Published online Apr 16, 2023. doi: 10.12998/wjcc.v11.i11.2474
Peer-review started: October 20, 2022
First decision: February 7, 2023
Revised: February 19, 2023
Accepted: March 20, 2023
Article in press: March 20, 2023
Published online: April 16, 2023
Processing time: 168 Days and 1.3 Hours
Sporadic cases of rheumatoid arthritis (RA) due to unsatisfactory responses to Abatacept (ABT) have been reported; however, the rescue therapy has not been finalized. Here, we present a case with difficult-to-treat RA (D2T RA) that was resistant to either a single ABT or a Janus kinase (JAK) inhibitor (Tofacitinib), but improved with a combination of ABT and JAK inhibitor (Baricitinib, BAT).
A 46-year-old Chinese woman who had RA for ten years that was resistant to Tocilizumab, Etanercept, Adalimumab, and ABT. According to the European League Against Rheumatism definition, the patient was diagnosed with D2T RA. It was then improved with a combination of ABT and a JAK inhibitor BAT.
ABT combined with BAT may be an acceptable strategy for treating D2T RA.
Core Tip: Although the combined use of Abatacept (ABT) and Janus kinase (JAK) inhibitors is not recommended in rheumatoid arthritis (RA) treatment guidelines, inflammatory cytokines have been found to compensate for the inhibitory effect of ABT on co-stimulatory signals, activate T-lymphocytes through the JAK/ Signal Transducers and Activators of Transcription pathway, and promote the inflammatory response. In the treatment of this patient, Baricitinib, as a JAK inhibitor, combined with ABT can be used as a rescue treatment for difficult-to-treat RA, especially for patients with poor responses to single ABT treatment.
- Citation: Qi JP, Jiang H, Wu T, Zhang Y, Huang W, Li YX, Wang J, Zhang J, Ying ZH. Difficult-to-treat rheumatoid arthritis treated with Abatacept combined with Baricitinib: A case report. World J Clin Cases 2023; 11(11): 2474-2481
- URL: https://www.wjgnet.com/2307-8960/full/v11/i11/2474.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i11.2474
New disease-modifying anti-rheumatic drugs (DMARDs) have drastically improved rheumatoid arthritis (RA) patients' quality of life[1]. However, 5%-20% of patients continue to show symptoms and clinical signs of autoimmune inflammatory activity despite the continuous treatment with various conventional synthetic, targeted synthetic, and biological DMARDs (cs, ts, and bDMARDs), Such patients are considered as difficult-to-treat RA (D2T RA) population[2,3], according to the European League Against Rheumatism (EULAR) definition of D2T RA (see Table 1 for a complete definition)[4]. D2T RA patients were found to have lower physical function and quality of life, along with substantial fatigue and discomfort than non-D2T RA patients, implying a larger illness load, more significant impairment effects, and early mortality[5]. The current treatment of D2T RA involves a repeated trial process of switching to another b/csDMARD after the first fails, as there are no specific management guidelines for these patients[6]. Therefore, establishing new treatment modalities for this population has become a top priority.
| EULAR definition of D2T RA |
| 1 Treatment according to the recommendations of the European League against rheumatism, treatment failure ≥ two biological / tsDMARDs (with different mechanism)1 after csDMARD treatment failure (unless there are contraindications)2. |
| 2 Signs indicating active/progressive diseases are defined as ≥ one of them: |
| (1) At least moderate disease activity (based on validated composite indicators, including joint counts, such as DAS28-ESR > 3.2 or CDAI > 10). |
| (2) Signs (including acute phase reactants and imaging) and / or symptoms indicating active disease (joint related or other). |
| (3) No reduction in glucocorticoid treatment (less than 7.5 mg / day prednisone or equivalent). |
| (4) Rapid radiographic progress (with or without signs of active disease)3. |
| (5) According to the above criteria, the disease is well controlled, but there are still RA symptoms, resulting in a decline in the quality of life. |
| 3 Rheumatologists and/or patients believe that there are problems in the management of signs and/or symptoms. |
Compared to other b/tsDMARDs, Abatacept (ABT), a novel T-cell costimulation modulator, created compelling clinical benefits and security in patients who did not respond to anti-tumor necrosis factor-α or methotrexate treatment[7,8]. However, ABT is not effective in all patients[9]. The poor response to ABT in D2T RA patients may be linked to inflammatory cytokines; however, the exact pathogenesis remains unknown. According to recent reports, combination therapy with other DMARDs is a more effective management option for patients who do not significantly respond to ABT[9]. Janus kinase (JAK) inhibitors are currently the routine therapy for RA patients on whom csDMARDs are ineffective, and are widely used as an alternative to biologics in patients with no risk factors for venous thromboembolism. Combination therapy has been demonstrated to be clinically and radiologically superior to monotherapy[10]. Although the use of ABT and JAK inhibitors in combination is not suggested in the RA treatment guidelines, it is considered a preliminary experiment because various biologics have been attempted in the past with no notable outcomes. Herein, we present a report of a patient who did not respond to multiple bDMARDs (Tocilizumab, Etanercept, Adalimumab, and ABT) and was successfully treated with a combination therapy of ABT and Baricitinib (BAT).
A 46-year-old woman presented with arthralgia for half a month.
Two years ago, following knee arthroplasty, she experienced profound weariness and stiffness in the morning, with swelling and soreness of several peripheral joints. Quickly, she had trouble moving, and was unable to crouch or rise without assistance. Subsequently, she was admitted to Zhejiang Provincial People’s Hospital on October 1, 2020.
The 46-year-old Hangzhou woman developed RA when she was 36 years old. After more than one year of treatment, the disease was nearly controlled.
The patient had a joint replacement two years ago without a family history.
A body temperature of 37.2°C, a blood pressure of 117/85 mmHg, a heart rate of 83 beats/min, and a respiratory rate of 19 times/min were noted. Swollen and painful joints on both sides of the knuckles, proximal interphalangeal joints, wrist joints and left knee joints.
On presentation to the clinician, the patient had elevated erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels of 118 mm/h (normal range; 0-26 mm/h) and 63.8 mg/L (normal range; 0-8 mg/L), respectively. The levels of rheumatoid factor and anti-cyclic citrullinated peptide antibody increased to 1590.0 IU/mL (normal range; 0-20 IU/mL) and 1351.6 U/mL (normal range; 0-25 U/mL), respectively. Serum immune complex levels, anti-neutrophil cytoplasmic antibody, anti-Sjogren syndrome A antibody (anti-SS-A) and anti-SS-B titers were also significantly increased. Clinical symptoms and serological tests were used to diagnose RA. The disease activity score of 28 joints with ESR (DAS28-ESR) was 6.05 (DAS28-ESR ≤ 2.6, remission; 2.6 < DAS28-ESR ≤ 3.2, mild activity; 3.2 < DAS28-ESR ≤ 5.1, moderate activity, and DAS28-ESR > 5.1, severe activity).
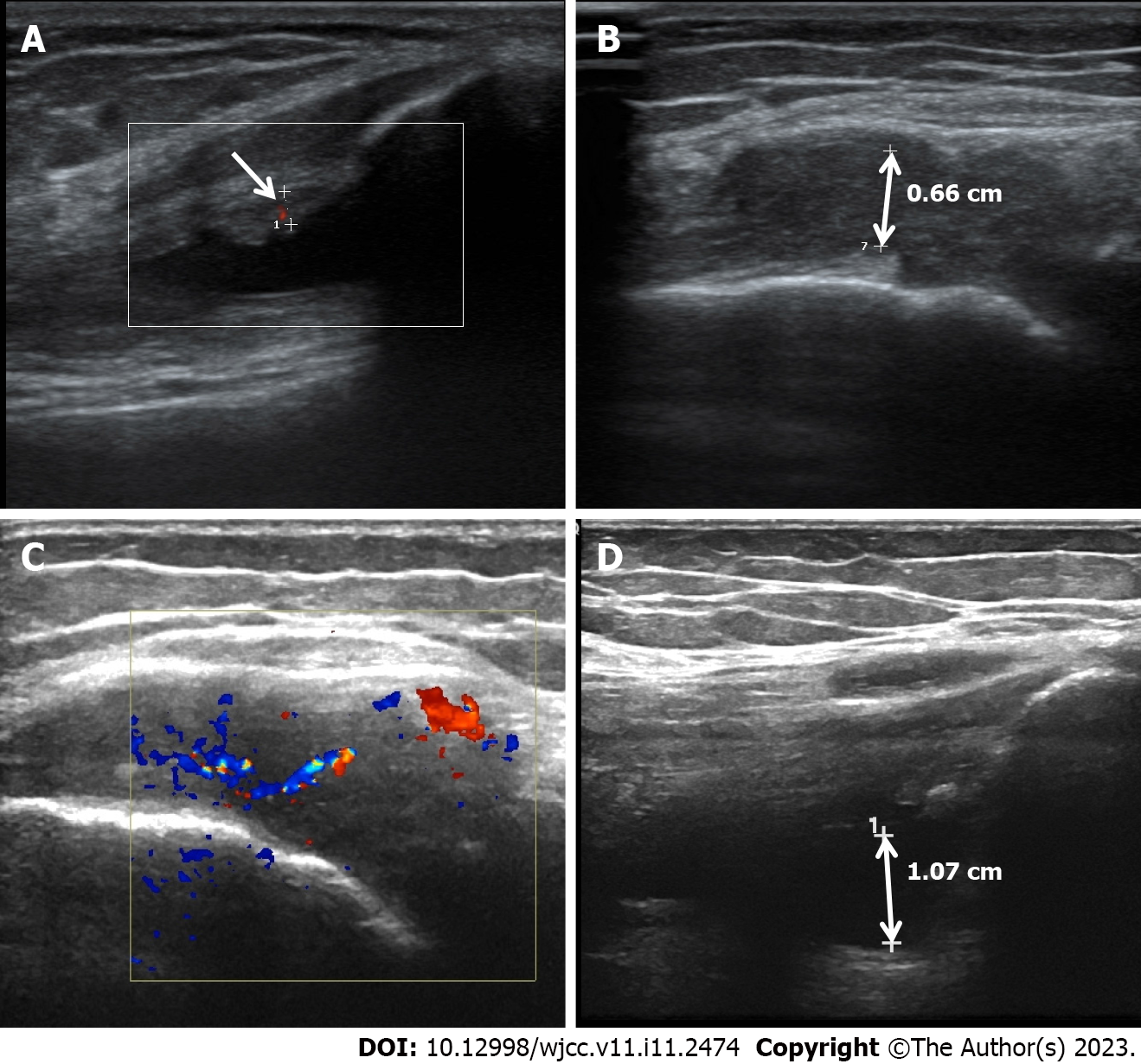
Ultrasonic studies revealed a thickened synovial membrane, suprapatellar bursa effusion, and degenerative changes in the left knee joint (Figure 1A and B).
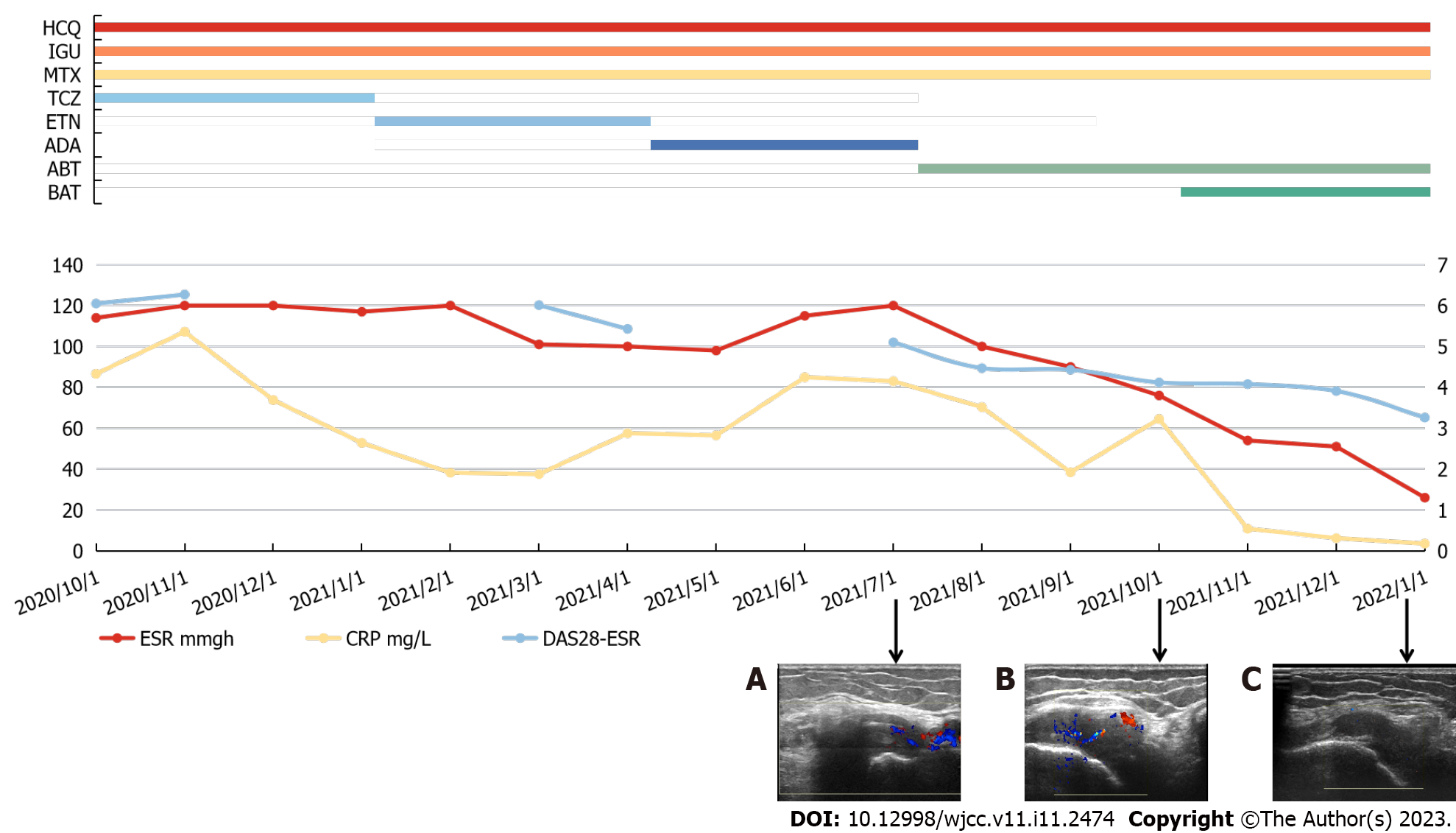
Subsequently, the patient was treated with ABT for 3 mo. Although the joint swelling and tenderness improved slightly, the DAS28-ESR decreased from 5.1 to 4.12, and ESR and CRP dropped to 89 mm/h and 64.5 mg/L, respectively, the disease was still in remission. The ultrasound test revealed the development of synovitis and pannus in the articular cavity of the left knee, and blood flow in the articular cavity was more abundant than before (Figure 1C and D). Difficulty in walking, squatting, and upright standing were still present. Comorbidities such as ankylosing spondylitis, psoriatic arthritis, osteoarthritis, lupus, and arthritis caused by other causes were excluded based on laboratory data, joint ultrasonography, and clinical picture. According to the EULAR definition of D2T RA, it was diagnosed as D2T RA.
Thereafter, BAT was introduced, considering that the combination of these medications may be successful if the patients do not have any contraindications, such as TB infection or viral hepatitis. After one month, the patient's DAS28-ESR score was 4.08, and ESR and CRP level were 76 mm/h and 10.8 mg/L, respectively. All three indicators constantly remained below this level for the next 3 mo. Furthermore, when compared to the prior time, the ultrasound test revealed that the development of synovitis and pannus in the left knee joint cavity had improved, and blood flow signals were significantly reduced [2021-7 (Figure 2A), 2021-10 (Figure 2B) and 2022-1 (Figure 2C) joint CDFI comparison]. During treatment, no significant side effects were observed.
The patient's ESR and CRP levels were within the normal thresholds (26 mm/h and 3.5 mg/L, respectively) after 3 mo, and DAS28-ESR was 3.26, indicating low-level activity. Moreover, the patients' autonomous walking, squatting, and standing abilities were significantly improved compared to before combination therapy. Entire clinical process and pharmacological dose of the patient is depicted in Figure 2. With the addition of BAT, the patient received effective and continuous treatment for the first time.
The pathophysiology of D2T RA is complex, and it is currently categorized into two groups: (1) Multidrug resistance caused by autoimmune disorders and environmental factors in RA patients, such as smoking, pharmacogenetics, or drug immunogenicity; and (2) Difficulties with intensive treatment, including comorbidities, poor medication compliance, financial constraints, and reluctance to intensify treatment[11]. Furthermore, from an immunogenetics standpoint, T-lymphocyte pathways play a significant role in inducing and perpetuating chronic relapsing arthritis of D2TRA[12]. Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) was first identified as an inhibitory signal that is delivered to stop the immune response and has the potential to adversely limit T-lymphocyte activation in various ways[13]. In several research investigations, the prevalence of D2T RA ranged from 5% to 20% of patients with RA[14]. Compared to RA patients, D2T RA patients have more impairment and die sooner. As a result, high-quality evidence is needed to guide D2 TRA patients' management and assist in the formulation of a structured and tailored treatment approach.
CTLA-4 was first identified as an inhibitory signal delivered to stop immune response and has the potential to adversely limit T-lymphocyte activation in a variety of ways[15]. ABT is a soluble, recombinant, completely humanized fusion protein made up of CTLA-4's extracellular domain and IgG1's Fc region. Interacts with co-stimulatory molecules CD80 Antigen and CD80 Antigen on antigen-presenting cells and inhibits T-lymphocyte activation by interfering with CD28 signaling. ABT has been demonstrated to be beneficial in combating a multitude of autoinflammatory disorders, including RA[16,17]. However, several clinical studies have proved that the single CTLA-4 therapy has a limited ability to block T-lymphocyte activation[18,19]. Inflammatory cytokines [Interleukin 6 (IL-6), IL-17, IL-18, and IL-1], which compensate for the loss of costimulatory signals in an inflammatory environment, can enhance the activation of allogeneic T-lymphocytes in a CD28-independent way. By signaling inflammatory cytokines, the JAK/ Signal Transducers and Activators of Transcription (STAT) system plays a vital role in CTLA-4 failure[20].
JAK inhibitors interact with the ATP-binding sites such as JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2) to suppress kinase phosphorylation and the JAK/STAT signaling pathway. However, it increases the risk of upper respiratory infections, herpes zoster, hematological abnormalities, and gastrointestinal problems[21]. Four JAK inhibitors are currently approved for RA[22]. As one of them, BAT can effectively inhibit JAK1 and JAK2, and moderately inhibit TYK2. Studies have shown that BAT with safety profiles may be more suitable for RA patients who are resistant to multiple bDMARDs and have a higher American College of Rheumatology (ACR) response rate than Tofacitinib (which mainly inhibits JAK1 and JAK3)[23,24]. It is known that IL-6 signaling is mediated by JAK1 and JAK2, and IL-17 and IL-18 signaling are mainly mediated by JAK2[25-28]. In the treatment of this patient, BAT may have inhibited the signaling of inflammatory cytokines by inhibiting the JAK/STAT pathway, cooperated with the inhibitory effect of ABT on costimulatory signals, and blocked the inflammatory response. With a more comprehensive exploration of the inflammatory molecules that antagonize CTLA4-Ig and the mechanisms underlying the synergism between BAT and CTLA4- Ig, it will be helpful to identify next-generation JAK inhibitors that will interact with other immunosuppressants more selectively and develop safer and more effective D2T RA management.
We report a case of D2T RA in which the effect was not significant after the replacement of multiple DMARDs, especially ABT, and the combination of ABT and JAK inhibitors was effective. Inflammatory cytokines can compensate for the inhibitory effect of ABT on costimulatory signals, activate T lymphocytes through the JAK/STAT pathway, and promote the inflammatory response. When considering the etiology and treatment of D2T RA patients, especially when the ABT response is not significant, this case can be used as a valuable reference.
Inflammatory cytokines can compensate for the inhibitory effect of ABT on co-stimulatory signals, activate T-lymphocytes through the JAK/STAT pathway, and promote the inflammatory response. In the treatment of this patient, BAT, as a JAK inhibitor, combined with ABT can be used as a rescue treatment for D2TRA, especially for patients with poor responses to single ABT treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dauyey K, Kazakhstan; Primadhi RA, Indonesia S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
| 1. | de Hair MJH, Jacobs JWG, Schoneveld JLM, van Laar JM. Difficult-to-treat rheumatoid arthritis: an area of unmet clinical need. Rheumatology (Oxford). 2018;57:1135-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 2. | Kearsley-Fleet L, Davies R, De Cock D, Watson KD, Lunt M, Buch MH, Isaacs JD, Hyrich KL; BSRBR-RA Contributors Group. Biologic refractory disease in rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann Rheum Dis. 2018;77:1405-1412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 115] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 3. | Buch MH. Defining refractory rheumatoid arthritis. Ann Rheum Dis. 2018;77:966-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 126] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 4. | Nagy G, Roodenrijs NMT, Welsing PM, Kedves M, Hamar A, van der Goes MC, Kent A, Bakkers M, Blaas E, Senolt L, Szekanecz Z, Choy E, Dougados M, Jacobs JW, Geenen R, Bijlsma HW, Zink A, Aletaha D, Schoneveld L, van Riel P, Gutermann L, Prior Y, Nikiphorou E, Ferraccioli G, Schett G, Hyrich KL, Mueller-Ladner U, Buch MH, McInnes IB, van der Heijde D, van Laar JM. EULAR definition of difficult-to-treat rheumatoid arthritis. Ann Rheum Dis. 2021;80:31-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 303] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 5. | Roodenrijs NMT, van der Goes MC, Welsing PMJ, Tekstra J, Lafeber FPJG, Jacobs JWG, van Laar JM. Difficult-to-treat rheumatoid arthritis: contributing factors and burden of disease. Rheumatology (Oxford). 2021;60:3778-3788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 97] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 6. | Smolen JS, Landewé RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, McInnes IB, Sepriano A, van Vollenhoven RF, de Wit M, Aletaha D, Aringer M, Askling J, Balsa A, Boers M, den Broeder AA, Buch MH, Buttgereit F, Caporali R, Cardiel MH, De Cock D, Codreanu C, Cutolo M, Edwards CJ, van Eijk-Hustings Y, Emery P, Finckh A, Gossec L, Gottenberg JE, Hetland ML, Huizinga TWJ, Koloumas M, Li Z, Mariette X, Müller-Ladner U, Mysler EF, da Silva JAP, Poór G, Pope JE, Rubbert-Roth A, Ruyssen-Witrand A, Saag KG, Strangfeld A, Takeuchi T, Voshaar M, Westhovens R, van der Heijde D. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79:685-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1929] [Cited by in RCA: 1751] [Article Influence: 350.2] [Reference Citation Analysis (0)] |
| 7. | Emery P, Burmester GR, Bykerk VP, Combe BG, Furst DE, Barré E, Karyekar CS, Wong DA, Huizinga TW. Evaluating drug-free remission with abatacept in early rheumatoid arthritis: results from the phase 3b, multicentre, randomised, active-controlled AVERT study of 24 months, with a 12-month, double-blind treatment period. Ann Rheum Dis. 2015;74:19-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 197] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 8. | Lundquist LM, Cole SW, Augustine JM. Critical appraisal of efficacy and safety of abatacept in the treatment of refractory rheumatoid arthritis. Open Access Rheumatol. 2012;4:9-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Suzuki M, Takahashi N, Kida D, Hirano Y, Kato T, Yabe Y, Oguchi T, Fujibayashi T, Hayashi M, Asai S, Ishiguro N, Kojima T. Clinical effectiveness and safety of additional administration of tacrolimus in rheumatoid arthritis patients with an inadequate response to abatacept: A retrospective cohort study. Int J Rheum Dis. 2019;22:2199-2205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Reddy V, Cohen S. Role of Janus Kinase inhibitors in rheumatoid arthritis treatment. Curr Opin Rheumatol. 2021;33:300-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Roodenrijs NMT, de Hair MJH, van der Goes MC, Jacobs JWG, Welsing PMJ, van der Heijde D, Aletaha D, Dougados M, Hyrich KL, McInnes IB, Mueller-Ladner U, Senolt L, Szekanecz Z, van Laar JM, Nagy G; whole EULAR Task Force on development of EULAR recommendations for the comprehensive management of difficult-to-treat rheumatoid arthritis. Characteristics of difficult-to-treat rheumatoid arthritis: results of an international survey. Ann Rheum Dis. 2018;77:1705-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 12. | Watanabe R, Hashimoto M, Murata K, Murakami K, Tanaka M, Ohmura K, Ito H, Matsuda S. Prevalence and predictive factors of difficult-to-treat rheumatoid arthritis: the KURAMA cohort. Immunol Med. 2022;45:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 13. | Tuncel J, Holmberg J, Haag S, Hopkins MH, Wester-Rosenlöf L, Carlsen S, Olofsson P, Holmdahl R. Self-reactive T cells induce and perpetuate chronic relapsing arthritis. Arthritis Res Ther. 2020;22:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Roodenrijs NMT, Hamar A, Kedves M, Nagy G, van Laar JM, van der Heijde D, Welsing PMJ. Pharmacological and non-pharmacological therapeutic strategies in difficult-to-treat rheumatoid arthritis: a systematic literature review informing the EULAR recommendations for the management of difficult-to-treat rheumatoid arthritis. RMD Open. 2021;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Mitsuiki N, Schwab C, Grimbacher B. What did we learn from CTLA-4 insufficiency on the human immune system? Immunol Rev. 2019;287:33-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 16. | Bonelli M, Scheinecker C. How does abatacept really work in rheumatoid arthritis? Curr Opin Rheumatol. 2018;30:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 17. | Hosseini A, Gharibi T, Marofi F, Babaloo Z, Baradaran B. CTLA-4: From mechanism to autoimmune therapy. Int Immunopharmacol. 2020;80:106221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 187] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 18. | Khalifian S, Raimondi G, Lee WA, Brandacher G. Taming inflammation by targeting cytokine signaling: new perspectives in the induction of transplantation tolerance. Immunotherapy. 2014;6:637-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, Massari P, Mondragon-Ramirez GA, Agarwal M, Di Russo G, Lin CS, Garg P, Larsen CP. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant. 2010;10:535-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 720] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 20. | Iglesias M, Khalifian S, Oh BC, Zhang Y, Miller D, Beck S, Brandacher G, Raimondi G. A short course of tofacitinib sustains the immunoregulatory effect of CTLA4-Ig in the presence of inflammatory cytokines and promotes long-term survival of murine cardiac allografts. Am J Transplant. 2021;21:2675-2687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Choy EH. Clinical significance of Janus Kinase inhibitor selectivity. Rheumatology (Oxford). 2019;58:953-962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 198] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 22. | You H, Xu D, Zhao J, Li J, Wang Q, Tian X, Li M, Zeng X. JAK Inhibitors: Prospects in Connective Tissue Diseases. Clin Rev Allergy Immunol. 2020;59:334-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 23. | Miyazaki Y, Nakano K, Nakayamada S, Kubo S, Inoue Y, Fujino Y, Tanaka Y. Efficacy and safety of tofacitinib versus baricitinib in patients with rheumatoid arthritis in real clinical practice: analyses with propensity score-based inverse probability of treatment weighting. Ann Rheum Dis. 2021;80:1130-1136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 24. | Lee YH, Song GG. Relative efficacy and safety of tofacitinib, baricitinib, upadacitinib, and filgotinib in comparison to adalimumab in patients with active rheumatoid arthritis. Z Rheumatol. 2020;79:785-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Taylor PC. Clinical efficacy of launched JAK inhibitors in rheumatoid arthritis. Rheumatology (Oxford). 2019;58:i17-i26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 125] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 26. | Clark JD, Flanagan ME, Telliez JB. Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J Med Chem. 2014;57:5023-5038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 465] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 27. | Kao CY, Chen Y, Thai P, Wachi S, Huang F, Kim C, Harper RW, Wu R. IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways. J Immunol. 2004;173:3482-3491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 327] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 28. | Lee KM, Kang JH, Yun M, Lee SB. Quercetin inhibits the poly(dA:dT)-induced secretion of IL-18 via down-regulation of the expressions of AIM2 and pro-caspase-1 by inhibiting the JAK2/STAT1 pathway in IFN-γ-primed human keratinocytes. Biochem Biophys Res Commun. 2018;503:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |