Published online Apr 16, 2023. doi: 10.12998/wjcc.v11.i11.2457
Peer-review started: September 20, 2022
First decision: November 11, 2022
Revised: December 4, 2022
Accepted: March 20, 2023
Article in press: March 20, 2023
Published online: April 16, 2023
Processing time: 198 Days and 0.6 Hours
Allergic bronchopulmonary aspergillosis (ABPA) is an immune-related pulmonary disease caused by sensitization of airway by Aspergillus fumigatus. The disease manifests as bronchial asthma and recurring pulmonary shadows, which may be associated with bronchiectasis. The diagnosis of ABPA mainly depends on serological, immunological, and imaging findings. Pathological examination is not necessary but may be required in atypical cases to exclude pulmonary tuberculosis, tumor, and other diseases through lung biopsy.
An 18-year-old man presented with recurrent wheezing, cough, and peripheral blood eosinophilia. Chest computed tomography showed pulmonary infiltration. There was a significant increase in eosinophils in bronchoalveolar lavage fluid. There was no history of residing in a parasite-endemic area or any evidence of parasitic infection. Pathologic examination of bronchoalveolar lavage fluid excluded fungal and mycobacterial infections. The patient was receiving medication for comorbid diseases, but there was no temporal correlation between medication use and clinical manifestations, which excluded drug-induced etiology. Histopathological examination of lung biopsy specimen showed no signs of eosinophilic granulomatosis with polyangiitis, IgG4-related diseases, or tumors. The diagnosis of ABPA was considered based on the history of asthma and the significant increase in serum Aspergillus fumigatus-specific immunoglobulin (Ig)E. Eosinophil-related diseases were excluded through pathological biopsy, which showed typical pathological manifestations of ABPA.
The possibility of ABPA should be considered in patients with poorly controlled asthma, especially those with eosinophilia, lung infiltration shadows, or bronchiectasis. Screening for serum IgE, Aspergillus fumigatus-specific IgE and IgG, and alveolar lavage can help avoid misdiagnosis.
Core Tip: Clinically, allergic bronchopulmonary aspergillosis should be considered for patients with poorly controlled asthma, especially those with elevated eosinophils, pulmonary infiltrates, or bronchiectasis. Serum total immunoglobulin (Ig)E (IgE), Aspergillus fumigatus-specific antibody IgE and IgG detection can help confirm the diagnosis.
- Citation: Zhang XX, Zhou R, Liu C, Yang J, Pan ZH, Wu CC, Li QY. Allergic bronchopulmonary aspergillosis with marked peripheral blood eosinophilia and pulmonary eosinophilia: A case report. World J Clin Cases 2023; 11(11): 2457-2463
- URL: https://www.wjgnet.com/2307-8960/full/v11/i11/2457.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i11.2457
Allergic bronchopulmonary aspergillosis (ABPA) is an immune-related pulmonary disease caused by airway sensitization by Aspergillus fumigatus, manifesting as bronchial asthma and recurring pulmonary shadows which may be associated with bronchiectasis. The disease is relatively rare and is often misdiagnosed or missed clinically. Early diagnosis and timely treatment with systemic glucocorticoids can control the disease and prevent irreversible lung damage. The estimated prevalence of ABPA in asthma patients worldwide is approximately 2.5%[1]. In the general population, secretory immunoglobulin (Ig)E in the bronchi or IgG in serum normally clears the inhaled Aspergillus spores; however, in patients with asthma or cystic fibrosis, the inhaled Aspergillus spores can colonize the airways, inducing immunity in the bronchi and lung interstitium. Inflammatory reactions, mainly type I and type III hypersensitivity reactions, promote the production of IgE, IgG, and IgA. This inflammatory response is mainly triggered due to the activation of eosinophils mediated by T helper 2 (Th2) cells[2]. The activation of this inflammatory response is mainly due to the activation of eosinophils mediated by Th2 cells[3]. The typical manifestations of ABPA are poorly controlled asthma or repeated pulmonary infiltrates with or without bronchiectasis. Up to one-third of all cases in developing countries are misdiagnosed as pulmonary tuberculosis[3]. In this article, we report a case of ABPA and review the clinical features of ABPA including clinical manifestations and serological, imaging, and pathological findings, which may help improve the diagnosis and treatment of this disease.
An 18-year-old man was admitted to the hospital on February 5, 2021 with a history of intermittent wheezing for the last 15 years, which was aggravated for the last 9 d.
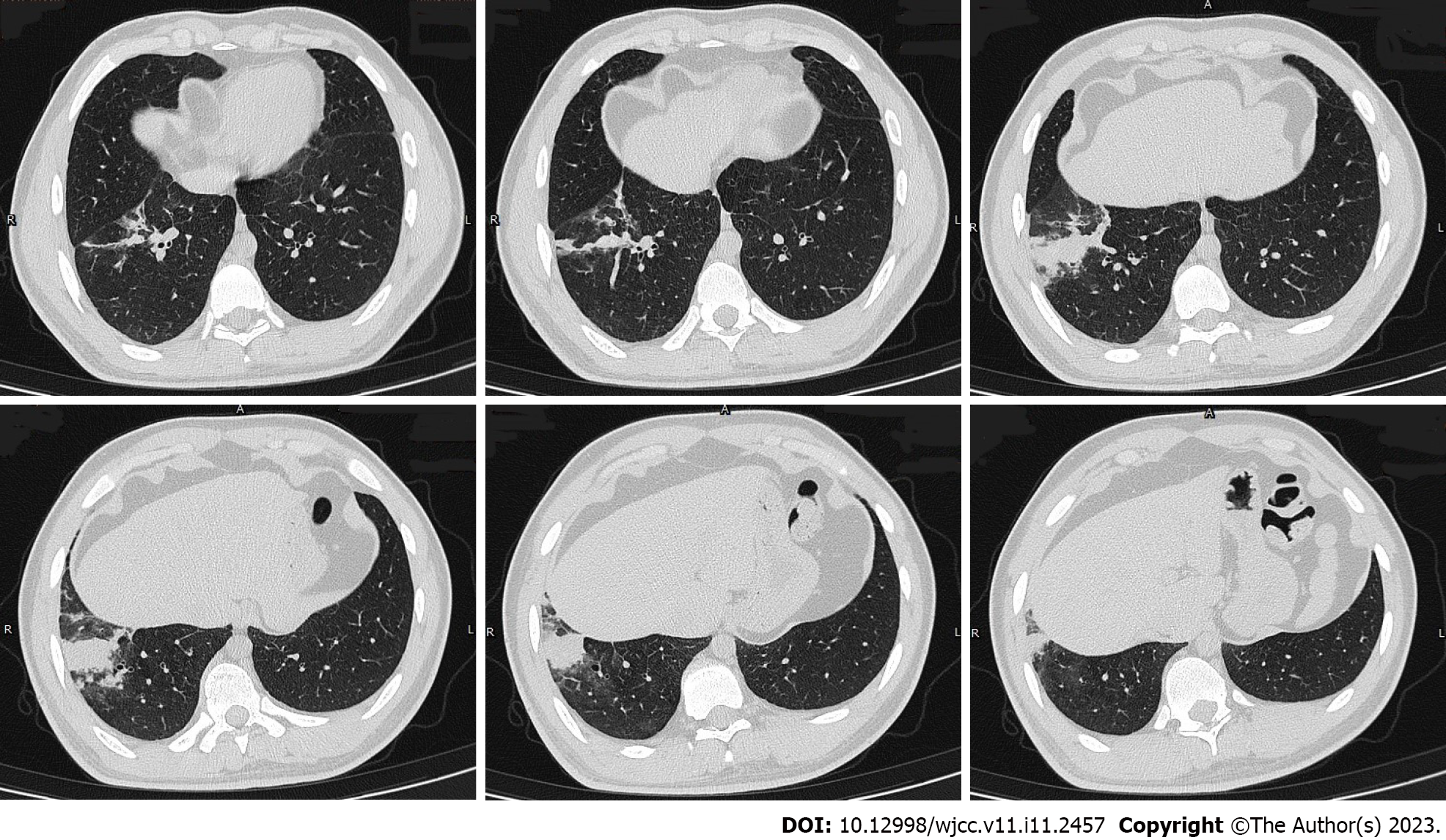
Fifteen years ago, the patient was diagnosed with bronchial asthma. He regularly received salmeterol and ficasone inhalation (50 μg/100 μg or 50 μg/2500 μg, 1 inhalation bid), but acute exacerbations usually occurred 1-2 times every year, typically between the months of October and December. Nine days before admission, the patient developed wheeze but had no fever. His white blood cell count was 10.56 × 109/L, neutrophil percentage was 45.3%, and eosinophil percentage was 17.6%. He was treated with an antibiotic (moxifloxacin) for 3 d, but there was no significant improvement in symptoms. Two days before admission, he had cough with a small amount of white colored sputum. Chest computed tomography (CT) showed consolidation in the anterior basal segment of the right lower lobe, and there were multiple small nodules with fuzzy margins around the periphery, some of which had merged into small patches of consolidation (Figure 1). Therefore, he was hospitalized for further evaluation and treatment.
The patient’s past history included congenital hip dislocation since childhood and developmental deformity of the lower extremities, due to which he was relying on a wheelchair for mobility. The patient was diagnosed with neurogenic bladder 6 years ago due to dysuria and obstructive renal insufficiency due to urinary retention. Cystostomy was performed 5 years ago. Hypertension was diagnosed 5 years ago (systolic blood pressure up to 160 mmHg). His blood pressure was controlled below 130/90 mmHg after treatment with oral fosinopril (10 mg, qd). Scoliosis deformity was discovered 4 years ago. He was also prescribed oral sertraline 50 mg once a night for treatment of depression 4 years ago. Two years ago, he was prescribed oral omeprazole for treatment of reflux esophagitis.
There was a positive family history of bronchial asthma (the patient's mother and grandmother were both affected).
Physical examination on admission revealed the following: Body temperature, 36.7 °C; pulse, 104 beats/min; respiratory rate, 18 beats/min; blood pressure 131/87 mmHg (1 mmHg = 0.133 kPa). The patient was conscious and had no signs of cyanosis. No dry or wet rales were heard in either lung. Cardio
Laboratory tests after admission revealed the following: White blood cell count, 11.75 × 109/L; hemoglobin 159 g/L; platelet count, 201 × 109/L; neutrophil percentage 41.7% (absolute value 4.9 × 109/L); eosinophil percentage 24% 6% (absolute value 2.89 × 109/L); serum procalcitonin 0.056 ng/mL; C-reactive protein 1.43 mg/dL; T-SPOT, G test, GM test, Mycoplasma pneumoniae antibody, and Chlamydia pneumoniae antibody were all negative; antineutrophil cytoplasmic antibodies and antinuclear antibody profiles were also negative; allergen total IgE > 5000 KU/L; Aspergillus fumigatus (m3) 66.4 (grade 5) KU/L; Aspergillus antibody IgG 276.2 AU/mL (reference value < 80) (Table 1).
| Test results | Reference range | |
| Peripheral blood eosinophil percentage | 28.7% | (0.4-8)% |
| Absolute value | 2.56 ×109/L | (0.02-0.52) × 109/L |
| Serum allergen total IgE | > 5000 KU/L | 0-60 |
| Serum Aspergillus fumigatus (m3) specific IgE | 66.4 (5 grade) KU/L | 0-0.35 |
| Serum Aspergillus fumigatus specific IgG | 276.2 AU/mL | < 80 |
| Bronchoalveolar lavage fluid total cells | 5.8 × 105/mL | |
| Macrophages | 40% | > 85% |
| Neutrophils | 2% | ≤ 3% |
| Lymphocytes | 23% | (10-15)% |
| Eosinophils | 35% | ≤ 1% |
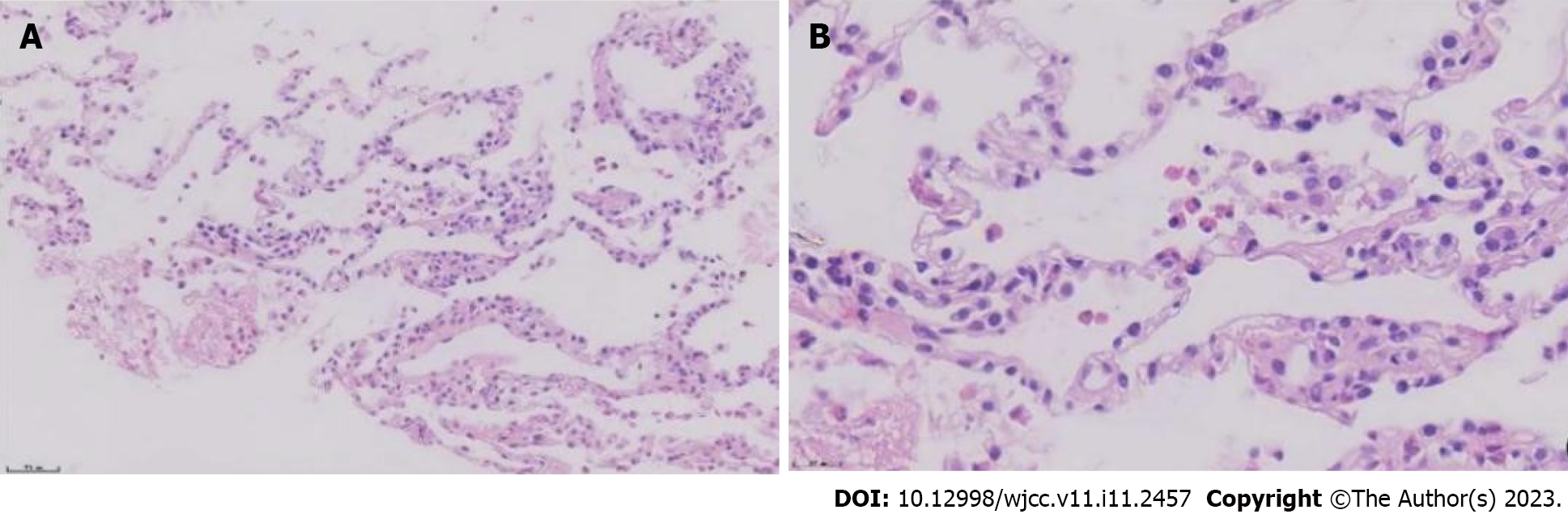
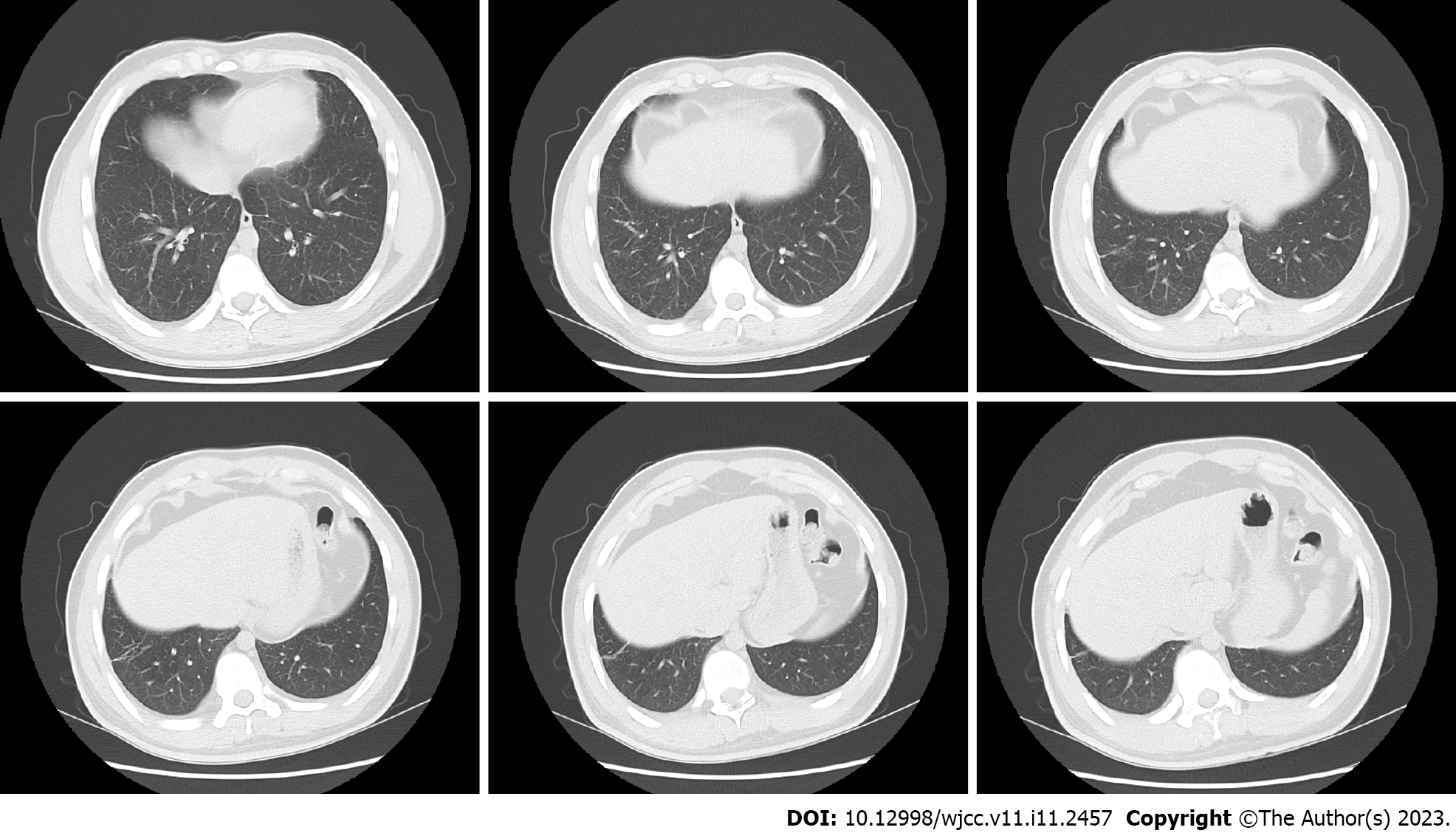
To further clarify the nature of the lung lesions, bronchoalveolar lavage fluid was examined. On cell count in the bronchoalveolar lavage fluid (Table 1), the proportion of eosinophils was 35%. Further ultrasound-guided percutaneous lung biopsy was performed; histopathological findings (Figure 2) were presence of alveolar structures, mild widening of alveolar septa, and infiltration of a large number of eosinophils and a small amount of scattered lymphoplasmacytic cells in the alveolar septum; interstitium. small amount of eosinophilic exudation was seen in some alveolar spaces, with no signs of necrosis, typical granulomas, or vasculitis. Based on the history of asthma, peripheral blood eosinophilia, significantly increased serum allergen total IgE, and positive Aspergillus fumigatus-specific IgE and IgG, a diagnosis of ABPA was established. After 1 mo of prednisone treatment, a routine blood test showed that the white blood cell count was 9.13 × 109/L, and the eosinophil percentage was 0.4% (absolute value 0.04 × 109/L). Chest CT (Figure 3) showed resorption of shadow in the right lower lobe; only fibrous cords were seen.
Based on the history of asthma, peripheral blood eosinophilia, significantly increased serum allergen total IgE, and positive Aspergillus fumigatus-specific IgE and IgG, a diagnosis of ABPA was established.
The patient was prescribed prednisone 20 mg qd orally for 2 mo, which was gradually tapered to 10 mg qd for 6 mo. In addition, he was prescribed antifungal treatment with itraconazole 0.2 g bid for 6 mo to reduce fungal colonization in the airway and the persistent inflammatory reaction caused by fungal antigen stimulation.
After 1 mo of prednisone treatment, the patient’s white blood cell count was 9.13 × 109/L, and the eosinophil percentage was 0.4% (absolute value 0.04 × 109/L). Chest CT (Figure 2) showed resorption of shadow in the right lower lobe; only fibrous cords were seen and the adjacent bronchi were stretched and dilated.
The main manifestations of this patient were recurrent wheezing and cough, eosinophilia, pulmonary infiltration shadow, and significantly increased eosinophils in bronchoalveolar lavage fluid. We considered increased eosinophils in bronchoalveolar lavage fluid as the breakthrough point and made differential diagnoses according to pulmonary eosinophilia.
The term pulmonary eosinophilia refers to a group of heterogeneous diseases with common characteristics of abnormal increase in eosinophils in the lung airway and lung parenchyma. The characteristics include: (1) Peripheral blood eosinophilia (absolute eosinophil count ≥ 500/μL) with abnormal pulmonary imaging examination; (2) increased eosinophils in the bronchoalveolar lavage fluid (> 10%); and (3) presence of eosinophils in lung tissue confirmed by bronchopulmonary biopsy or open chest lung biopsy[1]. Pulmonary eosinophilia is classified into secondary and idiopathic. Secondary causes include infectious diseases (including parasitic, fungal, and mycobacterial infections), allergic diseases (related to ABPA, drugs, or toxicants), connective tissue disorders (eosinophilic granulomatosis with polyangiitis, IgG4-related diseases, etc.), malignancies (lymphoma, leukemia, solid tumors, etc.), and other diseases (bronchial central granulomatosis, interstitial lung disease, lung transplantation, hematopoietic stem cell transplantation, secondary to radiotherapy, etc.). Idiopathic pulmonary eosinophilia includes acute eosinophilic pneumonia (AEP), chronic eosinophilic pneumonia (CEP), and idiopathic hypereosinophilic syndrome (IHES).
Our patient had no history of residing in a parasite endemic area or consumption of raw meat, fish, shrimp, or crab, and there was no evidence of parasitic infection. Pathologic examination of bronchoalveolar lavage fluid excluded fungal and mycobacterial infections. The patient was receiving medication for hypertension, hyperuricemia, depression, and gastroesophageal reflux disease; however, there was no temporal correlation between the medication and the patient's symptoms, eosinophilia, or pulmonary imaging changes. Therefore, any drug-induced etiology was not considered. Pathological examination of lung tissue revealed no signs of eosinophilic granulomatosis with polyangiitis, IgG4-related diseases, or tumors. The diagnosis of ABPA was considered based on the combination of history of asthma and significant increase of serum Aspergillus fumigatus IgE.
Elevated serum IgE was another feature of this patient. Serum IgE level can be elevated in many conditions, such as infectious diseases, atopic diseases, immunodeficiencies, inflammatory diseases, neoplasms, and drug-induced conditions; however, significantly elevated serum IgE (> 2000 KU/L or even 5000 KU/L) is seen in ABPA, IgE type multiple myeloma, and hyperimmunoglobulin E syndrome (HIES). IgE type multiple myeloma is extremely rare. Similar to other types of multiple myeloma, the clinical manifestations of IgE type multiple myeloma are anemia, renal dysfunction, hypercalcemia, and bone damage. Bone damage in patients with IgE type multiple myeloma manifests as osteoporosis, osteolytic damage, osteosclerosis, and pathological fracture[4], while osteolytic damage is more common in other types of multiple myeloma. HIES is a rare, primary immunodeficiency distinguished by the clinical triad of atopic dermatitis, recurrent staphylococcal skin infections, and recurrent pulmonary infections. Patients with HIES have characteristic facial features (facial asymmetry, prominent forehead, deep-set eyes, broad nasal bridge, and wide fleshy nasal tip) that appear in early adolescence or late childhood[5]. Our patient had no such symptoms, due to which IgE type multiple myeloma and HIES were excluded.
The diagnosis of ABPA is primarily based on serological, immunological, and imaging findings. Pathological examination is not necessary for diagnosis but may be required in atypical cases to exclude pulmonary tuberculosis, tumor, or other diseases through lung biopsy.
The main therapeutic goal in ABPA is to control asthma, reduce acute exacerbations, and prevent irreversible lung damage such as bronchiectasis and pulmonary fibrosis. The main therapeutic methods include systemic glucocorticoid therapy to suppress immune response, and use of antifungal drugs to reduce the colonization of respiratory tract[3]. At present, there is no clear consensus on the use of antifungal drugs in patients with ABPA, and these are recommended for patients with hormone dependence and relapse after discontinuation of hormone therapy. In addition, studies have also shown that omazumab (a humanized anti IgE monoclonal antibody) can reduce the serum IgE level of patients, the risk of acute exacerbation, and hormone dosage[6]. In terms of treatment follow-up, serum IgE is an important follow-up parameter apart from clinical symptoms and pulmonary imaging. The decrease in serum IgE indicates the effectiveness of treatment. Reducing the IgE to the normal range is often challenging in ABPA patients. Therefore, the goal of treatment is to reduce the IgE level by 35%-50%. In most cases, clinical and imaging improvements will be achieved. There are many published case reports of ABPA. However, there is a paucity of ABPA case reports describing the histopathological findings of lung biopsy. Thus, the present study presents the typical pathological manifestations of ABPA to readers.
In clinical practice, for patients with poorly controlled asthma, especially those with elevated eosinophils, lung infiltration shadows, or bronchiectasis, the possibility of ABPA should be considered. Screening of serum IgE, Aspergillus fumigatus-specific IgE and IgG, and alveolar lavage should be performed to avoid missed diagnosis and misdiagnosis. Serum total IgE and Aspergillus fumigatus-specific IgE and IgG detection can help confirm the diagnosis.
We thank Sun Yongchang, Zhu Hong and Wang Jianli (Department of Respiratory and Critical Medicine, Peking University Third Hospital), for their guidance on this article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Routray C, United States; Velázquez-Soto H, Mexico S-Editor: Chang KL L-Editor: Wang TQ P-Editor: Chang KL
| 1. | Denning DW, Pleuvry A, Cole DC. Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication chronic pulmonary aspergillosis in adults. Med Mycol. 2013;51:361-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 346] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 2. | Davidsen JR, Madsen PH, Laursen CB. Uncontrolled asthma and recurring pulmonary opacities: just asthma? Case Reports. 2014;bcr2013202428-bcr2013202428. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Agarwal R, Chakrabarti A, Shah A, Gupta D, Meis JF, Guleria R, Moss R, Denning DW; ABPA complicating asthma ISHAM working group. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy. 2013;43:850-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 571] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 4. | Wu XB, Zhou Y, Bao F, Wang X, Yu Y, Chen JX, Li j. IgE multiple myeloma, one case report. Zhonghua Neike Zazhi. 2020;59. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Hashemi H, Mohebbi M, Mehravaran S, Mazloumi M, Jahanbani-Ardakani H, Abtahi SH. Hyperimmunoglobulin E syndrome: Genetics, immunopathogenesis, clinical findings, and treatment modalities. J Res Med Sci. 2017;22:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Li JX, Fan LC, Li MH, Cao WJ, Xu JF. Beneficial effects of Omalizumab therapy in allergic bronchopulmonary aspergillosis: A synthesis review of published literature. Respir Med. 2017;122:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |