Published online Apr 16, 2023. doi: 10.12998/wjcc.v11.i11.2496
Peer-review started: November 28, 2022
First decision: January 19, 2023
Revised: February 1, 2023
Accepted: March 15, 2023
Article in press: March 15, 2023
Published online: April 16, 2023
Processing time: 128 Days and 15.9 Hours
Intraductal papillary mucinous neoplasm (IPMN) is a rare pancreatic tumor and has the potential to become malignant. Surgery is the most effective treatment at present, but there is no consensus on the site of resection. Heterotopic pancreas occurs in the gastrointestinal tract, especially the stomach and duodenum but is asymptomatic and rare. We report a case of ectopic pancreas with IPMN located in the jejunum.
A 56-year-old male patient suffered from severe pain, nausea and vomiting due to a traffic accident and sought emergency treatment at our hospital. Contrast-enhanced computed tomography of the whole abdomen suggested splenic congestion, which was considered to be splenic rupture. Emergency laparotomy was performed, and the ruptured spleen was removed during the operation. Unexpectedly, a cauliflower-like mass of about 2.5 cm × 2.5 cm in size was incidentally found about 80 cm from the ligament of Treitz during the operation. A partial small bowel resection was performed, and postoperative pathology confirmed the small bowel mass as heterotopic pancreas with low-grade IPMN.
Ectopic pancreas occurs in the jejunum and is pathologically confirmed as IPMN after surgical resection.
Core Tip: We report a patient who was admitted to the hospital because of splenic rupture caused by vehicle trauma. Exploratory laparotomy, splenectomy, small intestinal tumor resection, abdominal cavity irrigation and drainage were performed. Postoperative pathology confirmed a diagnosis of ectopic pancreas with intraductal papillary mucinous neoplasm.
- Citation: Huang JH, Guo W, Liu Z. Intraductal papillary mucinous neoplasm originating from a jejunal heterotopic pancreas: A case report. World J Clin Cases 2023; 11(11): 2496-2501
- URL: https://www.wjgnet.com/2307-8960/full/v11/i11/2496.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i11.2496
The heterotopic pancreas is anatomically separated from the main gland, and there is no continuity of blood vessels and ducts between the two[1]. Heterotopic pancreas usually occurs in the upper gastrointestinal tract, and the stomach, duodenum and proximal jejunum are the most common sites[2]. Although the disease is usually insidious and asymptomatic, some patients may experience abdominal pain, nausea, and vomiting. Heterotopic pancreas is often identified during other abdominal procedures or by accident during imaging examinations or autopsy and is more common in middle-aged males[2,3]. Ectopic pancreatic tissue also has the possibility of malignant transformation[4,5].
Intraductal papillary mucinous neoplasm (IPMN) is a pancreatic cystic tumor. It originates from pancreatic ductal epithelial cells, grows in a papillary pattern and can secrete mucin to form mucus[6,7]. According to epithelial dysplasia and malignant potential, IPMN can be divided into four types: adenoma; borderline carcinoma; carcinoma in situ; and invasive carcinoma[8,9]. The average age of onset of IPMN is 64 years, and the prevalence of IPMN is higher in males than in females[10]. IPMN is usually asymptomatic, but some patients may present with abdominal pain, weight instability, new-onset diabetes, pancreatitis and jaundice caused by pancreatic duct obstruction[7]. IPMN has the characteristics of malignant transformation and can eventually transform into invasive carcinoma[11]. Here, we report a rare case of pancreatic heterotopic location in the jejunum and ectopic pancreas pathologically confirmed as low-grade IPMN.
A 56-year-old male patient presented with trauma from a car accident 5 h prior to admission to the hospital.
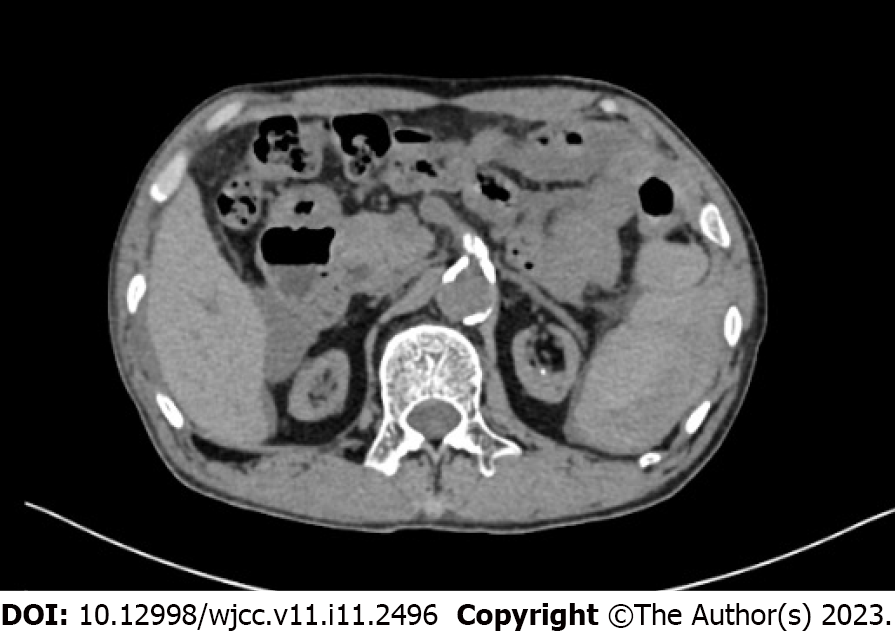
The patient presented to the emergency department of our hospital with severe abdominal pain accompanied by nausea and vomiting, severe pain in both lower limbs and inability to stand after being hit by a motor vehicle 5 h earlier. Abdominal computed tomography (CT) showed that the shape of the spleen was irregular, and the internal density was uneven. The spleen and stomach had multiple, high-density liquid shadows. It was considered that the spleen might have ruptured because there was perisplenic hemorrhage (Figure 1).
Ten years ago, the patient developed chronic nephritis and urine volume gradually decreased. Uremia developed 1 year later. The patient remained anuria and was treated with hemofiltration three times a week. He regularly took creatinine-lowering medications.
The patient’s personal and family history was not remarkable.
The patient had abdominal tenderness, rebound tenderness and muscle tension.
Laboratory examinations showed abnormalities in white blood cell count 13.64 × 109/L (reference range: 3.5 × 109-9.5 × 109/L), potassium 6.38 mmol/L (reference range 3.5-5.1 mmol/L), creatinine 1027 µmol/L (reference range 58-110 µmol/L), urea 30 mmol/L (reference range 3.2-7.1 mmol/L) and hemoglobin 85 g/L (reference range 130-175 g/L).
Abdominal CT showed that the shape of the spleen was irregular, its internal density was uneven, and multiple, high-density liquid shadows were seen in the spleen and stomach, which suggested spleen rupture and perisplenic hemorrhage (Figure 1).
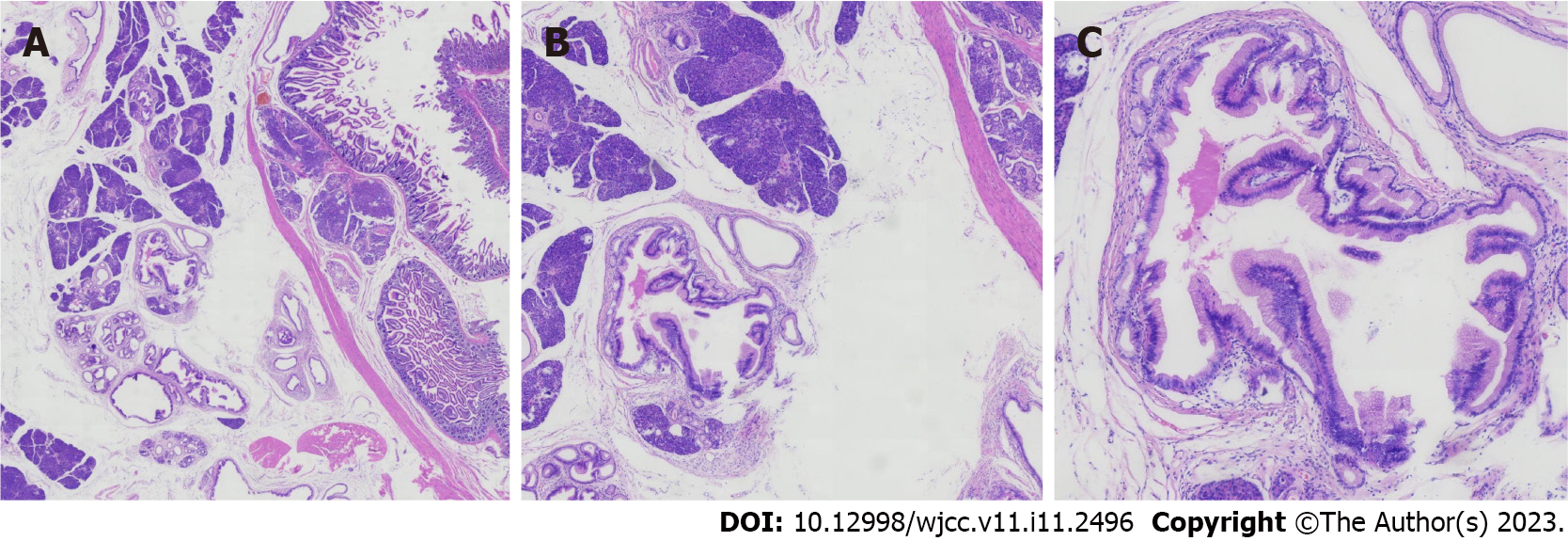
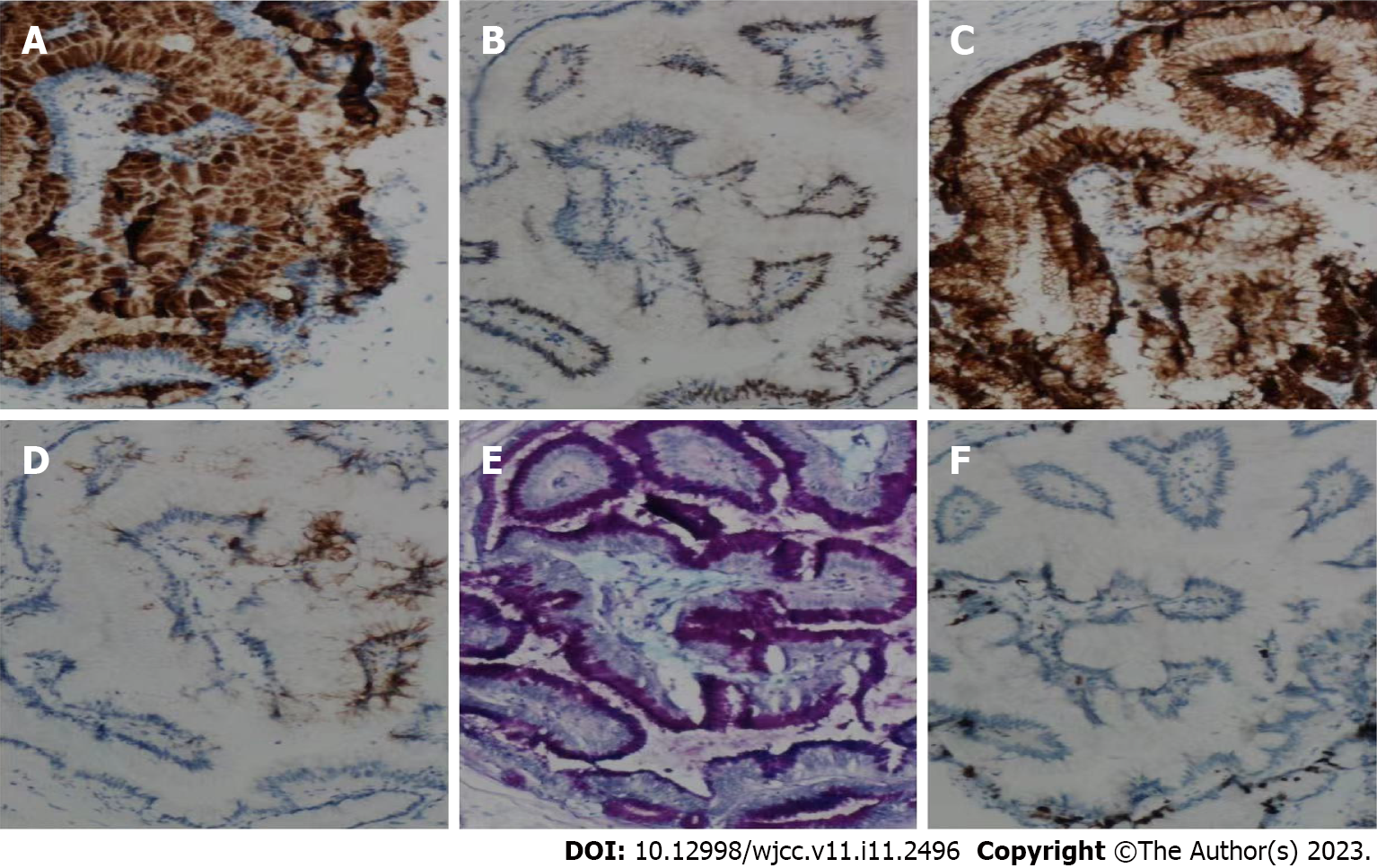
Under light microscopy, ectopic pancreatic tissue was seen in the muscular layer or submucosa of the intestinal wall, along with normal pancreatic ducts and acinar structures. Some of the ducts were dilated and lined by a single layer of gastric glandular epithelium, and some of the epithelia showed papillary growth with oval nuclei at the base. The cytoplasm was rich with mucus (Figure 2). The resected small intestinal mass suggested ectopic pancreatic tissue with low-grade IPMN. The immunohistochemistry of the tumor cells were diffusely positive for MUC5A, CDX2, CK7, CK20, AB-PAS and Ki67 (Figure 3). The patient was eventually diagnosed with low-grade IPMN, ectopic pancreas, splenic rupture and renal insufficiency.
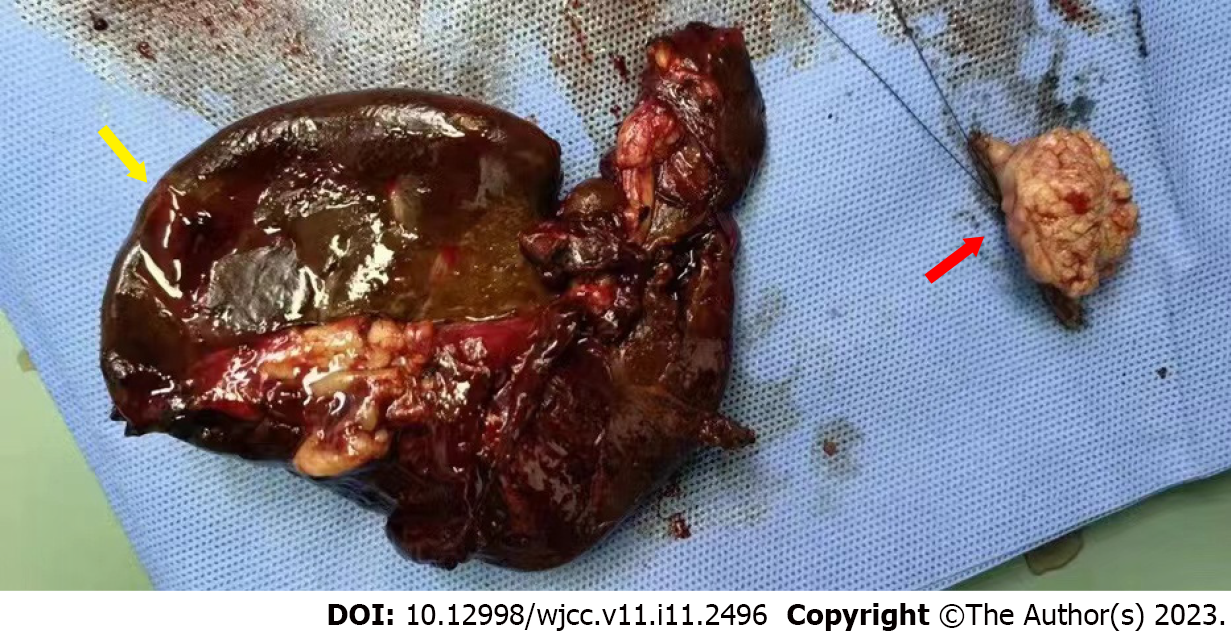
The patient underwent emergency laparotomy and splenectomy under general anesthesia. There was a 2.5 cm × 2.5 cm cauliform mass 80 cm from the small intestine to the ligament of Treitz, and partial small bowel resection was performed (Figure 4). During the operation, 100 mL liquid crystal, 2 U white and red blood cell suspension and 200 mL plasma were injected. There was 1500 mL blood loss and anuria. The patient was in critical condition because of severe trauma, blood loss and renal insufficiency. He was transferred to the intensive care unit and was given assisted ventilation, active blood transfusion, fluid replacement and anti-shock and anti-infection treatment. As the patient had a history of chronic renal insufficiency and was in the uremic stage, hemofiltration treatment was given in the intensive care unit.
The patient abandoned treatment.
Heterotopic pancreas is usually asymptomatic and < 2 cm, making the diagnosis difficult. The lack of effective and specific detection methods means that many patients may be misdiagnosed before surgery[12,13]. The most common CT finding is a submucosal oval mass with different lobes at the edge[2]. Submucosal tumors are usually seen on endoscopy. However, because they are covered by normal mucosa, a valid diagnosis depends on tissue validation of the submucosa[14]. The most widely accepted mechanism of ectopic pancreas is the dislocation theory, in which pancreatic tissue deposits fall into the developing gastrointestinal system and are separated from the main body of the pancreas[15,16].
According to the location of the lesion in the pancreatic duct, IPMN can be classified into three types: main duct; branch duct; and mixed IPMN. The key to differentiating IPMN from other cystic neoplasms of the pancreas, especially from serous and mucinous cystic neoplasms, depends on the location of the tumor in the pancreatic duct[17]. All pancreatic cysts > 10 mm should be examined by CT or enhanced magnetic resonance imaging (MRI) with magnetic resonance cholangiopancreatography (MRCP). MRI/MRCP is the preferred method of examination. In addition, endoscopic ultrasound can be used to perform cyst puncture to detect the content of carcinoembryonic antigen, amylase and other indicators in fluid samples to assist diagnosis or differential diagnosis.
Surgery is the most commonly used treatment, but the consensus guidelines published in 2006 recommended that asymptomatic branch duct IPMN < 30 mm with no solid nodules can be monitored and observed first. Therefore, the choice of treatment depends on the type of IPMN and the nature and size of the tumor[18].
Our patient is a special case of heterotopic pancreas with IPMN. Emergency splenectomy was performed because of the rupture of the spleen. A small intestinal mass was accidentally found during the operation, and small intestinal tumor resection was performed after explaining the condition to the family members during the operation and consent was obtained. Although the nature of the mass was confirmed as heterotopic pancreas with low-grade IPMN by postoperative pathology, we did not take the specimen of the pancreas for pathological examination during the operation as it is difficult diagnosing heterotopic pancreas, which was considered normal when the small intestinal mass was found. Therefore, more comprehensive considerations about the diagnosis and treatment of the disease are required.
Heterotopic pancreas with low-grade IPMN is a rare disease. This case may provide clinicians with a broader vision of heterotopic pancreas or IPMN to provide some new ideas for the diagnosis and differential diagnosis of pancreatic-related diseases.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Elkady N, Egypt; Ghazanfar A, United Kingdom S-Editor: Zhang H L-Editor: Filipodia P-Editor: Zhang H
| 1. | Christodoulidis G, Zacharoulis D, Barbanis S, Katsogridakis E, Hatzitheofilou K. Heterotopic pancreas in the stomach: a case report and literature review. World J Gastroenterol. 2007;13:6098-6100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in CrossRef: 107] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Rezvani M, Menias C, Sandrasegaran K, Olpin JD, Elsayes KM, Shaaban AM. Heterotopic Pancreas: Histopathologic Features, Imaging Findings, and Complications. Radiographics. 2017;37:484-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 3. | Sandrasegaran K, Maglinte DD, Cummings OW. Heterotopic pancreas: presentation as jejunal tumor. AJR Am J Roentgenol. 2006;187:W607-W609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Ginori A, Vassallo L, Butorano MA, Bettarini F, Di Mare G, Marrelli D. Pancreatic adenocarcinoma in duodenal ectopic pancreas: a case report and review of the literature. Pathologica. 2013;105:56-58. [PubMed] |
| 5. | Fukumori D, Matsuhisa T, Taguchi K, Minato M. Ectopic gastric pancreatic cancer: report of a case. Hepatogastroenterology. 2011;58:740-744. [PubMed] |
| 6. | Bernard P, Scoazec JY, Joubert M, Kahn X, Le Borgne J, Berger F, Partensky C. Intraductal papillary-mucinous tumors of the pancreas: predictive criteria of malignancy according to pathological examination of 53 cases. Arch Surg. 2002;137:1274-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM, Shimizu M, Wolfgang CL, Yamaguchi K, Yamao K; International Association of Pancreatology. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 1614] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 8. | Lahat G, Lubezky N, Haim MB, Nachmany I, Blachar A, Santo I, Nakache R, Klausner JM. Cystic tumors of the pancreas: high malignant potential. Isr Med Assoc J. 2011;13:284-289. [PubMed] |
| 9. | Le Borgne J, de Calan L, Partensky C. Cystadenomas and cystadenocarcinomas of the pancreas: a multiinstitutional retrospective study of 398 cases. French Surgical Association. Ann Surg. 1999;230:152-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 317] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 10. | Rossi RE, Massironi S. Intraductal papillary mucinous neoplasms of the pancreas: a clinical challenge. Expert Rev Gastroenterol Hepatol. 2018;12:1123-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Nagai E, Ueki T, Chijiiwa K, Tanaka M, Tsuneyoshi M. Intraductal papillary mucinous neoplasms of the pancreas associated with so-called "mucinous ductal ectasia". Histochemical and immunohistochemical analysis of 29 cases. Am J Surg Pathol. 1995;19:576-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 121] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Lin J, Yu Y, Chen Y, Zheng M, Zhou D. Heterotopic pancreatic cyst in the adrenal gland: A case report and review of literature. Medicine (Baltimore). 2018;97:e9414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Sathyanarayana SA, Deutsch GB, Bajaj J, Friedman B, Bansal R, Molmenti E, Nicastro JM, Coppa GF. Ectopic pancreas: a diagnostic dilemma. Int J Angiol. 2012;21:177-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Agale SV, Agale VG, Zode RR, Grover S, Joshi S. Heterotopic pancreas involving stomach and duodenum. J Assoc Physicians India. 2009;57:653-654. [PubMed] |
| 15. | Kim DW, Kim JH, Park SH, Lee JS, Hong SM, Kim M, Ha HK. Heterotopic pancreas of the jejunum: associations between CT and pathology features. Abdom Imaging. 2015;40:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Shin SS, Jeong YY, Kang HK. Giant heterotopic pancreas in the jejunal mesentery. AJR Am J Roentgenol. 2007;189:W262-W263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Del Chiaro M, Verbeke C. Intraductal papillary mucinous neoplasms of the pancreas: reporting clinically relevant features. Histopathology. 2017;70:850-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Crippa S, Arcidiacono PG, De Cobelli F, Falconi M. Review of the diagnosis and management of intraductal papillary mucinous neoplasms. United European Gastroenterol J. 2020;8:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |