Published online Apr 16, 2023. doi: 10.12998/wjcc.v11.i11.2567
Peer-review started: January 20, 2023
First decision: February 20, 2023
Revised: March 1, 2023
Accepted: March 22, 2023
Article in press: March 22, 2023
Published online: April 16, 2023
Processing time: 76 Days and 8.1 Hours
Pulp revascularization is a novel way to treat immature teeth with periapical disease, and the technique has become increasingly well established in recent years. By puncturing the periapical tissue, bleeding is induced, and a blood clot is formed in the root canal. The blood clot acts as a natural bioscaffold onto which mesenchymal stem cells from periapical tissue can be seeded and restore pulp vascularity, thus promoting root development as well as apical closure. Although the effect of pulp revascularization is ideal, there are certain requirements for the apical condition of the teeth. The apical barrier technique and apexification are still indispensable for teeth that cannot achieve ideal blood clot formation. In addition, a meta-analysis of several clinical studies concluded that pulp revascularization has no significant advantages over other treatments.
A 10-year-old girl complained of pain in the right upper and lower posterior teeth for 2 d. Clinical and radiological examinations revealed that both the right maxillary and mandibular second premolars were immature with periapical radiolucency. The right maxillary second premolar was treated by pulp revascularization, while the right mandibular second premolar was treated by conventional apical barrier surgery after revascularization failed. The purpose of this report is to compare the different root maturation processes induced by the pulp revascularization and apical barrier techniques in the same patient in homonymous teeth from different jaws. Twelve months of follow-up showed that the apical foramen of both teeth presented a clear tendency to close; however, the tooth treated with pulp revascularization showed a significant increase in root length as well as root canal wall thickness.
For the treatment of nonvital immature teeth, pulp revascularization showed a superior therapeutic effect in comparison with the apical barrier technique.
Core Tip: This report presents a case where pulp revascularization and the apical barrier technique were performed in the right maxillary and mandibular premolars. Twelve months of follow-up showed that both teeth were asymptomatic; however, the tooth treated with pulp revascularization showed a better outcome in root development.
- Citation: Chai R, Yang X, Zhang AS. Different endodontic treatments induced root development of two nonvital immature teeth in the same patient: A case report. World J Clin Cases 2023; 11(11): 2567-2575
- URL: https://www.wjgnet.com/2307-8960/full/v11/i11/2567.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i11.2567
Young permanent teeth take 3-5 years to complete root development and form the apical foramen after eruption. During this process, pulp or periapical tissue disease caused by infection or trauma may hinder or even stop root development. Flared and wide apical foramens have always been a great challenge for dental clinicians. Pulp revascularization is a novel therapeutic method that has been proven to be a clinically successful application of regenerative medicine. Numerous reports in recent years have demonstrated that revascularization is undoubtedly an ideal treatment for nonvital teeth with wide-open apexes. Sterilized drug irrigation replaces the conventional mechanical root canal preparation and maximizes the preservation of root apical tissue to provide conditions for subsequent tissue regeneration in the root canal. Previous studies have suggested that secondary hard tissue may be derived from residual pulp tissue at the root apex, Hertwig's epithelial root sheath cells and periapical stem cells[1]. These cells adhere to the autologous scaffold formed by the blood clot in the root canal; induce the formation of hard tissues, such as reparative dentin, cementoid tissue and bone-like tissue at the root apex; or directly induce the ingrowth of periapical hard tissue[2-5]. In this report, we describe a case of pulp revascularization use for the treatment of apical periodontitis in a young permanent tooth with a wide-open apex. After revascularization, the apical foramen closed, and the thickness of the dentinal wall increased significantly.
Although the therapeutic effect of pulp revascularization is satisfactory, if the apical blood supply of the affected tooth is poor, sufficient blood cannot be delivered during the treatment, which makes it difficult to conduct blood-clot induced revascularization. In our report, pulp revascularization was successfully performed on the maxillary second premolar, but microscopy did not reveal obvious blood inflow into the root canal of the mandibular second premolar, so we resorted to the use of the apical barrier technique. Both techniques are important treatments for nonvital teeth with wide-open apexes, and this case was a rare opportunity to evaluate and compare the therapeutic effect of pulp revascularization and the apical barrier technique in the same individual. A case report is presented to compare the different root maturation processes of pulp revascularization and the apical barrier technique in the same patient in homonymous teeth from different jaws.
A 10-year-old girl complained of pain in the right upper posterior teeth for 2 d before visiting; four months later, she visited again and complained of pain in the right lower posterior teeth for 2 d.
The patient reported that she felt discomfort in the right maxillary posterior region two days prior to visiting our department and went to see a dentist in another clinic. The diagnosis was central cusp deformity in the right maxillary and mandibular second premolars. The central cusps were partly ground off during the visit. The next day, she felt severe spontaneous pain in the right maxillary posterior region accompanied by swelling of the right maxillofacial area. Approximately 4 mo later, she complained of paroxysmal pain in the right mandibular posterior region, and the next day, the pain became persistent.
The patient denied any history of previous disease.
The patient denied a personal or family history of any systemic disease.
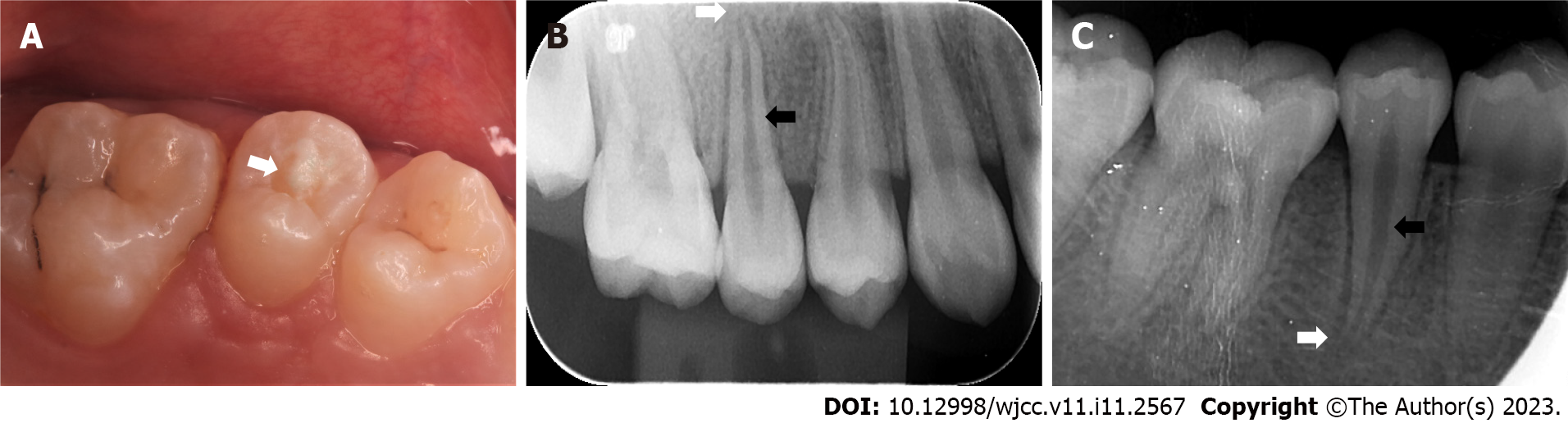
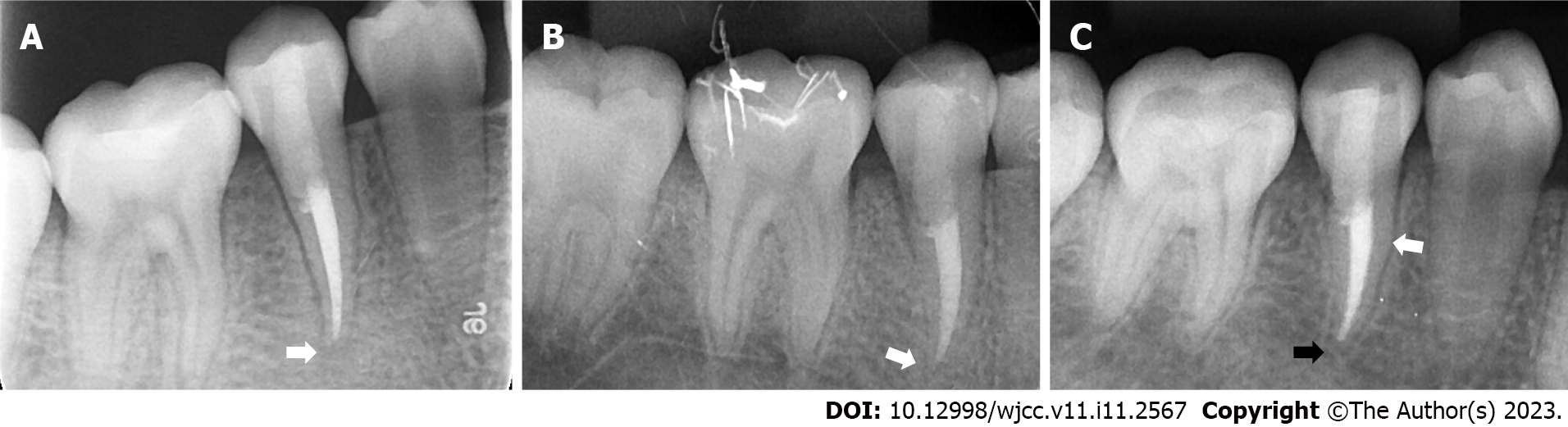
When the patient presented with pain in the right upper posterior teeth, the extraoral examination revealed mild swelling of the right maxillofacial region. Intraoral examination showed that the buccal gingiva around the apical region of the right maxillary premolars was swollen, the vestibular sulcus was shallow, and the residual partial central cusp deformity was visible on the occlusal surface (Figure 1A). The right maxillary second premolar was moderately loose and had a severe response to percussion.
When the patient presented with pain in the right lower posterior teeth, extraoral examination showed bilateral maxillofacial symmetry and no obvious swelling. Intraoral examination showed that the buccal gingiva around the apical area of the right mandibular premolars was red and slightly swollen. The right mandibular second premolar was slightly loose and had a severe response to percussion.
No laboratory examination was performed.
Intraoral radiographic examination revealed a hypodense area around the apex of the right maxillary second premolar, and the root was incompletely developed with an open apex (Figure 1B).
Periapical X-ray examination showed a hypodense area around the apex of the right mandibular second premolar with an open apex (Figure 1C).
Based on the patient’s history as well as clinical and radiographic examinations, a diagnosis of chronic apical periodontitis was made for the right maxillary second premolar and the right mandibular second premolar.
Treatment options, including the use of the pulp revascularization technique and the apical barrier technique, were offered to the patient. After fully understanding the advantages and disadvantages of the two techniques, the patient chose pulp revascularization. The timelines of the treatment of the affected teeth are presented in Tables 1 and 2.
| Timeline | Events | |
| March 11, 2021 | First treatment visit | Root canal irrigation and intracranial medication with calcium hydroxide paste |
| March 26, 2021 | Second treatment visit | Penetration of the periapical tissue to induce bleeding, sealing of the coronal third of the root canal with iRoot BP plus |
| June 29, 2021 | 3-mo follow-up | Significant reduction of the periapical hypodense area and obvious closure of the apex |
| September 28, 2021 | 6-mo follow-up | Complete healing of the periapical lesion and slight increase in dentinal wall thickness |
| April 1, 2022 | 12-mo follow-up | Significant thickening of the dentinal wall and obvious narrowing of the root canal |
| Timeline | Events | |
| July 19, 2021 | First treatment visit | Root canal irrigation and intracranial medication with calcium hydroxide paste |
| July 30, 2021 | Second treatment visit | Penetration of the periapical tissue without obvious bleeding |
| August 6, 2021 | Third treatment visit | Penetration of the periapical tissue for a second time without obvious bleeding and performance of the apical barrier technique |
| August 13, 2021 | Fourth treatment visit | Filling of the middle and coronal third of the root canal with hot gutta-percha and filling of the access cavity with light-cured composite resin |
| November 10, 2021 | 3-mo follow-up | Significant decrease in size of the periapical hypodense area |
| February 11, 2022 | 6-mo follow-up | Complete healing of the periapical hypodense area |
| August 25, 2022 | 12-mo follow-up | Narrowing of the periodontal ligament and complete closure of the apex |
First treatment visit: Local anesthesia was performed with 2% lidocaine hydrochloride. A rubber dam was used to isolate the affected tooth. Under a microscope, pulp chamber access was performed with a diamond bur. A large amount of pus was seen in the pulp chamber. The root canal was irrigated alternately with 1.5% sodium hypochlorite solution (Longly Biotechnology, Wuhan, China) and 17% ethylene diamine tetraacetic acid (EDTA) solution (Longly Biotechnology, Wuhan, China) for five minutes. After drying the canal with sterile paper tips, calcium hydroxide paste (ApexCal®, Ivoclar Vivadent, Liechtenstein) was placed into the root canal; a sterile cotton pellet was placed in the pulp chamber, and the access cavity was sealed with a temporary sealing paste (Cavit-G, 3M ESPE, St. Paul, MN, United States).
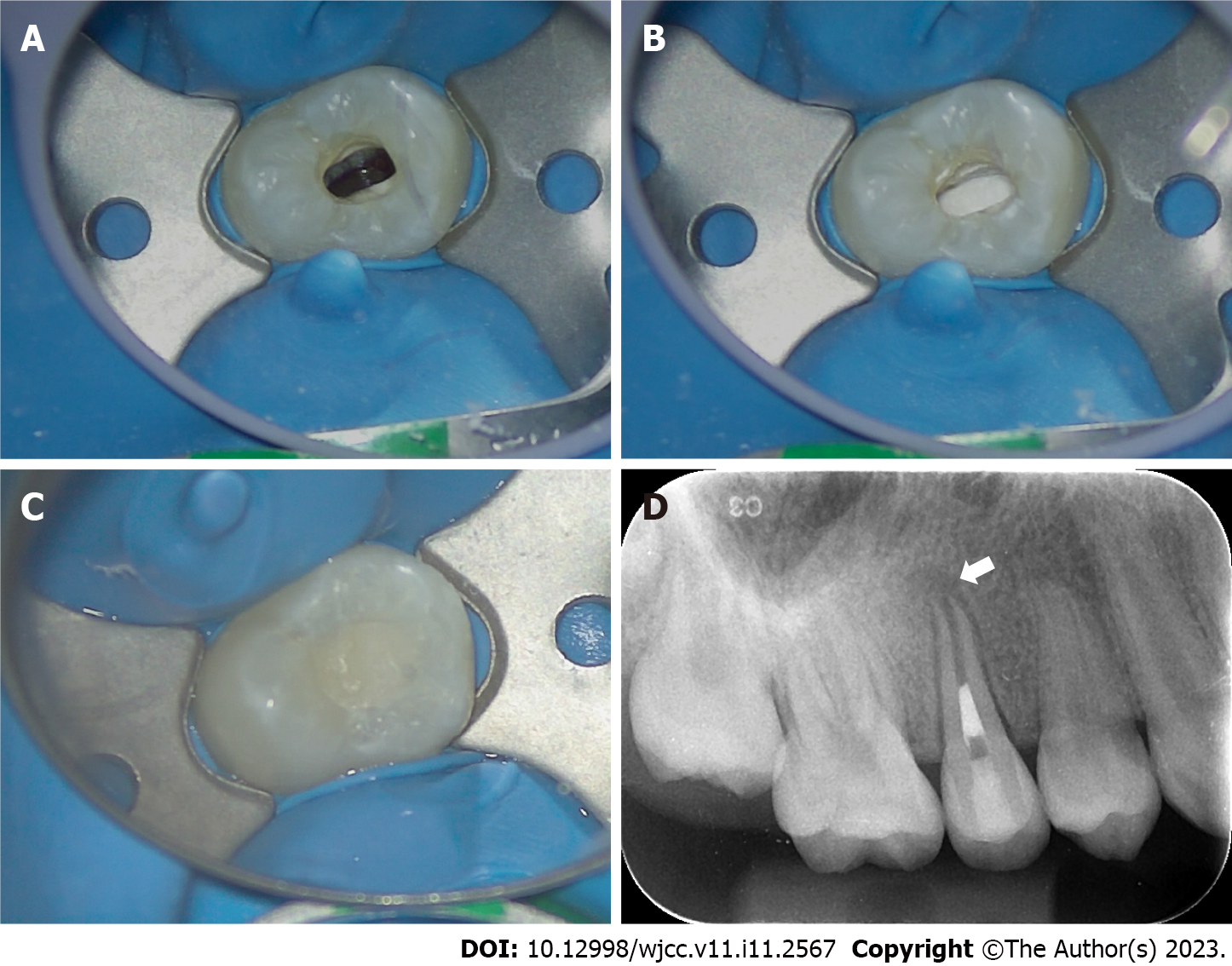
Second treatment visit: Two weeks later, the affected tooth had no percussion pain or abnormal mobility. Local anesthesia was performed with 2% lidocaine hydrochloride, and a rubber dam was used to isolate the affected tooth. Under a microscope, the temporary sealing material was removed, and the root canal was washed with an ultrasonic apparatus (Satelec P5 Newtron XS, Acteon, France). After copious irrigation with 17% EDTA solution (Longly Biotechnology, Wuhan, China) for five minutes, the root canal was dried with sterile paper tips. The periapical tissue was penetrated with a #20 K-file to induce bleeding until blood filled the root canal (Figure 2A). The coronal third of the root canal was sealed with iRoot BP plus (iRoot® BP+, Innovative BioCeramix, Canada) (Figure 2B). The access cavity was temporarily restored with glass ionomer cement (Fuji IX GP, GC, Japan) (Figure 2C). A postoperative periapical radiograph of the right maxillary second premolar was performed (Figure 2D).
First treatment visit: Local anesthesia was performed with 2% lidocaine hydrochloride. A rubber dam was used to isolate the affected tooth. Under a microscope, pulp chamber access was performed with a diamond bur. The root canal was irrigated alternately with 1.5% sodium hypochlorite solution (Longly Biotechnology, Wuhan, China) and 17% EDTA solution (Longly Biotechnology, Wuhan, China) for five minutes. After drying the canal with sterile paper tips, calcium hydroxide paste (ApexCal®, Ivoclar Vivadent, Liechtenstein) was placed into the root canal; a sterile cotton pellet was placed in the pulp chamber, and the access cavity was sealed with a temporary sealing paste (Cavit-G, 3M ESPE, St. Paul, MN, United States).
Second treatment visit: After two weeks, the affected tooth had no percussion pain or abnormal mobility. Local anesthesia was performed with 2% lidocaine hydrochloride, and a rubber dam was used to isolate the affected tooth. Under a microscope, the temporary sealing material was removed, and the root canal was washed with an ultrasonic apparatus (Satelec P5 Newtron XS, Acteon, France). After copious irrigation with 17% EDTA solution (Longly Biotechnology, Wuhan, China) for five minutes, the root canal was dried with sterile paper tips. The periapical tissue was penetrated with a #20 K-file to induce bleeding. However, after multiple attempts, there was no significant bleeding. The root canal was irrigated with 1.5% sodium hypochlorite solution (Longly Biotechnology, Wuhan, China) and then filled with calcium hydroxide paste (ApexCal®, Ivoclar Vivadent, Liechtenstein); a sterile cotton pellet was placed in the pulp chamber, and the access cavity was sealed with a temporary sealing paste (Cavit-G, 3M ESPE, St. Paul, MN, United States).
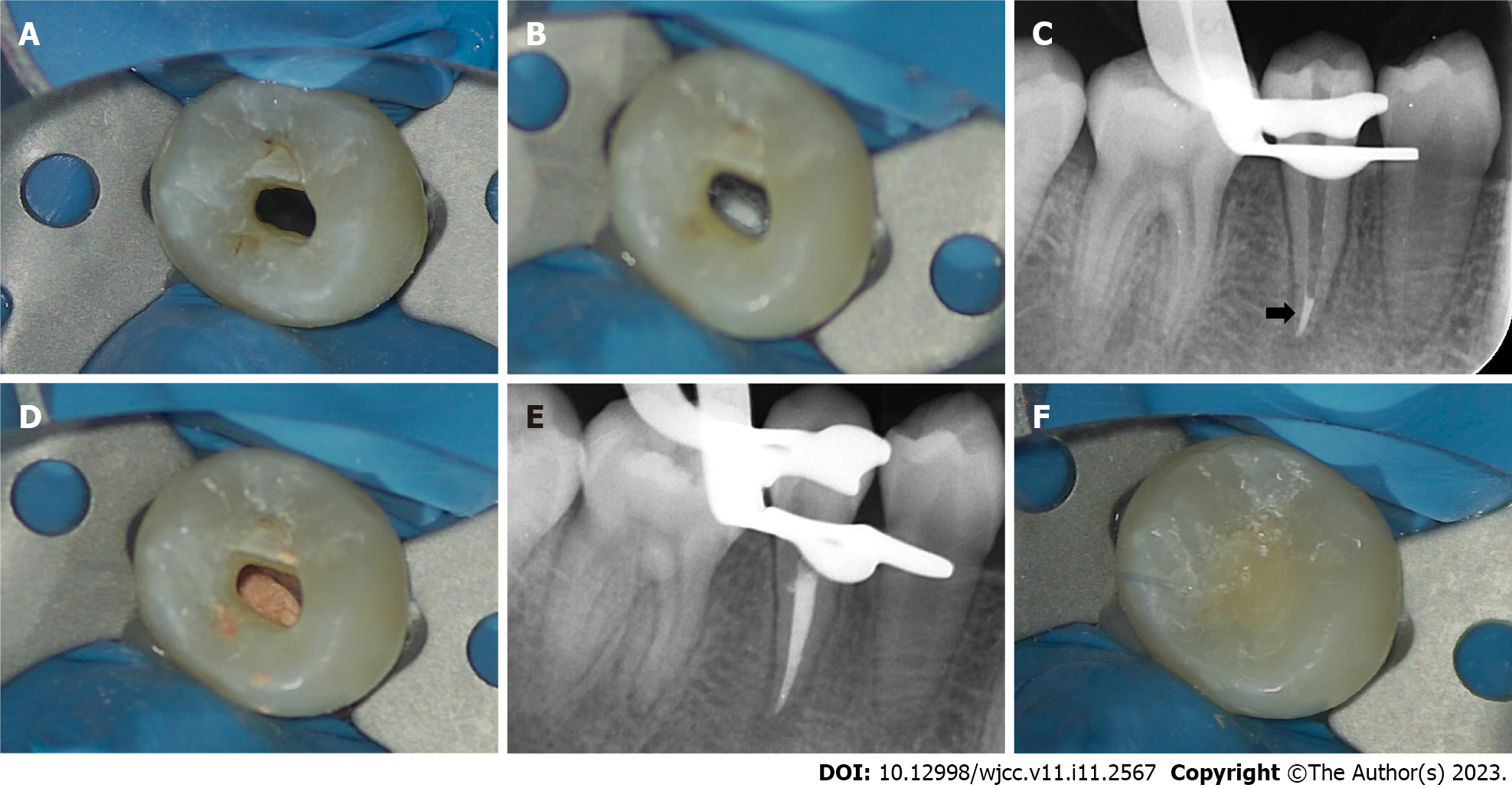
A week later, local anesthesia was performed with 2% lidocaine hydrochloride, and a rubber dam was used to isolate the affected tooth. Under a microscope, the temporary sealing material was removed, and the root canal was washed with an ultrasonic apparatus (Satelec P5 Newtron XS, Acteon, France). After copious irrigation with 17% EDTA solution (Longly Biotechnology, Wuhan, China) for five minutes, the root canal was dried with sterile paper tips. The periapical tissue was penetrated again with a #20 K-file to induce bleeding. After several attempts, there was still no obvious bleeding (Figure 3A). After discussion with the patient’s parents, we decided to switch the treatment plan to the apical barrier technique. Under a microscope, an appropriate amount of iRoot BP plus was placed into the root canal and pressed repeatedly to form an apical barrier with a thickness of approximately 4 mm at the root tip (Figure 3B and C). A moist sterile cotton pellet was placed in the pulp chamber, and the access cavity was sealed with a temporary sealing paste (Cavit-G, 3M ESPE, St. Paul, MN, United States).
After one week, a rubber dam was used to isolate the affected tooth. Under a microscope, the temporary sealing material was removed, and the root canal was washed with an ultrasonic apparatus (Satelec P5 Newtron XS, Acteon, France). After drying with sterile paper tips, the coronal and middle third of the root canal was filled with hot gutta-percha (Figure 3D and E), and the access cavity was restored with a flowable light-cured resin base (Z350XT Flowable, 3M™ Filtek™, United States) and light-cured composite resin (Z350XT, 3M™ Filtek™, United States) (Figure 3F).
At the 3-mo follow-up, the periapical radiograph revealed that the hypodense area around the root apex had significantly diminished, and the apex had obviously closed (Figure 4A). The glass ionomer cement was replaced by a flowable light-cured resin base (Z350XT Flowable, 3M™ Filtek™, United States) and light-cured composite resin (Z350XT, 3M™ Filtek™, United States).
At the 6-mo follow-up, there was complete healing of the periapical lesion. The thickness of the dentinal wall slightly increased, and there seemed to be some scattered calcifications in the root canal (Figure 4B).
At the 12-mo follow-up, the root canal had obviously narrowed, and the thickness of the dentinal wall had increased significantly (Figure 4C).
The affected tooth showed no response to cold, heat or electric stimulations.
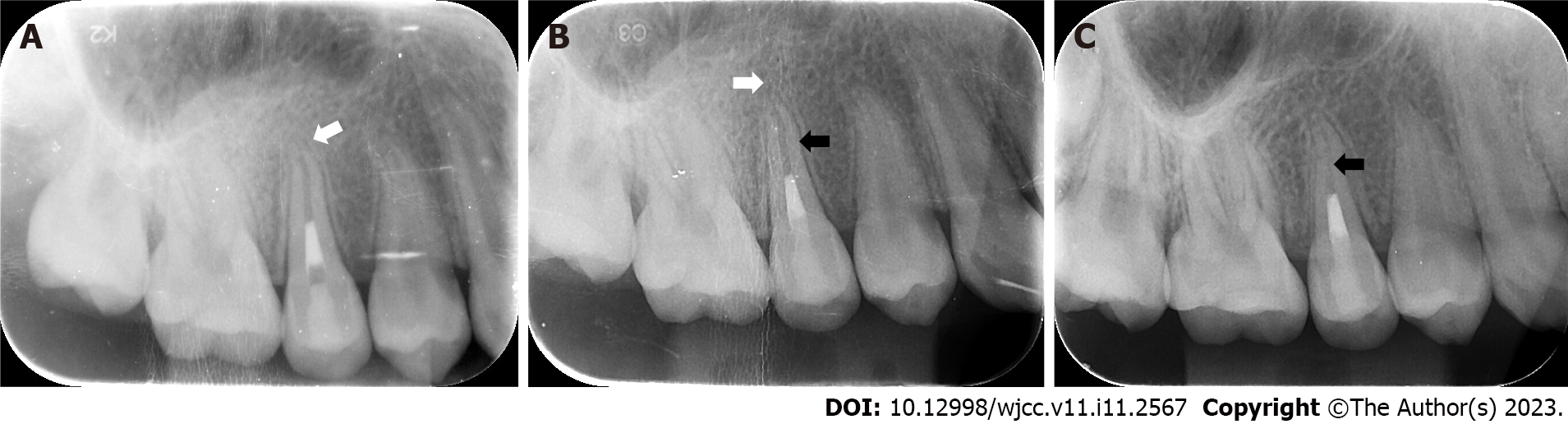
At the 3-mo follow-up, the periapical radiolucency area was significantly reduced (Figure 5A).
At the 6-mo follow-up, the periapical radiolucency area had completely healed (Figure 5B).
At the 12-mo follow-up, the periodontal ligament had narrowed, and complete closure of the apex was observed, with an obvious calcification layer generated just around the apical barrier materials (Figure 5C).
In the present case report, we described the maturation processes of homonymous teeth from different jaws after applying dental pulp revascularization and the apical barrier technique in the same patient. This was a valuable opportunity to observe the differences in the therapeutic effects between the two techniques. After 12 mo of follow-up, both teeth were asymptomatic; however, the different statuses of the roots indicated that pulp revascularization showed several advantages over the apical barrier technique. Pulp revascularization can stimulate the regeneration potential of periapical tissues, thereby promoting further development of the root, including root canal wall thickening, root elongation, and closure of the apical foramen[6,7]. In this way, the fragile and blunderbuss-like root can be rescued, and the incidence of tooth fracture and crown-to-root ratio incompatibility can be further reduced. In addition, after pulp revascularization, the tooth does not need root canal therapy, avoiding the risk of reinfection caused by reopening the pulp cavity.
Even if pulp revascularization fails, apexification and the apical barrier technique are alternative options. Traditionally, the procedure indicated for teeth with incomplete root formation is apexification; this technique is mainly performed by placing calcium hydroxide paste into the root canal for a certain time period to induce the formation of a calcified barrier[8]. Currently, apexification is regarded as the most classical treatment; however, long-term intracanal medication may decrease root strength due to the hygroscopic and proteolytic properties of calcium hydroxide[9,10]. The key to the apical barrier technique is to seal the wide-open apex tightly with bioceramic materials, such as iRoot BP plus or mineral trioxide aggregate[11], to prevent further infections. It is often a substantial technical challenge for dentists, and if the treatment fails, it is difficult to address[12]. The current view is that the root length and dentinal wall thickness will not change after treatment; thus, this technique does not considerably enhance the strength of the root. Therefore, with a comprehensive analysis of the pros and cons of apexification and the apical barrier technique, in this case, after the failure of revascularization, we resorted to the apical barrier technique to treat the mandibular second premolar. At the 6-mo follow-up, the hypodense area around the root apex had completely healed, demonstrating that successful application of the apical barrier technique can effectively control root canal infection and promote the healing of the periapical tissue. At the 12-mo follow-up, complete closure of the apex was observed, and there was an obvious calcification layer generated just around the apical barrier materials, likely due to the mineralization induction capacity of iRoot BP plus[13].
In this case, the different maturation processes of the two teeth demonstrated that pulp revascularization showed a superior therapeutic effect in nonvital young permanent teeth because pulp revascularization offers the possibility of additional tooth development rather than simple hard tissue deposition, which may greatly reduce the incidence of future tooth fractures. According to studies in regenerative medicine, therapeutic angiogenesis is a key factor in tissue regeneration[14-16]. Both the American Association of Endodontics and the European Society of Endodontology agree that pulp revascularization is the preferred treatment for teeth with incomplete root formation[17,18]. The blood clot-induced revascularization used in this study is the most commonly used, well-established technique. With the continuous development of regenerative medicine, revascularization can also be induced by, for example, platelet-rich fibrin, platelet-rich plasma, angiogenic peptide hydrogels, and buccal fat grafts[19-21]. Although pulp revascularization is highly recommended by many clinicians, its shortcomings, such as discoloration of the crown and root canal blockage, cannot be ignored[22-24]. Nicoloso et al[25] conducted a meta-analysis of several clinical studies and concluded that pulp revascularization has no significant advantages over other treatments. The optimal treatment for nonvital immature teeth remains uncertain. In the future, we need to pay close attention to new trends in regenerative medicine to create more innovative points for root regeneration; we also need to carefully analyze the condition of the affected teeth in clinical practice, balance the advantages and disadvantages of various treatment methods, and try to provide the most suitable choice for patients.
In this case, pulp revascularization showed a superior outcome of inducing root development in comparison with the apical barrier technique. However, more attention should be given to regenerative endodontics to optimize treatment for nonvital immature teeth.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: He YH, China; Sekhar P, India S-Editor: Hu YR L-Editor: A P-Editor: Hu YR
| 1. | Chueh LH, Huang GT. Immature teeth with periradicular periodontitis or abscess undergoing apexogenesis: a paradigm shift. J Endod. 2006;32:1205-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 184] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 2. | Torabinejad M, Alexander A, Vahdati SA, Grandhi A, Baylink D, Shabahang S. Effect of Residual Dental Pulp Tissue on Regeneration of Dentin-pulp Complex: An In Vivo Investigation. J Endod. 2018;44:1796-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Zhu X, Wang Y, Liu Y, Huang GT, Zhang C. Immunohistochemical and histochemical analysis of newly formed tissues in root canal space transplanted with dental pulp stem cells plus platelet-rich plasma. J Endod. 2014;40:1573-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Lui JN, Lim WY, Ricucci D. An Immunofluorescence Study to Analyze Wound Healing Outcomes of Regenerative Endodontics in an Immature Premolar with Chronic Apical Abscess. J Endod. 2020;46:627-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Becerra P, Ricucci D, Loghin S, Gibbs JL, Lin LM. Histologic study of a human immature permanent premolar with chronic apical abscess after revascularization/revitalization. J Endod. 2014;40:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 158] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 6. | Zhou R, Wang Y, Chen Y, Chen S, Lyu H, Cai Z, Huang X. Radiographic, Histologic, and Biomechanical Evaluation of Combined Application of Platelet-rich Fibrin with Blood Clot in Regenerative Endodontics. J Endod. 2017;43:2034-2040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Nawal RR, Utneja S, Sharma V, Yadav S, Talwar S. Long-term follow-up of traumatized immature necrotic permanent teeth treated with regenerative endodontic protocol using platelet-rich fibrin: A prospective case series. J Conserv Dent. 2020;23:417-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Shaik I, Dasari B, Kolichala R, Doos M, Qadri F, Arokiyasamy JL, Tiwari RVC. Comparison of the Success Rate of Mineral Trioxide Aggregate, Endosequence Bioceramic Root Repair Material, and Calcium Hydroxide for Apexification of Immature Permanent Teeth: Systematic Review and Meta-Analysis. J Pharm Bioallied Sci. 2021;13:S43-S47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Arruda MEF, Neves MAS, Diogenes A, Mdala I, Guilherme BPS, Siqueira JF Jr, Rôças IN. Infection Control in Teeth with Apical Periodontitis Using a Triple Antibiotic Solution or Calcium Hydroxide with Chlorhexidine: A Randomized Clinical Trial. J Endod. 2018;44:1474-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Guerrero F, Mendoza A, Ribas D, Aspiazu K. Apexification: A systematic review. J Conserv Dent. 2018;21:462-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Plascencia H, Díaz M, Gascón G, Garduño S, Guerrero-Bobadilla C, Márquez-De Alba S, González-Barba G. Management of permanent teeth with necrotic pulps and open apices according to the stage of root development. J Clin Exp Dent. 2017;9:e1329-e1339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Asgary S, Fayazi S. Endodontic Surgery of a Symptomatic Overfilled MTA Apical Plug: A Histological and Clinical Case Report. Iran Endod J. 2017;12:376-380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Zhang J, Zhu LX, Cheng X, Lin Y, Yan P, Peng B. Promotion of Dental Pulp Cell Migration and Pulp Repair by a Bioceramic Putty Involving FGFR-mediated Signaling Pathways. J Dent Res. 2015;94:853-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3471] [Cited by in RCA: 4023] [Article Influence: 287.4] [Reference Citation Analysis (0)] |
| 15. | Sarkar B, Nguyen PK, Gao W, Dondapati A, Siddiqui Z, Kumar VA. Angiogenic Self-Assembling Peptide Scaffolds for Functional Tissue Regeneration. Biomacromolecules. 2018;19:3597-3611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Mastrullo V, Cathery W, Velliou E, Madeddu P, Campagnolo P. Angiogenesis in Tissue Engineering: As Nature Intended? Front Bioeng Biotechnol. 2020;8:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 17. | Galler KM, Krastl G, Simon S, Van Gorp G, Meschi N, Vahedi B, Lambrechts P. European Society of Endodontology position statement: Revitalization procedures. Int Endod J. 2016;49:717-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 253] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 18. | American Association of Endodontists. AAE Clinical Considerations for a Regenerative Procedure. 2016. Available from: https://www.aae.org/specialty/clinical-resources/guide-clinical-endodontics/. |
| 19. | Nicoloso GF, Pötter IG, Rocha RO, Montagner F, Casagrande L. A comparative evaluation of endodontic treatments for immature necrotic permanent teeth based on clinical and radiographic outcomes: a systematic review and meta-analysis. Int J Paediatr Dent. 2017;27:217-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Siddiqui Z, Sarkar B, Kim KK, Kadincesme N, Paul R, Kumar A, Kobayashi Y, Roy A, Choudhury M, Yang J, Shimizu E, Kumar VA. Angiogenic hydrogels for dental pulp revascularization. Acta Biomater. 2021;126:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 21. | Khazaei S, Khademi A, Torabinejad M, Nasr Esfahani MH, Khazaei M, Razavi SM. Improving pulp revascularization outcomes with buccal fat autotransplantation. J Tissue Eng Regen Med. 2020;14:1227-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Bezgin T, Yilmaz AD, Celik BN, Kolsuz ME, Sonmez H. Efficacy of platelet-rich plasma as a scaffold in regenerative endodontic treatment. J Endod. 2015;41:36-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 23. | Kim JH, Kim Y, Shin SJ, Park JW, Jung IY. Tooth discoloration of immature permanent incisor associated with triple antibiotic therapy: a case report. J Endod. 2010;36:1086-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 221] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 24. | Gelman R, Park H. Pulp revascularization in an immature necrotic tooth: a case report. Pediatr Dent. 2012;34:496-499. [PubMed] |
| 25. | Nicoloso GF, Goldenfum GM, Pizzol TDSD, Scarparo RK, Montagner F, de Almeida Rodrigues J, Casagrande L. Pulp Revascularization or Apexification for the Treatment of Immature Necrotic Permanent Teeth: Systematic Review and Meta-Analysis. J Clin Pediatr Dent. 2019;43:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |