Published online Apr 16, 2023. doi: 10.12998/wjcc.v11.i11.2549
Peer-review started: December 27, 2022
First decision: January 20, 2023
Revised: February 2, 2023
Accepted: March 22, 2023
Article in press: March 22, 2023
Published online: April 16, 2023
Processing time: 100 Days and 6.9 Hours
Lichen amyloidosis (LA) is a chronic, severely pruritic skin disease, which is the most common form of primary cutaneous amyloidosis. The treatment of LA has been considered to be difficult. LA may be associated with atopic dermatitis (AD), and in this setting, the treatment options may be more limited. Herein, we report four cases of LA associated with AD successfully treated by dupilumab.
In this article, we describe four cases of patients who presented with recurrent skin rash accompanied by severe generalized intractable pruritus, diagnosed with refractory LA coexisting with chronic AD. Previous treatments had not produced any apparent improvement. Thus, we administered dupilumab injection subcutaneously at a dose of 600 mg for the first time and 300 mg every 2 wk thereafter. Their lesions all markedly improved.
Dupilumab may be a new useful treatment for LA coexisting with AD.
Core Tip: Lichen amyloidosis (LA) is a chronic, severely pruritic skin disease, which is the most common subtype of primary cutaneous amyloidosis characterized by deposition of amyloid protein on the skin without visceral involvement. The treatment of LA has been considered difficult, with high relapse rates. In this article, we present four cases of LA associated with atopic dermatitis (AD) treated with dupilumab who achieved remarkable results. Our observations suggested that dupilumab may shed light on the systematic treatment of refractory LA patients with AD.
- Citation: Zhu Q, Gao BQ, Zhang JF, Shi LP, Zhang GQ. Successful treatment of lichen amyloidosis coexisting with atopic dermatitis by dupilumab: Four case reports. World J Clin Cases 2023; 11(11): 2549-2558
- URL: https://www.wjgnet.com/2307-8960/full/v11/i11/2549.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i11.2549
Lichen amyloidosis (LA) is the most common subtype of primary cutaneous amyloidosis (PCA) characterized by deposition of amyloid protein in the skin without visceral involvement. PCA is commonly observed in Southeast Asian countries and LA appears to be more prevalent in those of Chinese descent. LA typically presents as persistent hyperkeratotic skin-colored or hyperpigmented papules usually located on the calves and other extensor surfaces, e.g., anterior thighs or forearms, which can then coalesce into corrugated patches that often have a rippled or ridged pattern accompanied by severe itching. At the onset, lesions are usually unilateral, but overtime a bilateral symmetric distribution can develop. Histologically, LA appears as eosinophilic amorphous deposits that are restricted to the upper dermis particularly the papillary dermis with epidermal acanthosis and orthohyperkeratosis. The treatment of LA has been considered difficult, with a high relapse rate. Generally, many medical and physical treatments such as laser and light therapies have been tried with unsatisfactory results. LA associated with atopic dermatitis (AD) has been reported previously. We present four cases of LA associated with AD treated with dupilumab that demonstrated remarkable therapeutic achievements (Table 1).
| Cases | Age/gender | Duration | Absolute value of eosinophils at baseline | Duration of treatment for absolute value of eosinophils decreased to normal range | Total serum IgE at baseline | Duration of treatment for total IgE decreased to normal range | EASI at baseline | EASI at 16 wk of treatment | VAS at baseline | VAS at 16 wk of treatment | DLQI at baseline | DLQI at 16 wk of treatment |
| Case 1 | 37 yr/male | 14 yr | 6.30 × 109/L | 6 wk | 277.30 IU/mL | 10 wk | 62 | 16 | 10 | 1 | 22 | 3 |
| Case 2 | 67 yr/male | 10 yr | 0.10 × 109/L | - | 54.20 IU/mL | - | 36 | 10 | 8 | 0 | 19 | 1 |
| Case 3 | 20 yr/male | 6 yr | 0.20 × 109/L | - | 1002 IU/mL | 12 wk | 16 | 4 | 6 | 0 | 16 | 2 |
| Case 4 | 54 yr/female | 5 yr | 2.0 × 109/L | 10 wk | 893.60 IU/mL | 14 wk | 23 | 8 | 7 | 0 | 17 | 1 |
Case 1: The patient was a 37-year-old man who presented with recurrent skin rash accompanied by severe generalized intractable pruritus for more than 14 years.
Case 2: A 67-year-old man presented with recurrent rashes on his back and limbs, which was most consistent with the characteristics of the nummular variant of AD, with generalized and intense pruritus for 10 years.
Case 3: A 20-year-old man visited our department presenting with multiple pruritic brownish papules that were mostly located on the anterior chest, back, and both thighs for 6 years.
Case 4: A 54-year-old woman presented with skin lesions along with pruritus which appeared 3 years prior to current presentation.
Case 1: The patient had received treatment with a variety of oral antihistamines and topical hormone creams for external use, but without satisfactory result.
Case 2: Prior treatment consisting of halometasone ointment for external use, oral antihistamine, and narrow band ultraviolet B phototherapy showed no benefit, and the rash continued to be worsened.
Case 3: The patient had received treatment with oral epinastine and mometasone furoate for external use, but without obvious effects.
Case 4: The patient had a history of irregular consumption of oral methylprednisolone tablets and epinastine hydrochloride capsules, but without satisfactory effects.
Case 1: The patient had suffered allergic rhinitis for more than 14 years.
Case 2: The patient had a past history of allergic rhinitis for 20 years.
Case 3: The patient had suffered allergic rhinitis for 1 year.
Case 4: No significant past history or any illness.
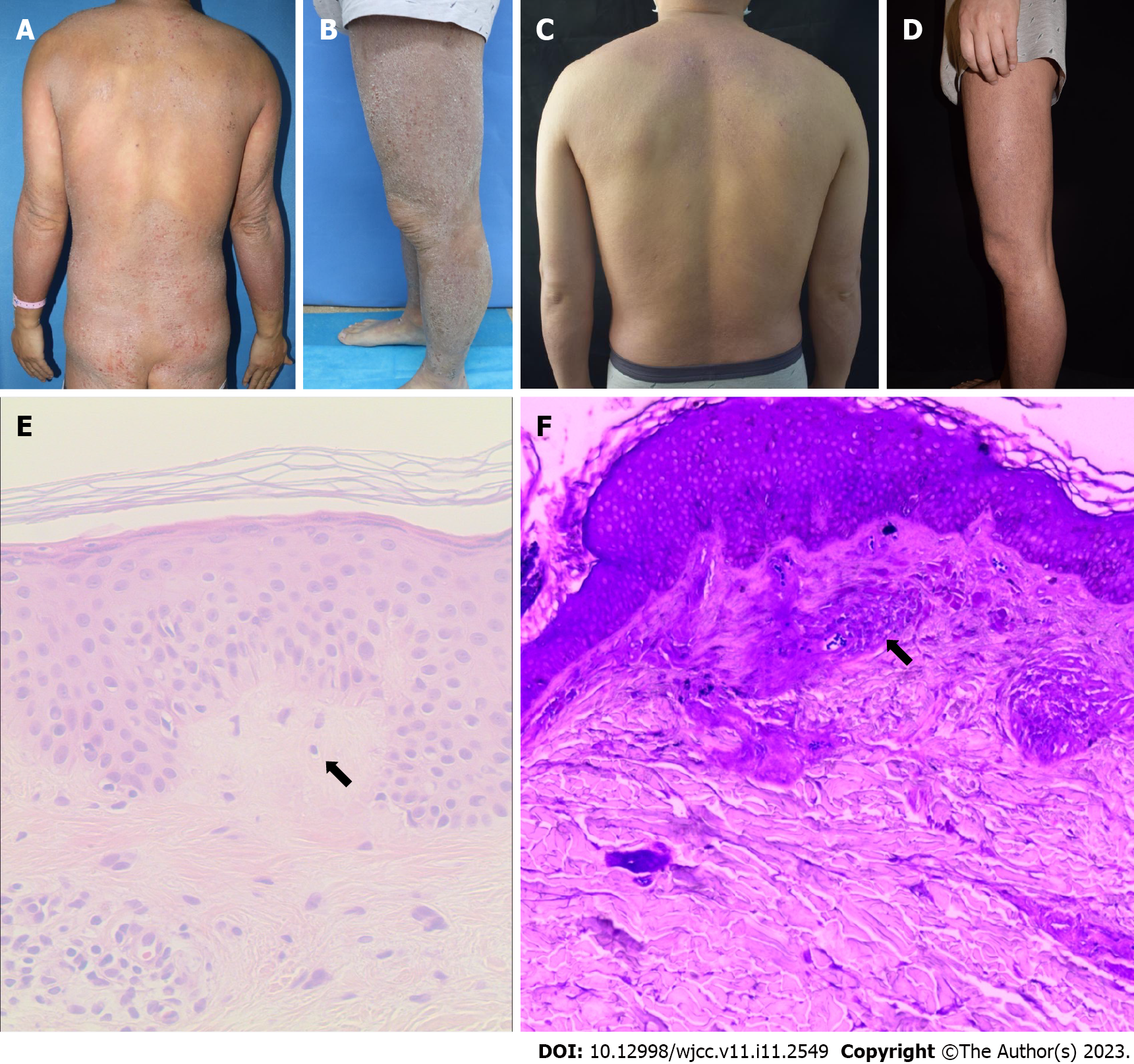
Case 1: A dense distribution of hard and solid confluent plaques and papules was seen throughout the body, covered with dry and thick scales (Figure 1A-D).
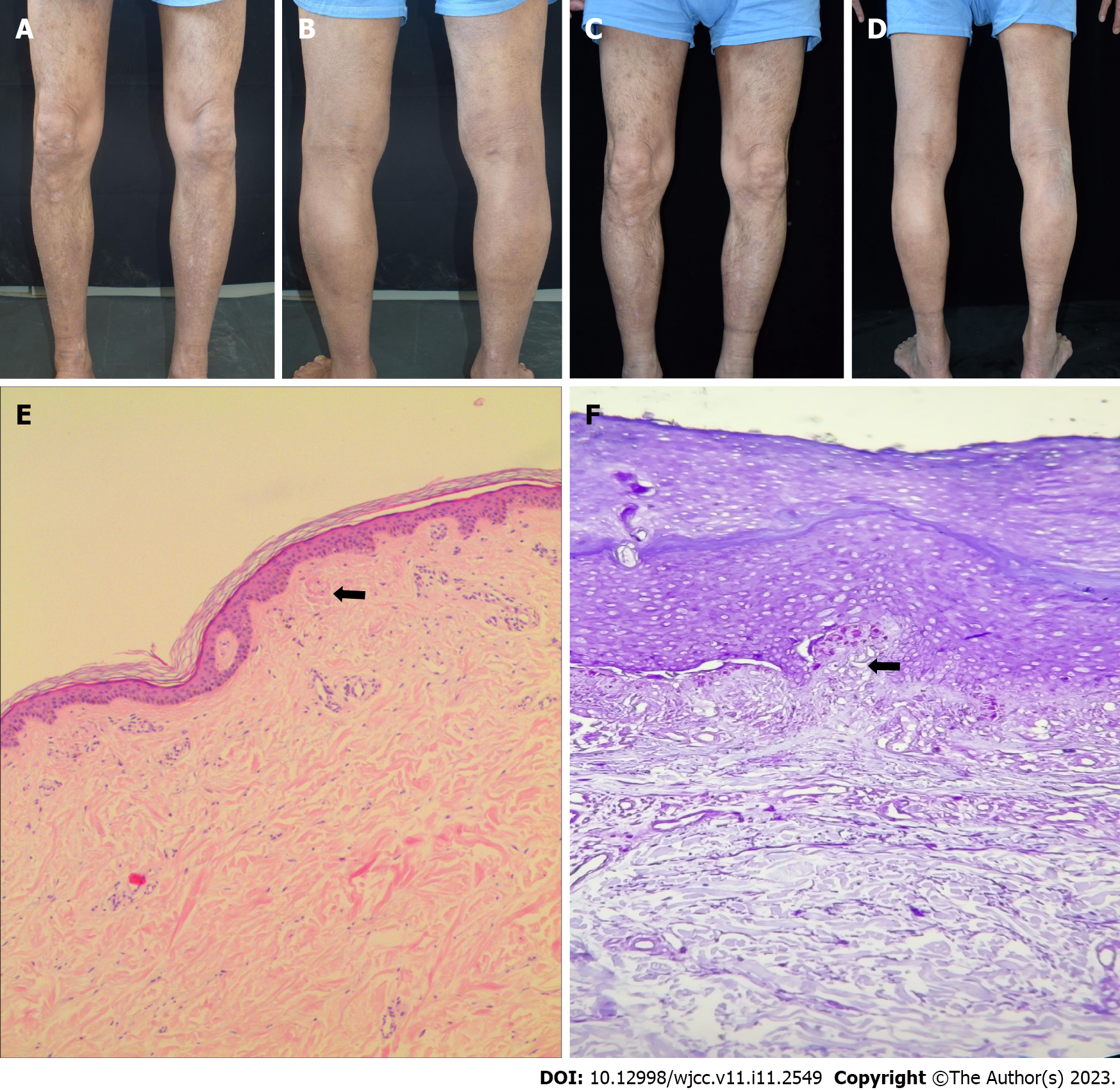
Case 2: Cutaneous examination revealed a dense distribution of hard and solid papules and irregular erythematous patches with unclear boundaries distributed in the back and limbs, which were covered with dry scales. Most of the skin was mossy and rough (Figure 2A-D).
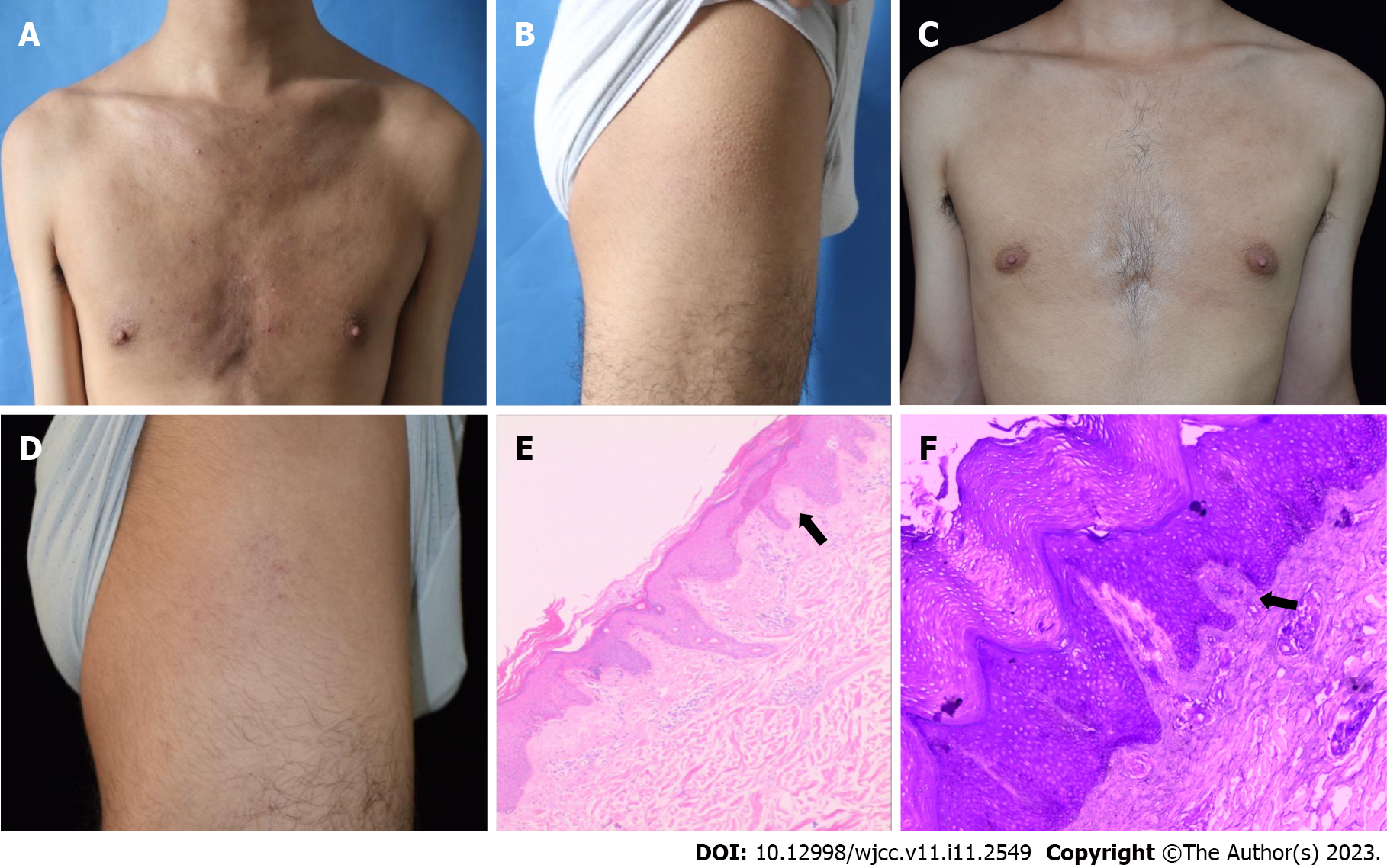
Case 3: Upon dermatological examination, a dense distribution of solid and unmixing papules was seen on the back, anterior chest, and both thighs, which were covered with white scales. The skin showed lichenoid changes with a rippled pattern, which were more prominent on the lateral side (Figure 3A-D).
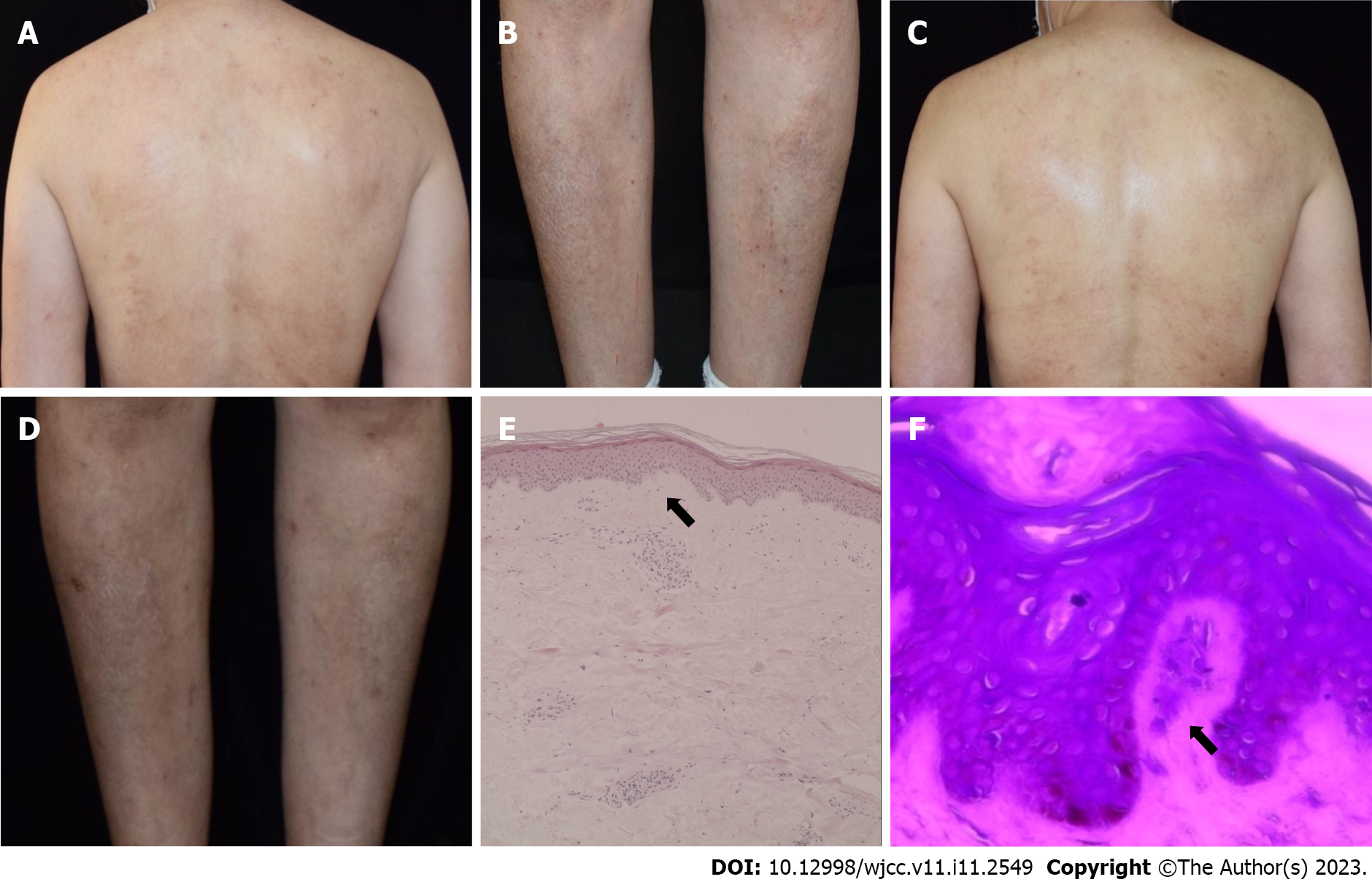
Case 4: A physical examination showed localized discrete brownish papules in association with erythema of different sizes on the neck, trunk, and lower limbs. Lichenoid skin changes and bloody crusts could be seen on the back and lateral aspects of shins. In addition, multiple firm and pigmented papules were symmetrically and densely distributed on the anterolateral surfaces of the calves (Figure 4A-D).
Case 1: Laboratory examination demonstrated a total serum IgE level of 277.30 IU/mL and an absolute eosinophilic value of 6.30 × 109/L. The histopathological examination of the specimen obtained from the back area showed mild hyperkeratosis of the epidermis, irregular hyperplasia of the spinous layer, spongy edema, complete basal cells, an equal number of lymphocytes, and histocytes infiltrating into the blood vessels in the superficial dermis. The dermal papilla and superficial dermis also showed uniformly reddish masses stained with crystal violet confirming amyloid deposits (Figure 1E and F).
Case 2: The biopsy of the right leg revealed epidermal hyperkeratosis, irregular hyperplasia of the spinous layer, increased basal pigment cells, red mass material deposition in the dermal papilla, and superficial perivascular lymphatic and histiocytic infiltration. Crystal violet staining was positive for amyloid bodies (Figure 2E and F).
Case 3: The LA lesions are usually located primarily on the shins or other extensor aspects of the extremities; the thighs, the upper back, abdominal area, and chest wall may also be involved[1,2]. While LA is often limited to localized areas of the body, it can present in a generalized fashion[3]. Due to the atypical presentation and the lack of age-specific distribution of lesions for the diagnosis of AD, a skin biopsy was performed on the back skin to aid in the diagnosis. Pathological findings included hyperkeratosis with incomplete keratosis and irregular hyperplasia of the spinous layer. Moreover, a pink, amorphous material can be seen in the dermal papilla. Crystal violet staining was positive for amyloid deposits (Figure 3E and F).
Case 4: A biopsy specimen obtained from a papular lesion of the right shin revealed hyperkeratotic and hyperplastic epidermis and homogeneous pinkish materials in the dermal papilla. Crystal violet staining was positive for amyloid deposits (Figure 4E and F).
Case 1: The patient had the following essential features for the diagnosis of AD based on the American Academy of Dermatology (AAD) diagnostic criteria 2014 including: Pruritus and a history of chronic and relapsing eczema. Besides, supporting features for the diagnosis of AD consisted of a personal history of allergic rhinitis, immunoglobulin E reactivity, and xerosis. The lesions among AD adults are frequently localized to the extremities; however, the lesions of this patient were generalized and involved the whole body. According to the eczema area and severity index, the severity of AD in this patient was estimated to be very severe, with a score between 7.1 and 21 denoting moderate, 21.1 to 50 indicating severe, and 50.1 to 72 designating very severe. Thus, the patient was considered to have concurrent refractory LA and AD.
Case 2: The patient had the following essential features for the diagnosis of AD based on the AAD diagnostic criteria 2014: Pruritus and a history of a chronic and relapsing eczema, with typical morphology and age-specific patterns. In addition, supportive features included a personal history of allergic rhinitis and xerosis. Thus, a diagnosis of LA coexisting with chronic AD was made.
Case 3: Important supportive features included a personal history of allergic rhinitis, early age of onset, immunoglobulin E reactivity, and "goose bump" appearance on thighs and xerosis. A diagnosis of LA associated with chronic AD was made based on the clinical and histopathological findings.
Case 4: The patient had the following essential features: Pruritus and a history of chronic and relapsing eczema, with typical morphology and age-specific patterns. Other important characteristics were a family history of allergic conjunctivitis and xerosis. Based on these findings, the patient was diagnosed with LA associated with chronic AD.
LA coexisting with AD.
Previous treatments with topical corticosteroids and an antihistamine had not produced any apparent improvement; thus, we administered dupilumab injection subcutaneously at a dose of 600 mg for the first time and 300 mg every 2 wk thereafter.
Treatment was then initiated with dupilumab at a dose of 600 mg, which was injected subcutaneously, and continued at a dose of 300 mg every 2 wk thereafter.
The patient started on subcutaneous injection of dupilumab at a dose of 600 mg for the first time and 300 mg every 2 wk thereafter.
We decided to treat the patient’s lesions with subcutaneous injections of dupilumab at a dose of 600 mg initially, which was continued at a dose of 300 mg every 2 wk thereafter.
After 16 wk of treatment, the plaques steadily flattened and decreased in size and number, and the skin became smooth. The itching continued to improve without any obvious adverse reactions. The patient continues to receive dupilumab maintenance therapy by injection of 300 mg every 4 wk.
After 16 wk of treatment, the patient had experienced complete resolution of pruritus. He had visual clinical improvement of his skin lesions with steadily flattening and lightening of the papules and plaques. No obvious adverse reactions were observed.
At week 16, the patient showed a marked reduction of pruritus, and subsequent progressive improvement of both AD and LA.
After 16 wk of treatment, the papules steadily flattened and decreased in number, and the itching disappeared. Moreover, no obvious adverse reactions were observed. She is still under treatment.
LA has been implicated to be associated with other skin conditions including lichen planus and especially AD[4]. It is also associated with HIV, Type A multiple endocrine tumor, vascular lymphoid hyperplasia with eosinophilia, ankylosing spondylitis, autoimmune thyroiditis, hyperthyroidism, and other autoimmune disorders[5-7]. LA treatment is generally directed to relieve pruritus and inflammation, such as low doses of systemic cyclosporine, topical corticosteroids, and oral retinoids such as acitretin. Owing to the drying effects of retinoid on the skin and thereby the potential for exacerbation of AD, the therapeutic options for treating co-existing LA and AD are more limited than those for LA alone[8]. Successful treatment of AD-associated LA with oral cyclosporine and subsequent narrow band ultraviolet B therapy has been reported[9]. Three cases of LA with AD have also been successfully treated with CO2 laser[10]. Koh et al[8] also reported a case of LA with AD successfully treated with alitretinoin, which has lower mucocutaneous side-effects, as compared to other vitamin A derivatives.
Aoki et al[11] reported a case of generalized LA with AD in a female patient, whose intractable pruritus decreased within 4 wk after treatment with dupilumab, her eczematous lesions significantly improved, and the LA papules flattened and decreased after 3 mo of treatment. Humeda et al[12] reported a case of generalized PCA in a male patient without an atopic disease history who had a 4-year history of severe itching. After treatment with dupilumab, both itching and rash were markedly improved. An excellent response to dupilumab was observed in the treatment of extensive LA associated with AD in our patients. No adverse effect or relapse till date was observed. Thus, dupilumab should be considered more often in the treatment of extensive, recalcitrant cases of LA associated with AD. The pathogenesis of PCA is still not clearly established, but it is considered to involve both genetic and environmental factors. Frictional epidermal damage, apoptosis, viral infection, and other triggers have been implicated in the disease etiology in previous reports. Chronic irritation, itching, and scratching causing prolonged epidermal trauma may lead to dermal amyloid deposition by inducing degradation of keratinocytes, thereby leading to amyloid formation by dermal macrophages and fibroblasts through conversion of keratin peptides to amyloid fibrils that react with antikeratin antibodies[13,14]. Thus, scratching seems to be the most important step in its pathogenesis. Hence, treatment modalities should be centered on minimizing skin trauma and relieving pruritus. A previous study has suggested that small fiber neuropathy and interleukin (IL)-31 receptor overexpression may play a role in the intense pruritus associated with PCA[11]. IL-31, a key mediator of pruritus, signals through a receptor composed of two subunits, IL-31 receptor A and oncostatin M receptor β (OSMR β). A familial study of some cases of PCA has revealed mutations in both genes encoding the subunits of IL-31 receptors (IL31RA and OSMR β)[15]. It has been demonstrated that IL-4, IL-13, and IL-31 are associated with sensory nerve sensitization and itching in AD patients, leading to scratching that further exacerbates inflammation and barrier dysfunction[16]. IL-31 is significantly overexpressed in the skin samples of patients with AD. Thus, it is possible that the IL-31 signaling pathway is involved in the pathogenesis of both PCA and AD, and patients with abnormalities in this pathway may show phenotypes of both diseases[7].
Dupilumab, a human anti-IL-4 receptor-α monoclonal antibody that blocks signaling of both IL-4 and IL-13[17], has been approved by the Food and Drug Administration for use in the treatment of adult patients with moderate-to-severe AD whose disease is not adequately controlled with topical medications or when those therapies are not advisable. Previous analyses have shown that treatment with dupilumab normalizes the AD transcriptome by downregulating markers of type 2 inflammation such as IL-13 and IL-3. It effectively treats itching by directly blocking IL-4 and IL-13 on sensory neurons and indirectly through inhibition of IL-31 production by T2-helper cells by blocking IL-4[18]. We hypothesized that dupilumab may also be effective in treating LA with AD. In our four cases of LA with AD, we observed a convincing antipruritic effect and improvement of skin lesions after injection of dupilumab. The advantages of this treatment are practicability, convenience, and low side-effects. In general, AD symptomatology and distribution depend on the patient’s age at the time of presentation. Adults may present with dry and scaly patches on the extremities[19]. Our cases demonstrated that dupilumab may be a good therapy for LA coexisting with AD regardless of the generalized eczema type or typical age-specific patterns of clinical phenotypes of adult AD.
Dupilumab may shed light on the systematic treatment of refractory LA patients associated with AD but further case studies are required to evaluate the effectiveness of this drug for these cases. Meanwhile, the mechanism by which dupilumab improves LA remains elusive but it seems that the interruption of the vicious cycle of pruritus and skin trauma as well as its direct and indirect effects on the cytokines involved in the pathophysiology of this disorder are all implicated.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Dermatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Sabooniha F, Iran; Sideris N, Greece S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Yan JP
| 1. | Dousset L, Seneschal J, Boniface K, Charreau S, Ezzedine K, Milpied B, Mossalayi MD, McGrath JA, Lecron JC, Taïeb A. A Th2 cytokine interleukin-31 signature in a case of sporadic lichen amyloidosis. Acta Derm Venereol. 2015;95:223-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Panchaprateep R, Tusgate S, Munavalli GS, Noppakun N. Fractional 1,550 nm Ytterbium/Erbium fiber laser in the treatment of lichen amyloidosis: clinical and histological study. Lasers Surg Med. 2015;47:222-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Tursen U, Kaya TI, Dusmez D, Ikizoglu G. Case of generalized lichen amyloidosis. Int J Dermatol. 2003;42:649-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Hongcharu W, Baldassano M, Gonzalez E. Generalized lichen amyloidosis associated with chronic lichen planus. J Am Acad Dermatol. 2000;43:346-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Gönül M, Cakmak SK, Kayaçatin S. Generalized lichen amyloidosis and hyperthyroidism: coincidence or association. Postepy Dermatol Alergol. 2013;30:265-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Akar A, Taştan HB, Demiriz M, Erbil H. Lack of effect of cyclosporine in lichen amyloidosis associated with atopic dermatitis. Eur J Dermatol. 2002;12:612-614. [PubMed] |
| 7. | Lee DD, Huang CK, Ko PC, Chang YT, Sun WZ, Oyang YJ. Association of primary cutaneous amyloidosis with atopic dermatitis: a nationwide population-based study in Taiwan. Br J Dermatol. 2011;164:148-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Koh WS, Oh EH, Kim JE, Ro YS. Alitretinoin treatment of lichen amyloidosis. Dermatol Ther. 2017;30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 9. | Kang MJ, Kim HS, Kim HO, Park YM. A case of atopic dermatitis-associated lichen amyloidosis successfully treated with oral cyclosporine and narrow band UVB therapy in succession. J Dermatolog Treat. 2009;20:368-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Chu H, Shin JU, Lee J, Park CO, Lee KH. Successful treatment of lichen amyloidosis accompanied by atopic dermatitis by fractional CO(2) laser. J Cosmet Laser Ther. 2017;19:345-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Aoki K, Ohyama M, Mizukawa Y. A case of lichen amyloidosis associated with atopic dermatitis successfully treated with dupilumab: A case report and literature review. Dermatol Ther. 2021;34:e15005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 12. | Humeda Y, Beasley J, Calder K. Clinical resolution of generalized lichen amyloidosis with dupilumab: a new alternative therapy. Dermatol Online J. 2020;26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Kobayashi H, Hashimoto K. Amyloidogenesis in organ-limited cutaneous amyloidosis: an antigenic identity between epidermal keratin and skin amyloid. J Invest Dermatol. 1983;80:66-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 88] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | He A, Zampella JG, Kwatra SG. Interleukin-31 receptor and pruritus associated with primary localized cutaneous amyloidosis. Br J Dermatol. 2016;175:433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Tanaka A, Arita K, Lai-Cheong JE, Palisson F, Hide M, McGrath JA. New insight into mechanisms of pruritus from molecular studies on familial primary localized cutaneous amyloidosis. Br J Dermatol. 2009;161:1217-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 16. | Haddad EB, Cyr SL, Arima K, McDonald RA, Levit NA, Nestle FO. Current and Emerging Strategies to Inhibit Type 2 Inflammation in Atopic Dermatitis. Dermatol Ther (Heidelb). 2022;12:1501-1533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 17. | Shirley M. Dupilumab: First Global Approval. Drugs. 2017;77:1115-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 18. | Miake S, Tsuji G, Takemura M, Hashimoto-Hachiya A, Vu YH, Furue M, Nakahara T. IL-4 Augments IL-31/IL-31 Receptor Alpha Interaction Leading to Enhanced Ccl 17 and Ccl 22 Production in Dendritic Cells: Implications for Atopic Dermatitis. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 19. | Frazier W, Bhardwaj N. Atopic Dermatitis: Diagnosis and Treatment. Am Fam Physician. 2020;101:590-598. [PubMed] |