative resolutions that could assist the decision-making and treatment in their clinical practice. Coe et al[9] performed a retrospective study and concluded that gastroenterologists should consider treating patients with IBD and IDA with IV iron as it is safe and effective[9]. Our study confirms that IV medication has proved to be an important therapeutic strategy, which can help quickly and safely in the treatment of IDA when prescribed by physicians accompanying this group of patients. A suggested approach to managing patients with CD and IDA is illustrated in Figure 3.
It is important to point out that although other studies have resulted in an increase in Hb levels of ≥ 2 g/dL, the assessment was not done with just a single dose of medication[10,20,23]. Our study showed that 44% of patients had an increase of ≥ 2 g/dL with a single dose of 50 mg/mL, which demonstrates that FCM can be an important therapeutic strategy when a significant increase in parameters is needed in a short period of follow-up, either to relieve symptoms or to prepare CD patients for surgical procedures and post-surgical recovering.
Iron and ferritin serum levels increased in 86% of patients, and TAST levels increased by 50% after treatment. This early response is in agreement with previous studies that performed this assessment. However, these studies evaluated a complete response between 4 to 8 wk of treatment. Our data point to this response in most patients 15 d after treatment[20,26].
The effect of biological therapy on anemia in patients with IBD is seldom discussed in the literature. Demonstrated evidence showed a significantly improving in anemia in patients using biological therapy for other chronic inflammatory diseases such as arthritis and ankylosing spondylitis[27]. Due to chronic inflammation, anemia is usually characterized as anemia of chronic disease (ACD) in these conditions. However, although ACD is associated with IBD, the most prominent impact is due to iron deficiency, and patients have recurrent anemia even after treatment with immunomodulators[28].
A recent pediatric study showed no statistical difference in Hb levels between IBD patients who responded or did not respond to ant-Tumor necrosis factor treatment[29]. A study of adult patients with IBD demonstrated that although biological therapy had significant beneficial effects on disease activity, the research found no significant change in the prevalence of anemia. Furthermore, one-fifth of patients without anemia at baseline developed anemia after one year of therapy[30]. Our results are in agreement with these recent studies. We demonstrated that although patients had iron levels below normal at baseline, the use of biological therapy had a negative correlation with baseline serum iron levels, indicating that these patients had more severe anemia.
A study evaluating anemia in Korean patients with IBD found no significant association between patients’ clinical characteristics and anemia[31]. Bergamaschi et al[32], in 2010, did not relate anemia in CD to the location or behavior of the disease[32]. In 2020, a study analyzed the prevalence and risk factors of anemia and iron deficiency in patients with IBD in Brazil and concluded that patients with the penetrating disease phenotype in CD were associated with a lower risk of anemia[33]. Our study demonstrates that patients with an ileal and colonic location correlate negatively with basal serum iron levels, demonstrating more severe anemia. Patients with stenosing and fistulizing phenotypes positively correlated with baseline serum iron levels and TAST.
We also compared treatment effects with FCM and previous treatment with ferric hydroxide sucrose. We observed no statistical difference in Hb and hematocrit levels after treatment with ferric hydroxide sucrose, while those levels showed a significant increase with a single dose of FCM. Although other studies evaluated the efficacy of the two medications[9,34], our study compared the efficacy of the treatment in the same group of patients, demonstrating that FCM is an important therapeutic strategy in refractory patients who received ferric hydroxide sucrose.
The present study reported a median of 12 applications (4-35) with previous treatment with ferric hydroxide sucrose. However, when the same patients were treated with FCM, a single infusion guaranteed a significant increase in the hematological parameters. Evstatiev et al[34] reported in their study treatment with 1 to 3 infusions of FCM, while the ferric hydroxide sucrose treatment lasted 11 infusions[34]. Onken et al[35] also showed a similar result; patients treated with ferric sucrose received an average of 5 infusions, while patients treated with FCM underwent two infusions[35].
Thus, CD patients can be exposed to fewer interventions since FCM has better efficacy with a significant increase in hematological parameters, as well as iron, ferritin, and TAST levels with usually a single dose. The consequence is a decreased number of patients’ visits to hospitals or private clinics, contributing to the quality of life and emotional well-being.
Currently, the value of an ampoule of FCM (+/- R$ 641.90 – Brazilian currency) is approximately 10 times more expensive than an ampoule of ferric hydroxide sucrose (+/- R$ 64.70 - Brazilian currency), which restricts patient adherence to treatment since this medication is not available by the Unified Health System through State Public Pharmacies in Brazil. However, studies indicate that as a final result of the treatment, FCM has a lower cost when compared to ferric sucrose. Vicente et al[36] concluded that the overall cost of FCM treatment is significantly advantageous compared to ferric hydroxide sucrose, such as the relatively lower number of infusions of FCM and the quick increase of serum Hb levels after iron replacement[36].
Toblli and Di Gennaro[24] in 2015, evaluated the economic impact of oral iron replacement treatment vs FCM in patients with chronic kidney disease. As a result, the study demonstrated that the cumulative cost during the 6 mo study period with FCM was United States $ 3.070 per patient, whereas compared to oral iron administration over the same period, the cost was US $ 17.670. The study also found that using FCM to treat IDA resulted in savings of US $ 14.600 (82.6%) per patient[24].
Another study published in 2021 by Aksan et al[37] analyzed and compared the cost-effectiveness of IV and oral iron treatment in patients with IDA associated with IBD and concluded that FCM is designed to be the most cost-effective IV iron therapy in Switzerland and with better clinical response to treatment[37]. Basha et al[25] in 2021 evaluated the efficacy and cost-effectiveness of FCM vs. iron sucrose. The retrospective study assessed patients who were followed up for 12 mo in a tertiary center, and as a result, although the cost of the medication FCM is 6.5 times higher than iron sucrose, at the end of the period, the treatment with FCM has a lower cost in bed or nursing[25]. Table 4 shows the main characteristics differentiating FCM from iron sucrose[38,39].
| Ferric carboxymaltase[38] | Iron hydroxide sucrose[39] | |
| Concentration | Up to 20 mL corresponding to 1000 mg of iron | 10 mL corresponding to 200 mg of iron |
| Dosage | 1 time a week | 1 to 3 times a week (depending on hemoglobin level) |
| Infusion time | Up to 200 mg of iron - there is no established administration time from 200 mg to 500 mg of iron – the rate of 100 mg per minute above 500 mg up to 1000 mg of iron -66 mg per min | Up to 200 mg of iron - 6.6 mg per min, 300 mg of iron -3.3 mg per min, 400 mg - 2.6 mg per min, 500 mg - 2.3 mg per min |
| Molecule | Stable iron is in the form of a complex of non-dextran iron with a polynuclear ferric hydroxide core with a carbohydrate linker. Because of the high stability of the complex, there is only a small amount of weakly bound iron (also called free iron). The structure of the nucleus is similar to that of ferritin, so the complex is intended to provide a controlled supply of usable iron for ferric transport and storage of proteins in the body | Trivalent form as a macromolecular colloidal complex of ferric hydroxide saccharate. The polynuclear ferric hydroxide core is superficially surrounded by a large number of non-covalently linked sucrose molecules, resulting in a complex whose molecular mass is approximately 43 kDa |
| Pharmacokinetics | After administration of a single dose of iron carboxymaltose of 100 to 1000 mg of iron in patients with anemia, peak serum iron concentrations were between 37 and 333 mcg per mL | After the injection of 100mg of iron in healthy individuals, the maximum plasma concentration, on average, of 538 µmol per L, 10 min after the injection |
| Volume of distribution | The volume of distribution of the central compartment corresponds to the plasma volume (approximately 3 L). It is retained mainly in the reticuloendothelial system of the bone marrow, liver, and spleen. The average residence time varied between 11 and 17 h | The central compartment volume of distribution correlates well with serum volume (approximately 3 L). The volume of distribution at a steady state was about 8 L, which indicates the low distribution of iron in body fluids |
| Half-life | 7 and 12 h | 6 h |
| Route of administration | Intravenous injectable solution | Intravenous injectable solution |
The literature demonstrates the effectiveness of FCM in many diseases. Several studies have shown the important role that medication performs in cardiovascular diseases[40,41]. However, in the gastrointestinal tract, few studies have analyzed the effectiveness of FCM, especially in CD. Thus, our study contributed to a greater understanding of medication use in this disease, helping clinical practice.
The limitations of our study lay in the retrospective character of the research, as data collected through electronic medical records or outpatient databases may be scarce. As described above, we could only analyze the serum levels of Hb and hematocrit in comparing the two medications. Another limitation is that the study evaluated a small number of patients despite being larger than other Brazilian studies. In addition, it was neither possible to correlate the degree of CD activity through the CDAI, CRP, and erythrocyte sedimentation rate values with the severity of anemia nor to determine if the increase of the hematological parameters after FCM treatment correlates with an improvement in quality of life assessed by validated questionnaires, such as IBD Questionnaire[29].
From the analysis performed in this retrospective study, a better understanding of the effects of FCM in the treatment of IDA in CD patients has emerged. The study also showed that FCM is an important therapeutic strategy, as it achieves superior results compared to the administration of iron hydroxide sucrose in patients with refractory IDA. However, there are still many gaps to be addressed in future studies about the molecular mechanism of IDA in CD. We do not yet know if IV iron replacement, besides improving the patient’s quality of life and well-being, can affect the activity of the disease and help the patient to enter clinical and endoscopic remission. Our findings support FCM as an important therapeutic strategy to treat anemia and improve CD patients’ clinical status.
Crohn’s disease (CD) is a chronic inflammatory disorder of the gastrointestinal tract, and anemia is one of the clinical manifestations. Iron deficiency anemia (IDA) in CD is due to chronic blood loss from the ulcerated intestinal mucosa associated with diarrhea, decreased iron intake due to dietary restriction, and/or deficiency in transluminal iron absorption during the disease activity. Currently, intravenous (IV) iron replacement is the best option for the treatment of IDA in CD. Recent pharmacology progress has led ferric carboxymaltose (FCM) to correct the IDA through its pharmacokinetic characteristics.
The administration of FCM can be performed in high doses safely and well-tolerated. Given that, this study was designed to evaluate the effectiveness of FCM in the treatment of IDA in CD patients in a tertiary center in Brazil, where inflammatory bowel disease has become increasingly frequent. This clinical approach can make the quality of life of CD patients better.
The objective of this study was to analyze, through medical records, the clinical and epidemiological data of a cohort of patients with active CD who received IV FCM in the treatment of IDA to elucidate the effectiveness of the drug and compare it to iron hydroxide sucrose treatment.
It is a retrospective, observational study, which included 25 patients with active CD, severe anemia, and refractory to previous conventional treatments. Patients were evaluated two times: During previous treatment with ferric hydroxide sucrose and treatment with FCM. Epidemiological and clinical data were analyzed, besides hematimetric parameters.
The parameters of IDA assessment significantly improve after treatment with FCM. Serum hemoglobin (Hb) levels increased in 93% of patients, and in 44%, there was an increase of ≥ 2 g/dL in a single application. Moreover, 86% of the patients showed increased serum iron and ferritin and 50% in transferrin saturation. The serum iron levels at baseline showed a negative association with the ileal and colonic CD and use of biologics and a positive association with patients who developed CD later in life after the age of 40 (A3) and with a stenosing (B2) and fistulizing (B3) phenotype. The Hb and hematocrit values after ferric hydroxide sucrose treatment remained similar to those found before treatment.
FCM is an important therapeutic strategy for treating IDA in CD patients, achieving satisfactory results in refractory cases.
The study showed that FCM is an important therapeutic strategy to treat IDA in CD patients. However, there are still many gaps to be addressed in future studies about the molecular mechanism of IDA in CD. We do not yet know if IV iron replacement, besides improving the patient’s quality of life and well-being, can affect the activity of the disease and help the patient to enter clinical and endoscopic remission. Our findings support FCM as an important therapeutic strategy to treat anemia and improve the clinical status of CD patients.
We thank Professor Torriani T for revising the English version of our manuscript and Professor Azevedo AT for revising the statistical methods of this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: European Crohn's Colitis Organisation; Brazilian Society of Colorectal Surgeons; Brazilian Research Group for Inflammatory Bowel Diseases.
Specialty type: Medicine, research and experimental
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rodrigo L, Spain; Rosati A, Italy S-Editor: Li L L-Editor: A P-Editor: Zhang YL
| 1. | Khan I, Samson SE, Grover AK. Antioxidant Supplements and Gastrointestinal Diseases: A Critical Appraisal. Med Princ Pract. 2017;26:201-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Ye Y, Pang Z, Chen W, Ju S, Zhou C. The epidemiology and risk factors of inflammatory bowel disease. Int J Clin Exp Med. 2015;8:22529-22542. [PubMed] |
| 3. | Nielsen OH, Ainsworth M, Coskun M, Weiss G. Management of Iron-Deficiency Anemia in Inflammatory Bowel Disease: A Systematic Review. Medicine (Baltimore). 2015;94:e963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Forbes A, Escher J, Hébuterne X, Kłęk S, Krznaric Z, Schneider S, Shamir R, Stardelova K, Wierdsma N, Wiskin AE, Bischoff SC. ESPEN guideline: Clinical nutrition in inflammatory bowel disease. Clin Nutr. 2017;36:321-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 426] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 5. | Filmann N, Rey J, Schneeweiss S, Ardizzone S, Bager P, Bergamaschi G, Koutroubakis I, Lindgren S, Morena Fde L, Moum B, Vavricka SR, Schröder O, Herrmann E, Blumenstein I. Prevalence of anemia in inflammatory bowel diseases in european countries: a systematic review and individual patient data meta-analysis. Inflamm Bowel Dis. 2014;20:936-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 6. | Alves RA, Miszputen SJ, Figueiredo MS. Anemia in inflammatory bowel disease: prevalence, differential diagnosis and association with clinical and laboratory variables. Sao Paulo Med J. 2014;132:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Zeitz J, Fournier N, Labenz C, Biedermann L, Frei P, Misselwitz B, Scharl S, Vavricka SR, Sulz MC, Fried M, Rogler G, Scharl M. Risk Factors for the Development of Fistulae and Stenoses in Crohn Disease Patients in the Swiss Inflammatory Bowel Disease Cohort. Inflamm Intest Dis. 2017;1:172-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | World Health Organization. Nutritional Anaemias: Tools for Effective Prevention and Control. Switzerland: World Health Organization, 2017: 7. |
| 9. | Coe CL, Meyers MH, Beaulieu DB, Scoville E, Schwartz DA, Horst SN, Dalal RL. Gastroenterologist-Lead Management of Iron Deficiency Anemia in Inflammatory Bowel Disease Is Effective, Safe, and May Increase Quality of Life. Crohns Colitis 360. 2020;2:otaa051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Stein J, Aksan A, Klemm W, Nip K, Weber-Mangal S, Dignass A. Safety and Efficacy of Ferric Carboxymaltose in the Treatment of Iron Deficiency Anaemia in Patients with Inflammatory Bowel Disease, in Routine Daily Practice. J Crohns Colitis. 2018;12:826-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Nielsen OH, Soendergaard C, Vikner ME, Weiss G. Rational Management of Iron-Deficiency Anaemia in Inflammatory Bowel Disease. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Martin J, Radeke HH, Dignass A, Stein J. Current evaluation and management of anemia in patients with inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2017;11:19-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Goldberg ND. Iron deficiency anemia in patients with inflammatory bowel disease. Clin Exp Gastroenterol. 2013;6:61-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Tolkien Z, Stecher L, Mander AP, Pereira DI, Powell JJ. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PLoS One. 2015;10:e0117383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 480] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 15. | Gasche C, Berstad A, Befrits R, Beglinger C, Dignass A, Erichsen K, Gomollon F, Hjortswang H, Koutroubakis I, Kulnigg S, Oldenburg B, Rampton D, Schroeder O, Stein J, Travis S, Van Assche G. Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm Bowel Dis. 2007;13:1545-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 313] [Article Influence: 17.4] [Reference Citation Analysis (3)] |
| 16. | Gomollón F, Gisbert JP, García-Erce JA. Intravenous iron in digestive diseases: a clinical (re)view. Ther Adv Chronic Dis. 2010;1:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Geisser P, Burckhardt S. The pharmacokinetics and pharmacodynamics of iron preparations. Pharmaceutics. 2011;3:12-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 18. | García-López S, Bocos JM, Gisbert JP, Bajador E, Chaparro M, Castaño C, García-Erce JA, Gomollón F. High-dose intravenous treatment in iron deficiency anaemia in inflammatory bowel disease: early efficacy and impact on quality of life. Blood Transfus. 2016;14:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 19. | ANVISA - AGÊNCIA NACIONAL DE VIGILÂNCIA SANITÁRIA. Detalhe do Produto : FERINJECT. 2020. [cited 3 March 2023]. Available from: https://consultas.anvisa.gov.br/#/medicamentos/25351770404201123/. |
| 20. | Kulnigg S, Stoinov S, Simanenkov V, Dudar LV, Karnafel W, Garcia LC, Sambuelli AM, D'Haens G, Gasche C. A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: the ferric carboxymaltose (FERINJECT) randomized controlled trial. Am J Gastroenterol. 2008;103:1182-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 251] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 21. | Kulnigg-Dabsch S, Schmid W, Howaldt S, Stein J, Mickisch O, Waldhör T, Evstatiev R, Kamali H, Volf I, Gasche C. Iron deficiency generates secondary thrombocytosis and platelet activation in IBD: the randomized, controlled thromboVIT trial. Inflamm Bowel Dis. 2013;19:1609-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Fucilini LMP, Genaro LM, Sousa DCE, Coy CSR, Leal RF, Ayrizono MLS. EPIDEMIOLOGICAL PROFILE AND CLINICAL CHARACTERISTICS OF INFLAMMATORY BOWEL DISEASES IN A BRAZILIAN REFERRAL CENTER. Arq Gastroenterol. 2021;58:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Sobrado CW, Cançado RD, Sobrado LF, Frugis MO, Sobrado MF. TREATMENT OF ANEMIA AND IMPROVEMENT OF QUALITY OF LIFE AMONG PATIENTS WITH CROHN'S DISEASE: experience using ferric carboxymaltose. Arq Gastroenterol. 2015;52:255-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Toblli JE, Di Gennaro F. Switching patients with non-dialysis chronic kidney disease from oral iron to intravenous ferric carboxymaltose: effects on erythropoiesis-stimulating agent requirements, costs, hemoglobin and iron status. PLoS One. 2015;10:e0125528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Basha A, Ibrahim MIM, Hamad A, Chandra P, Omar NE, Abdullah MAJ, Aldapt MB, Hussein RM, Mahfouz A, Adel AA, Shwaylia HM, Ekeibed Y, AbuMousa R, Yassin MA. Efficacy and cost effectiveness of intravenous ferric carboxymaltose versus iron sucrose in adult patients with iron deficiency anaemia. PLoS One. 2021;16:e0255104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Pallis AG, Mouzas IA, Vlachonikolis IG. The inflammatory bowel disease questionnaire: a review of its national validation studies. Inflamm Bowel Dis. 2004;10:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Furst DE, Kay J, Wasko MC, Keystone E, Kavanaugh A, Deodhar A, Murphy FT, Magnus JH, Hsia EC, Hsu B, Xu S, Rahman MU, Doyle MK. The effect of golimumab on haemoglobin levels in patients with rheumatoid arthritis, psoriatic arthritis or ankylosing spondylitis. Rheumatology (Oxford). 2013;52:1845-1855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Sjöberg D, Holmström T, Larsson M, Nielsen AL, Holmquist L, Rönnblom A. Anemia in a population-based IBD cohort (ICURE): still high prevalence after 1 year, especially among pediatric patients. Inflamm Bowel Dis. 2014;20:2266-2270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Assa A, Hartman C, Weiss B, Broide E, Rosenbach Y, Zevit N, Bujanover Y, Shamir R. Long-term outcome of tumor necrosis factor alpha antagonist's treatment in pediatric Crohn's disease. J Crohns Colitis. 2013;7:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 30. | Koutroubakis IE, Ramos-Rivers C, Regueiro M, Koutroumpakis E, Click B, Schwartz M, Swoger J, Baidoo L, Hashash JG, Barrie A, Dunn MA, Binion DG. The Influence of Anti-tumor Necrosis Factor Agents on Hemoglobin Levels of Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2015;21:1587-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Lee DS, Bang KB, Kim JY, Jung YS, Park JH, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI, Choi KY, Park DI. The prevalence and clinical characteristics of anemia in Korean patients with inflammatory bowel disease. Intest Res. 2016;14:43-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Bergamaschi G, Di Sabatino A, Albertini R, Ardizzone S, Biancheri P, Bonetti E, Cassinotti A, Cazzola P, Markopoulos K, Massari A, Rosti V, Porro GB, Corazza GR. Prevalence and pathogenesis of anemia in inflammatory bowel disease. Influence of anti-tumor necrosis factor-alpha treatment. Haematologica. 2010;95:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 33. | Parra RS, Feitosa MR, Ferreira SDC, Rocha JJRD, Troncon LEA, FÉres O. ANEMIA AND IRON DEFICIENCY IN INFLAMMATORY BOWEL DISEASE PATIENTS IN A REFERRAL CENTER IN BRAZIL: PREVALENCE AND RISK FACTORS. Arq Gastroenterol. 2020;57:272-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Evstatiev R, Marteau P, Iqbal T, Khalif IL, Stein J, Bokemeyer B, Chopey IV, Gutzwiller FS, Riopel L, Gasche C; FERGI Study Group. FERGIcor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology. 2011;141:846-853.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 244] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 35. | Onken JE, Bregman DB, Harrington RA, Morris D, Buerkert J, Hamerski D, Iftikhar H, Mangoo-Karim R, Martin ER, Martinez CO, Newman GE, Qunibi WY, Ross DL, Singh B, Smith MT, Butcher A, Koch TA, Goodnough LT. Ferric carboxymaltose in patients with iron-deficiency anemia and impaired renal function: the REPAIR-IDA trial. Nephrol Dial Transplant. 2014;29:833-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 36. | Vicente AB, Decimoni TC, Quero AA. Cost-minimization analysis of the ferric carboxymaltose (i.v.) compared with iron sucrose (i.v.) in the treatment of anemia under supplementary health care perspective. J Bras Econ Saúde. 2015;7:28-37. [DOI] [Full Text] |
| 37. | Aksan A, Schoepfer A, Juillerat P, Vavricka S, Bettencourt M, Ramirez de Arellano A, Gavata S, Morin N, Valentine WJ, Hunt B. Iron Formulations for the Treatment of Iron Deficiency Anemia in Patients with Inflammatory Bowel Disease: A Cost-Effectiveness Analysis in Switzerland. Adv Ther. 2021;38:660-677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 38. | Ferric Carboxymaltose. [Leaflet]. New Zealand: Vifor Pharma Pty Ltd. Nov 2022. [cited 3 March 2023]. Available from: https://www.medsafe.govt.nz/Consumers/cmi/f/ferinject.pdf. |
| 39. | Ferric Hydroxide Sucrose. [Leaflet]. São Paulo: ALTANA Pharma Ltda. Nov 2022. [cited 3 March 2023]. Available from: http://cmar.med.br/bulas/NORIPURUM.pdf. |
| 40. | Ponikowski P, van Veldhuisen DJ, Comin-Colet J, Ertl G, Komajda M, Mareev V, McDonagh T, Parkhomenko A, Tavazzi L, Levesque V, Mori C, Roubert B, Filippatos G, Ruschitzka F, Anker SD; CONFIRM-HF Investigators. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency†. Eur Heart J. 2015;36:657-668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 655] [Cited by in RCA: 867] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 41. | McDonagh T, Damy T, Doehner W, Lam CSP, Sindone A, van der Meer P, Cohen-Solal A, Kindermann I, Manito N, Pfister O, Pohjantähti-Maaroos H, Taylor J, Comin-Colet J. Screening, diagnosis and treatment of iron deficiency in chronic heart failure: putting the 2016 European Society of Cardiology heart failure guidelines into clinical practice. Eur J Heart Fail. 2018;20:1664-1672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |