Published online Apr 16, 2023. doi: 10.12998/wjcc.v11.i11.2482
Peer-review started: December 17, 2022
First decision: January 3, 2023
Revised: January 31, 2023
Accepted: March 21, 2023
Article in press: March 21, 2023
Published online: April 16, 2023
Processing time: 110 Days and 5.6 Hours
Reports on perioperative anesthesia management in pediatric patients with difficult airways are scarce. In addition to relatively more difficulties in the technique of endotracheal intubation, the time for manipulation is restricted compared to adults. Securing the airways safely and avoiding the occurrence of hypoxemia in these patients are of significance.
A 9-year-old boy with spastic cerebral palsy, severe malnutrition, thoracic scoliosis, thoracic and airway malformation, laryngomalacia, pneumonia, and epilepsy faced the risk of anesthesia during palliative surgery. After a thorough preoperative evaluation, a detailed scheme for anesthesia and a series of intu
Dealing with difficult airways in the pediatric population, proper sedation allows time to intubate without interrupting spontaneous breathing. The appropriate endotracheal intubation method based on the patient’s unique characteristics is the key factor in successful management of these rare cases.
Core Tip: A loss of control of the pediatric airway can result in catastrophic consequences if not addressed promptly. Spastic cerebral palsy is often associated with complicated airways in pediatric patients, which can be classified as difficult intubations of anticipated difficult airways. Sedation with spontaneous breathing and fiberoptic bronchoscope-guided endotracheal intubation is a valuable method for such patients. Herein, we describe the entire process of airway management and analyze the failure of the first intubation attempt.
- Citation: Chen JX, Shi XL, Liang CS, Ma XG, Xu L. Anesthesia management in a pediatric patient with complicatedly difficult airway: A case report. World J Clin Cases 2023; 11(11): 2482-2488
- URL: https://www.wjgnet.com/2307-8960/full/v11/i11/2482.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i11.2482
The pediatric difficult airway is a massive challenge for anesthesiologists and is one of the main causes of perioperative respiratory complications[1]. Cerebral palsy (CP) comprises a group of permanent disorders affecting the development of movement causing limited activity. Spasticity account for nearly 80% of pediatric CP cases, whereby comorbidities and functional limitations are prevalent and disabling[2]. Life-threatening complications are primarily associated with recurrent chest infections, upper airway obstruction (UAO), and scoliosis leading to progressive lung disease, along with complications of epilepsy[3]. These characteristic features are major risk assessments for anesthesiologists for predicting a difficult airway.
Cases of airway management in spastic CP are infrequently reported worldwide. Herein, we describe anesthesia management in a pediatric case of a difficult airway caused by spastic CP, outlining the detailed process of airway management. We discuss the results of our literature review for evidence related to fiberoptic bronchoscope (FOB)-guided endotracheal intubation, the choice of sedation method, and other management strategies for the pediatric difficult airway, to encourage anesthesiologists to pay more attention to these patients.
A 9-year-old boy (14 kg) was admitted with feeding difficulties after birth caused by spastic CP.
After birth, the patient had persistent feeding difficulties, accompanied by repeated coughing and vomiting after eating. He was diagnosed with spastic CP along with severe malnutrition, thoracic scoliosis, laryngomalacia, pneumonia, and multiple site deformities, including those of the airway, thorax, hip joint, and both hands and feet. In addition to epilepsy and taking clonazepam 1 mg, phenobarbital 25 mg, levetiracetam 150 mg, and sodium valproate oral liquid 5 mL twice daily, he had a history of aspiration pneumonia and copious purulent sputum, for which he was prescribed antibiotics for 9 d. He was scheduled to undergo implantation of an implantable venous access port and gastrostomy to improve feeding and nutrition. This was not a typical elective operation and was difficult to adjust to a conventionally safe state, because the pneumonia was protracted and nursing conditions were limited.
The patient was diagnosed with spastic CP along with severe malnutrition, thoracic scoliosis, laryngomalacia, pneumonia, and multiple site deformities, including those of the airway, thorax, hip joint, and both hands and feet.
The patient had been abandoned as a toddler, and his birth and family histories were uncertain.
The patient’s general physical examination revealed typical facial dysmorphism, thoracic deformities, scoliosis, oxycephaly, and hip dislocation. He showed a Mallampati class IV airway with severely limited neck movement, thyromental distance of fewer than three fingers, and 20-mm-inter-incisor distance. Auscultation indicated an obvious UAO with distinct sputum sounds, and oxygen saturation (SpO2) was 85%-90% on 3 L/min of supplemental oxygen using a nasal oxygen cannula. Preoperative evaluation exhibited a class III physical status of American Society of Anesthesiologists with a difficult airway.
Routine blood tests showed a hemoglobin (Hb) level of 9.7 g/dL, hematocrit of 33.3%, mean corpuscular volume of 73.9 fL, mean corpuscular Hb of 21.6 pg, and mean corpuscular Hb concentration of 29.2 g/dL. Other blood test results showed no significant abnormalities.
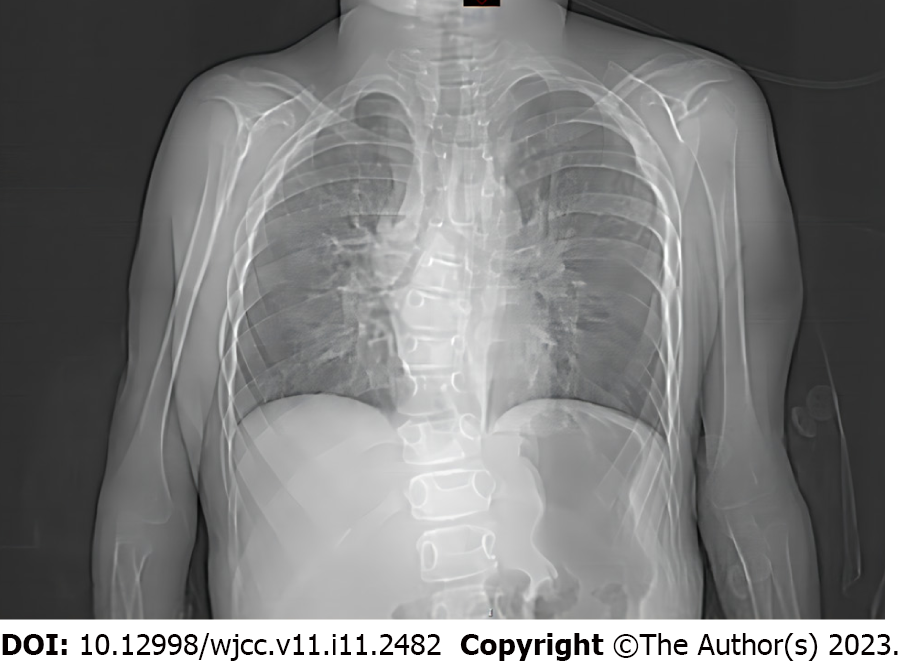
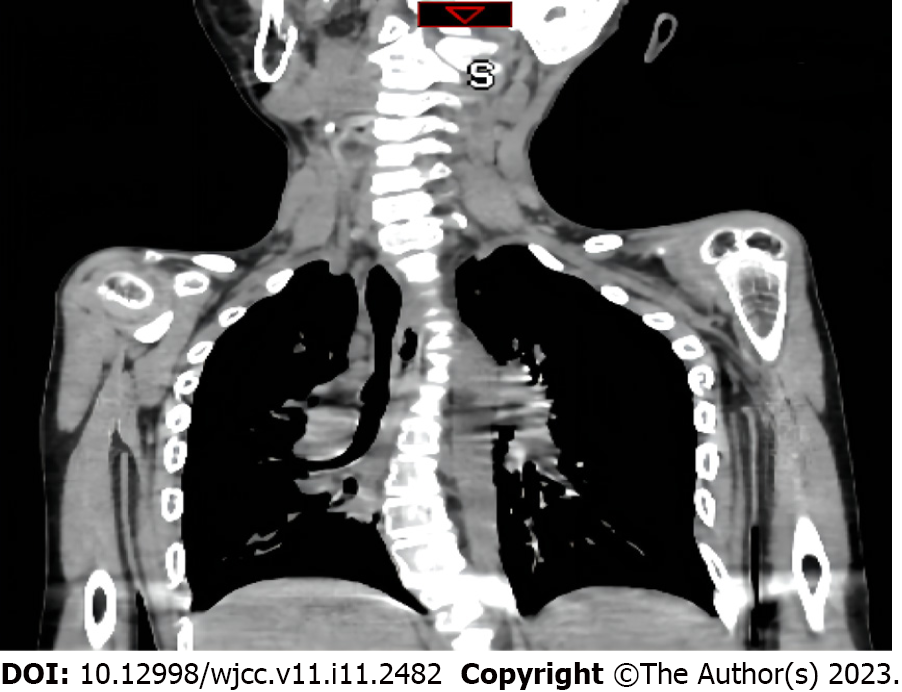
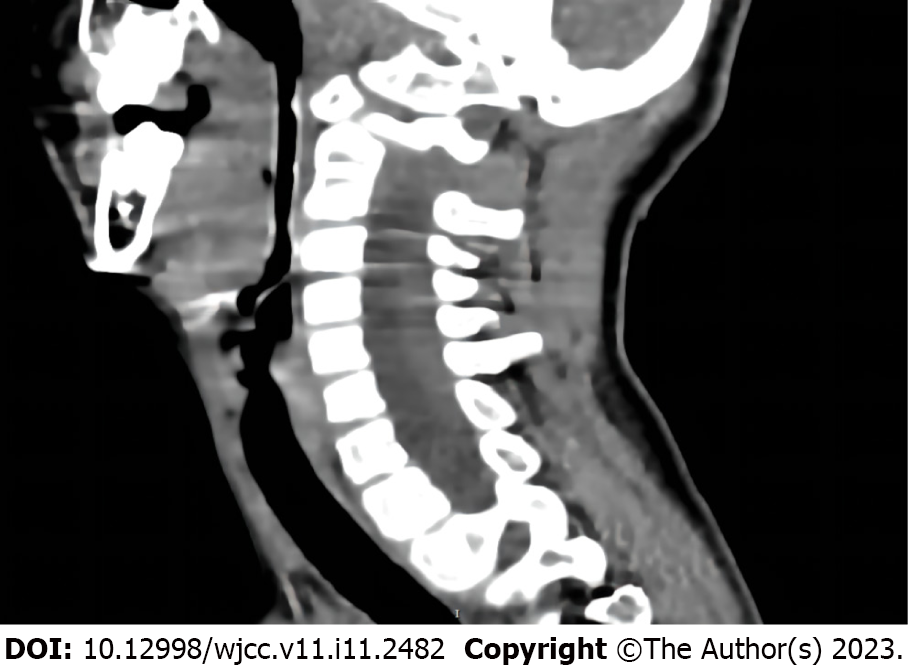
Chest radiography demonstrated pneumonia, scoliosis, and right deviation of the trachea (Figure 1). Computed tomography (CT) scans revealed scoliosis, osteoporosis of the spine, significant atrophy of the muscles of the back in the bilateral thoracolumbar region with fat infiltration, and thoracic and tracheal malformation (Figure 2). Lateral cervical spine CT scans displayed laryngomalacia and malformations of the pharynx and cervical spine (Figure 3).
The final diagnosis of the present case was spastic CP, along with feeding difficulties, severe malnutrition, laryngomalacia, epilepsy, severe pneumonia, anemia, congenital scoliosis, congenital thoracic deformity, congenital bilateral hip dislocation, and ectrodactyly.
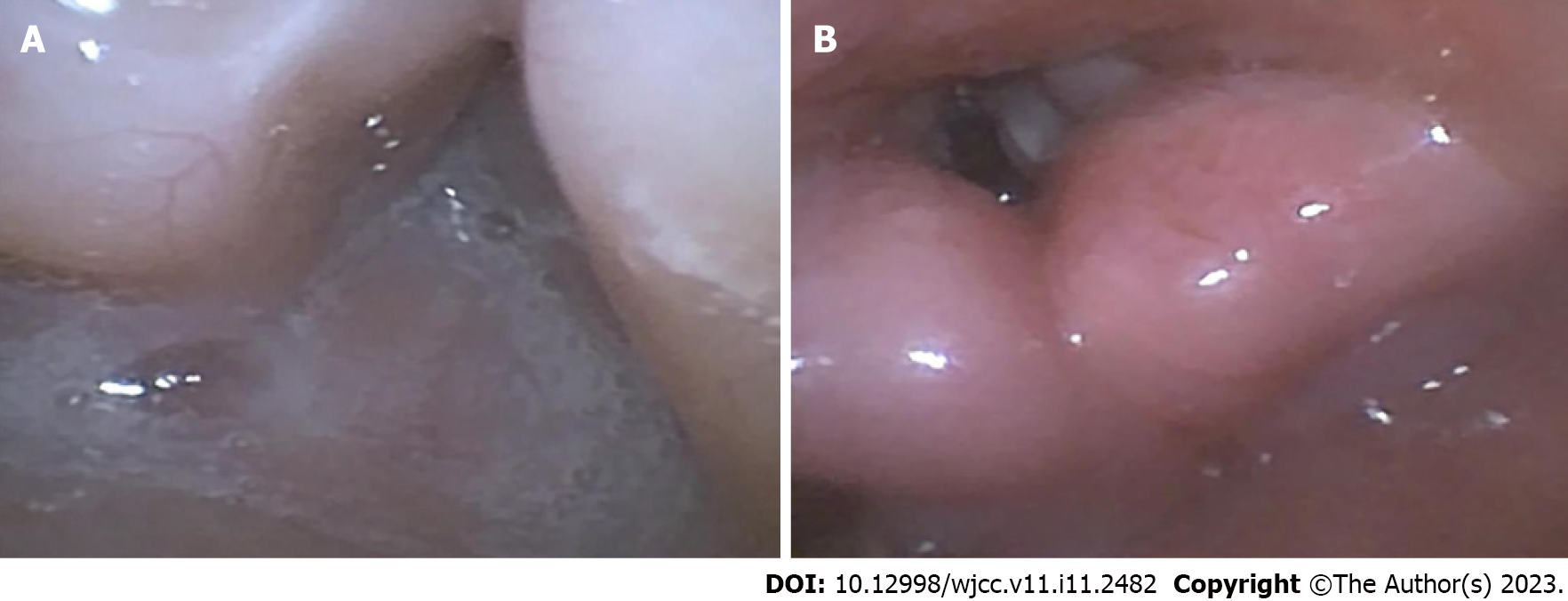
A series of intubation tools including supraglottic airway devices, video laryngoscope, an FOB, and invasive airway equipment (percutaneous tracheostomy) were utilized and a skilled anesthesiologist was present. In the operating room, the patient was placed in a supine position, and standard monitoring devices for measuring SpO2, noninvasive blood pressure, and electrocardiogram were installed. Suction device and large suction tubes were ready for sudden regurgitation and aspiration. The patient's position was adjusted to 30°-40° head height and oxygen flow rate was adjusted to 8 L/min. The mask was gently placed on his mouth and nose without additional pressure, and oxygen was continuously inhaled for 5 min. After pre-oxygenation, gradual anesthesia was induced with penehyclidine hydrochloride 0.15 mg, esketamine 8 mg, and midazolam 1.4 mg. The patient was adequately sedated and showed spontaneous breathing. Due to the patient's limited neck motion, narrow inter-incisor distance, and malformed pharynx, we initially attempted a nasotracheal intubation guided by an FOB with a tip diameter of 2.8 mm. The procedure was performed by an anesthesiologist proficient in FOB intubation. Following intranasal administration of xylometazoline, the FOB passed from the nostril to the larynx. Even after making a few adjustments, no view of the epiglottis or the vocal cords was available. This was due to incorrect angles and copious secretions (Figure 4A). As a result, the FOB was removed and pre-oxygenation and aspiration of secretions were immediately commenced. Subsequently, we again tried to pass the FOB from the oral cavity to the pharynx. The assistant gently used a jaw-thrust maneuver to expand the patient's pharyngeal cavity. The glottis could be seen clearly when the forepart advanced from the oropharynx to the pharynx (Figure 4B). Propofol and rocuronium were administrated after confirming that the forepart reached the mid-trachea and the endotracheal anterograde intubation was advanced with FOB view. A cuffed endotracheal tube of size 5.0 was used for the endotracheal intubation. After confirming that the endotracheal tube was in the trachea with a capnograph and auscultation, 20 mg of methylprednisolone was administered to the patient to alleviate airway stress reactions. Anesthesia was maintained with 2% sevoflurane and remifentanil 0.2 μg/kg/min. The operation lasted approximately 4 h and was successful without any incidents. The intraoperative fluid intake was 450 mL and the urine output was 80 mL. After the operation, the patient was sent to the intensive care unit with a tracheal tube inserted for mechanical ventilator support. He was treated with aminomethylbenzoic acid, phenylethylamine for hemostasis, ceftriaxone for infection, and omeprazole for acid inhibition. The patient was successfully extubated after receiving assistance from a mechanical ventilator for 2 d.
The patient was transferred to the general ward on postoperative day 4 and discharged from the hospital 1 wk after surgery without any complications.
Spastic CP is the most common cause of upper motor neuron syndrome among children, accounting for nearly 80% of cases[4]. In patients with spastic CP, motor impairments are often accompanied by seizures and secondary musculoskeletal issues, and abnormalities in sensation, perception, cognition, communication, and behavior[5,6]. The progressive nature of the disorder results in a combination of obstructive and restrictive patterns of pulmonary diseases[7]. The main causes of UAO in patients are abnormal laryngeal structure and muscle hypertonia, tongue prolapsing posteriorly over the larynx, and excessive respiratory secretions, resulting in decreased tidal volume and carbon dioxide accumulation[8]. Moreover, musculoskeletal deformities of the neck result in a severe decrease in neck motion and limited mouth opening, making endotracheal intubation difficult[5]. The resulting patho
When facing such a case of a pediatric difficult airway, a comprehensive preoperative evaluation is the first and most crucial step of anesthesia management. Unfortunately, no specific scales or suggested measurements for pediatric airway evaluation exist[1]. Despite these limitations, we can evaluate the airway based on previous medical records, facial and jaw features, and anatomical and ultrasonographic measurements[9,10].
An experienced and competent specialist needs to lead the team to formulate an anesthesia plan and discuss alternative strategies before any intervention. Flexible FOB-guided endotracheal intubation is a highly reliable solution for pediatric difficult airways, particularly for difficult or impossible ventilation[11,12]. Studies with observational findings for FOB-guided intubation indicated success rates ranging from 78%-100%[13]. Moreover, FOB-assisted anterograde intubation is almost noninvasive[14]. Unlike adults who can receive FOB-guided endotracheal intubation in the awake state, children require sedation or general anesthesia. Pediatric patients have lower oxygen reserve and higher oxygen consumption during apnea compared to adults[15], so it is better to use appropriate sedation with spontaneous breathing during elective pediatric FOB-aided difficult endotracheal intubation. In this case, we chose esketamine 0.5 mg/kg and midazolam 0.1 mg/kg for sedation, which allowed suitable sedation without interrupting spontaneous breathing. Midazolam provides proper sedation and prevented seizures during the procedure[16]. Esketamine induces a state of dissociative sedation, resulting in strong analgesia, sedation, immobilization, and amnesia whilst maintaining spontaneous respiration and cardiopulmonary stability[17]. Esketamine relatively preserves the protective airway reflexes of patients[18], and is a comparatively safe and effective sedative. However, there is no relevant report on the use of esketamine in the management of pediatric difficult airways. Esketamine produced a good sedative effect that facilitated subsequent procedures in this case. Dexmedetomidine, propofol, fentanyl, and inhalational anesthetic agents are classic sedatives for the management of a difficult airway. Their combinations and dosages need to be adjusted individually to prevent interruption of breathing. If the induction of anesthesia is inappropriate, the airway may turn into a difficult mask ventilation airway or even an emergency airway. Therefore, anesthesiologists should choose the appropriate sedation method according to their experience and the specific patient’s situation. Before the procedure began, we also pretreated the nasal cavity with xylometazoline and penehyclidine hydrochloride. Xylometazoline is a topical vasoconstrictor that can reduce nasal bleeding during procedures which may affect the visual field[19]; penehyclidine hydrochloride can inhibit salivary gland and airway gland secretions.
The anesthesiologist team analyzed the reasons for the failure of the first attempt at intubation. Fiberoptic nasotracheal intubation was the first choice owing to the cervical deformity and restricted mouth opening of the patient. However, in the process, despite the use of anticholinergics and full secretion suction before the procedure, the patient had severe respiratory inflammation and constant copious secretions, which severely affected the visual field. Furthermore, the longer and more curved path from the nasal cavity to the glottis, combined with structural abnormalities in the pharynx, made the adjustment of the anterior end of the FOB more difficult. On the second attempt, we opted for transoral FOB-guided intubation and a jaw-thrust maneuver was performed by the assistant. When the mandible was displaced forward, it pulled the tongue outward, thereby enlarging the pharyngeal cavity and offering more space for the adjustment of the FOB's forepart. There were no adverse events like decreased oxygen saturation, blood pressure, and heart rate during the whole process.
Managing different difficult airways depends on the patient's condition and the individual abilities and habits of the anesthesiologist. A visual laryngoscope can help complete most common intubations, and FOB is a standard protocol for patients who have difficulty with laryngoscope exposure. Awake intubation can be used in adults with a difficult airway, but it is difficult for children to cooperate awake intubation. In the pediatric difficult airway, we need the child to keep sedation with spontaneous breathing to create a suitable operating environment. Midazolam combined with esketamine may be an effective induction regimen.
There are only a few articles on the anesthetic management of children with spastic CP. However, patients with spastic CP present several challenges during anesthetic management that may even be fatal on some occasions. When confronted with a pediatric difficult airway, it is essential to have both an experienced specialist and a coordinated team to conduct a complete preoperative evaluation and implement various appropriate management strategies. Under sedation with spontaneous breathing, FOB-guided endotracheal intubation is a standard treatment for a pediatric difficult airway. Anesthesiologists need to individually select the sedation protocol and FOB approach according to the specific patient’s situation to reduce the occurrence of respiratory interruption, hypoxemia, drop in blood pressure, and/or other adverse events.
The authors thank the patient and his guardian for the consent to publish this case.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Anesthesiology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: He YH, China; Wu SH, Taiwan S-Editor: Li L L-Editor: Wang TQ P-Editor: Li L
| 1. | Cook TM, Woodall N, Frerk C; Fourth National Audit Project. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: anaesthesia. Br J Anaesth. 2011;106:617-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1171] [Cited by in RCA: 1243] [Article Influence: 88.8] [Reference Citation Analysis (1)] |
| 2. | Novak I, Morgan C, Adde L, Blackman J, Boyd RN, Brunstrom-Hernandez J, Cioni G, Damiano D, Darrah J, Eliasson AC, de Vries LS, Einspieler C, Fahey M, Fehlings D, Ferriero DM, Fetters L, Fiori S, Forssberg H, Gordon AM, Greaves S, Guzzetta A, Hadders-Algra M, Harbourne R, Kakooza-Mwesige A, Karlsson P, Krumlinde-Sundholm L, Latal B, Loughran-Fowlds A, Maitre N, McIntyre S, Noritz G, Pennington L, Romeo DM, Shepherd R, Spittle AJ, Thornton M, Valentine J, Walker K, White R, Badawi N. Early, Accurate Diagnosis and Early Intervention in Cerebral Palsy: Advances in Diagnosis and Treatment. JAMA Pediatr. 2017;171:897-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 980] [Cited by in RCA: 1004] [Article Influence: 125.5] [Reference Citation Analysis (0)] |
| 3. | Wilkinson DJ, Baikie G, Berkowitz RG, Reddihough DS. Awake upper airway obstruction in children with spastic quadriplegic cerebral palsy. J Paediatr Child Health. 2006;42:44-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Skoutelis VC, Kanellopoulos AD, Kontogeorgakos VA, Dinopoulos A, Papagelopoulos PJ. The orthopaedic aspect of spastic cerebral palsy. J Orthop. 2020;22:553-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Graham HK, Rosenbaum P, Paneth N, Dan B, Lin JP, Damiano DL, Becher JG, Gaebler-Spira D, Colver A, Reddihough DS, Crompton KE, Lieber RL. Cerebral palsy. Nat Rev Dis Primers. 2016;2:15082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 586] [Article Influence: 65.1] [Reference Citation Analysis (0)] |
| 6. | Morgan C, Fetters L, Adde L, Badawi N, Bancale A, Boyd RN, Chorna O, Cioni G, Damiano DL, Darrah J, de Vries LS, Dusing S, Einspieler C, Eliasson AC, Ferriero D, Fehlings D, Forssberg H, Gordon AM, Greaves S, Guzzetta A, Hadders-Algra M, Harbourne R, Karlsson P, Krumlinde-Sundholm L, Latal B, Loughran-Fowlds A, Mak C, Maitre N, McIntyre S, Mei C, Morgan A, Kakooza-Mwesige A, Romeo DM, Sanchez K, Spittle A, Shepherd R, Thornton M, Valentine J, Ward R, Whittingham K, Zamany A, Novak I. Early Intervention for Children Aged 0 to 2 Years With or at High Risk of Cerebral Palsy: International Clinical Practice Guideline Based on Systematic Reviews. JAMA Pediatr. 2021;175:846-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 224] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 7. | Siriwat R, Deerojanawong J, Sritippayawan S, Hantragool S, Cheanprapai P. Mechanical Insufflation-Exsufflation Versus Conventional Chest Physiotherapy in Children With Cerebral Palsy. Respir Care. 2018;63:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Marpole R, Blackmore AM, Gibson N, Cooper MS, Langdon K, Wilson AC. Evaluation and Management of Respiratory Illness in Children With Cerebral Palsy. Front Pediatr. 2020;8:333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 9. | Altun D, Kara H, Bozbora E, Ali A, Dinç T, Sonmez S, Buget M, Aydemir L, Basaran B, Tuğrul M, Çamci E. The Role of Indirect Laryngoscopy, Clinical and Ultrasonographic Assessment in Prediction of Difficult Airway. Laryngoscope. 2021;131:E555-E560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Apfelbaum JL, Hagberg CA, Connis RT, Abdelmalak BB, Agarkar M, Dutton RP, Fiadjoe JE, Greif R, Klock PA, Mercier D, Myatra SN, O'Sullivan EP, Rosenblatt WH, Sorbello M, Tung A. 2022 American Society of Anesthesiologists Practice Guidelines for Management of the Difficult Airway. Anesthesiology. 2022;136:31-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 571] [Article Influence: 190.3] [Reference Citation Analysis (0)] |
| 11. | Cabrini L, Baiardo Redaelli M, Ball L, Filippini M, Fominskiy E, Pintaudi M, Putzu A, Votta CD, Sorbello M, Antonelli M, Landoni G, Pelosi P, Zangrillo A. Awake Fiberoptic Intubation Protocols in the Operating Room for Anticipated Difficult Airway: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Anesth Analg. 2019;128:971-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 12. | Yang SZ, Huang SS, Yi WB, Lv WW, Li L, Qi F. Awake fiberoptic intubation and use of bronchial blockers in ankylosing spondylitis patients. World J Clin Cases. 2021;9:6705-6716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Kaufmann J, Laschat M, Engelhardt T, Hellmich M, Wappler F. Tracheal intubation with the Bonfils fiberscope in the difficult pediatric airway: a comparison with fiberoptic intubation. Paediatr Anaesth. 2015;25:372-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Cui XL, Wang SY, Xue FS. Fiberoptic and retrograde intubation in difficult pediatric airway: useful suggestions. J Neurosurg Anesthesiol. 2014;26:257-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Riva T, Pedersen TH, Seiler S, Kasper N, Theiler L, Greif R, Kleine-Brueggeney M. Transnasal humidified rapid insufflation ventilatory exchange for oxygenation of children during apnoea: a prospective randomised controlled trial. Br J Anaesth. 2018;120:592-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 16. | Kacar Bayram A, Yan Q, Isitan C, Rao S, Spencer DD, Alkawadri R. Effect of anesthesia on electrocorticography for localization of epileptic focus: Literature review and future directions. Epilepsy Behav. 2021;118:107902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Xin N, Xu H, Yue C. Comparison between dexmedetomidine and esketamine in pediatric dentistry surgery. Transl Pediatr. 2021;10:3159-3165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 18. | Patel D, Talbot C, Luo W, Mulvaney S, Byrne E. The use of esketamine sedation in the emergency department for manipulation of paediatric forearm fractures: A 5 year study. Injury. 2021;52:1321-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Sryma PB, Mittal S, Tiwari P, Mohan A, Hadda V, Guleria R, Madan K. Topical nasal xylometazoline for flexible bronchoscopy (VAIN): A randomized, double-blind, placebo-controlled trial. Respir Investig. 2021;59:350-355. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |