Published online Apr 16, 2023. doi: 10.12998/wjcc.v11.i11.2435
Peer-review started: January 5, 2023
First decision: January 30, 2023
Revised: February 7, 2023
Accepted: March 23, 2023
Article in press: March 23, 2023
Published online: April 16, 2023
Processing time: 91 Days and 4.7 Hours
Isotretinoin is a widely prescribed drug for various dermatological conditions, especially acne. Blood tests are routinely performed to monitor its side effects. However, the optimal testing schedule remains controversial.
To evaluate the laboratory monitoring tests and schedules of patients with acne taking isotretinoin to determine the most efficient testing regimen.
We retrospectively reviewed electronic medical records from the Dermatology Department of Dongguk University Ilsan Hospital from 2005 to 2020 for patients prescribed isotretinoin for acne who underwent monthly blood tests.
Aspartate aminotransferase (AST), alanine aminotransferase (ALT), and triglyceride (TG) levels significantly changed between 5 and 6 mo when the total prescription period and dose variables were considered altogether. The high-density lipoprotein level also significantly changed between 5 and 6 mo. However, low-density lipoprotein (LDL) and total cholesterol levels significantly changed between 1 and 2 mo.
We recommend testing AST, ALT, and TG levels once every 5 to 6 mo. We also suggest testing LDL and total cholesterol levels during the first and second months.
Core Tip: Isotretinoin is a widely prescribed drug for various dermatological conditions, especially acne. Blood tests are routinely performed to monitor its side effects. However, the optimal testing schedule remains controversial. This study investigated the optimal blood test type and frequency for patients taking isotretinoin for acne. This article will contribute to Asian-specific isotretinoin laboratory guidelines and further studies.
- Citation: Park YJ, Shin HY, Choi WK, Lee AY, Lee SH, Hong JS. Optimal laboratory testing protocol for patients with acne taking oral isotretinoin. World J Clin Cases 2023; 11(11): 2435-2442
- URL: https://www.wjgnet.com/2307-8960/full/v11/i11/2435.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i11.2435
Oral isotretinoin is a natural vitamin A metabolite (a retinoid) approved for use in 1982 by the United States Food and Drug Administration to treat acne vulgaris[1-4]. Isotretinoin inhibits sebaceous cell proliferation, differentiation, and lipid synthesis in vivo[1]. It also suppresses acne by reducing inflammation caused by Propionibacterium acnes and hyperkeratosis of the hair follicle sebaceous gland system[1].
Isotretinoin is a very effective treatment for acne. However, side effects such as teratogenicity, hyperlipidemia, pancreatitis, and leukopenia are possible and require periodic laboratory monitoring[1-10]. The 2016 laboratory monitoring guidelines in the US do not recommend routine monitoring of the complete blood cell count (CBC) during oral isotretinoin administration for acne[1,5,6]. However, the United States and European guidelines recommend monitoring serum lipid profiles and liver function enzymes[1]. Specifically, they recommended performing a liver and lipid panel before treatment, 1 mo after treatment, and every 3 mo thereafter[1].
In Korea, isotretinoin doses and routine laboratory monitoring protocols vary, which has likely contributed to the increase in unnecessary hospital visits, thus increasing medical expenses, which is detrimental to the finances of the national health insurance. In Korea, although the adverse effects of isotretinoin therapy for acne are known, a cohort study analyzing laboratory changes at a single institution does not exist, nor do Asian-specific guidelines regarding isotretinoin dose and monitoring protocols.
This study retrospectively investigated hematological monitoring tests and intervals for patients taking isotretinoin for acne at a single institution to determine the most effective protocol.
This retrospective study analyzed medical records obtained from Dongguk University Ilsan Hospital’s electronic medical records database. We included patients who visited the dermatology outpatient department at this hospital from 2005 to 2020 and were prescribed isotretinoin for acne based on the Korean standard classification of disease codes L700 (acne vulgaris), L701 (acne conglobata), and L709 (acne, unspecified). Among them, patients who had not taken isotretinoin for > 1 mo or had irregular laboratory monitoring instead of terminating laboratory monitoring at 1-mo intervals because side effects were excluded from the analysis. For example, patients who underwent laboratory testing at 2 mo after the first dose, or those who underwent laboratory testing at 1st month and 4th month sporadically, rather than serial laboratory monitoring, were excluded.
Data on age, sex, prescription dose, prescription period, and underlying disease were collected. Patients who underwent monthly laboratory monitoring for aspartate aminotransferase (AST), alanine aminotransferase (ALT), triglyceride (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), and total cholesterol levels were included. Meanwhile, patients with irregular laboratory testing intervals during isotretinoin administration were excluded from the study.
The Institutional Review Board of Dongguk University Ilsan Hospital approved this study (IRB No. DUIH 2022-05-002).
All blood tests were performed in US units. Normal AST levels were 10-40 units/L for men and 9-25 units/L for women. Normal ALT ranges were 10-55 units/L for men and 7-30 units/L for women. Furthermore, the normal range (men and women) for the TG level was 40-200 mg/dL. Finally, HDL levels ≥ 40 mg/dL, LDL levels ≤ 100 mg/dL, and total cholesterol levels < 200 mg/dL were considered normal.
Laboratory values for AST, ALT, TG, HDL, LDL, and total cholesterol levels were identified for each patient and were categorized by month of therapy for those performed while the patient was receiving isotretinoin. The tendency of the laboratory values to change was determined through the graph slope of the median value.
Independent variables (e.g., sex, total prescription date and dose, daily average prescription dose, underlying disease type, and monthly interval per blood test) were analyzed by repeated-measures analysis of variance. Significance was set at P < 0.05.
Among the 112 patients with acne, 30 were included in the analysis according to the exclusion criteria. Nineteen (63.3%) were male, and 11 (36.7%) were female. The average age was 22.8 years. The total number of prescription days was 60-180 d, with a mean of 140 d. The total prescribed dose was 600-3600 mg, with a mean total of 1825.4 mg. The lowest daily dose was 5 mg/d, and the highest daily dose was 20 mg/d; the average daily dose was 13.2 mg.
In total, 23 patients had no underlying diseases. However, one patient had androgenetic alopecia, one had asthma, one had Crohn’s disease, one had ulcerative colitis, one had seborrheic dermatitis, and two had urticaria (Table 1).
| Characteristics | Mean (min-max) | n(%) | |
| Age | 22.8 (15-38) | 30 (100) | |
| Sex | Male | 19 (63) | |
| Female | 11 (37) | ||
| Total | 30 (100) | ||
| Total prescription days | 339.1 (230-1216) | ||
| Total prescription dose (mg) | 4029.7 (230-10495) | ||
| Daily prescription dose (mg/d) | 13.2 (8.01-20) | ||
| Underlying disease | None | 23 (77) | |
| Androgenetic alopecia | 1 (3) | ||
| Asthma | 1 (3) | ||
| Crohn’s disease | 1 (3) | ||
| Ulcerative colitis | 1 (3) | ||
| Seborrheic dermatitis | 1 (3) | ||
| Urticaria | 2 (6) | ||
Blood tests were performed for 30 patients 1 mo after taking isotretinoin (Table 2). Overall, 2 (7%) patients had abnormal AST levels, 2 (7%) had abnormal HDL levels, 21 (70%) had abnormal LDL levels, and 6 (20%) had abnormal total cholesterol levels. All the patients had normal ALT and TG levels.
| Laboratory | Month 1 (total number: 30), n (%) | Month 2 (total number: 26), n (%) | Month 3 (total number: 21), n (%) | Month 4 (total number: 20), n (%) | Month 5 (total number: 18), n (%) | Month 6 (total number: 13), n (%) |
| AST | 2 (7) | 2 (8) | 4 (19) | 1 (5) | 0 (0) | 1 (8) |
| ALT | 0 (0) | 1 (4) | 1 (5) | 1 (5) | 0 (0) | 1 (8) |
| TG | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 0 (0) | 0 (0) |
| HDL | 2 (7) | 2 (8) | 1 (5) | 0 (0) | 1 (6) | 0 (0) |
| LDL | 21 (70) | 19 (73) | 14 (67) | 14 (70) | 11 (61) | 7 (54) |
| Total cholesterol | 6 (20) | 4 (15) | 5 (24) | 3 (15) | 5 (28) | 3 (23) |
Blood tests were performed after 2 mo of isotretinoin administration (n = 26). Overall, 2 (8%), 1 (4%), 2 (8%), 19 (73%), and 4 (15%) patients had abnormal AST, ALT, HDL, LDL, and total cholesterol levels, respectively. All the patients had normal TG levels.
Blood tests were performed after 3 mo of isotretinoin administration (n = 21). Overall, 4 (19%), 1 (5%), 1 (5%), 14 (67%), and 5 (24%) patients had abnormal AST, ALT, HDL, LDL, and total cholesterol levels, respectively. One (5%) patient had an abnormal TG level.
Blood tests were performed 4 mo after isotretinoin administration (n = 20). Overall, 1 (5%), 1 (5%), 14 (70%), and 3 (15%) patients had abnormal AST, ALT, LDL, and total cholesterol levels, respectively. All the patients had normal HDL and TG levels.
Blood tests were performed after 5 mo of isotretinoin administration (n = 18). Overall, 1 (6%), 11 (61%), and 5 (28%) patients had abnormal HDL, LDL, and total cholesterol levels, respectively. All the patients had normal AST, ALT, and TG levels.
Blood tests were performed after 6 mo of isotretinoin administration (n = 10). Overall, 1 (8%), 1 (8%), 7 (54%), and 3 (23%) patients had abnormal AST, ALT, LDL, and total cholesterol levels, respectively. All the patients had normal HDL and TG levels.
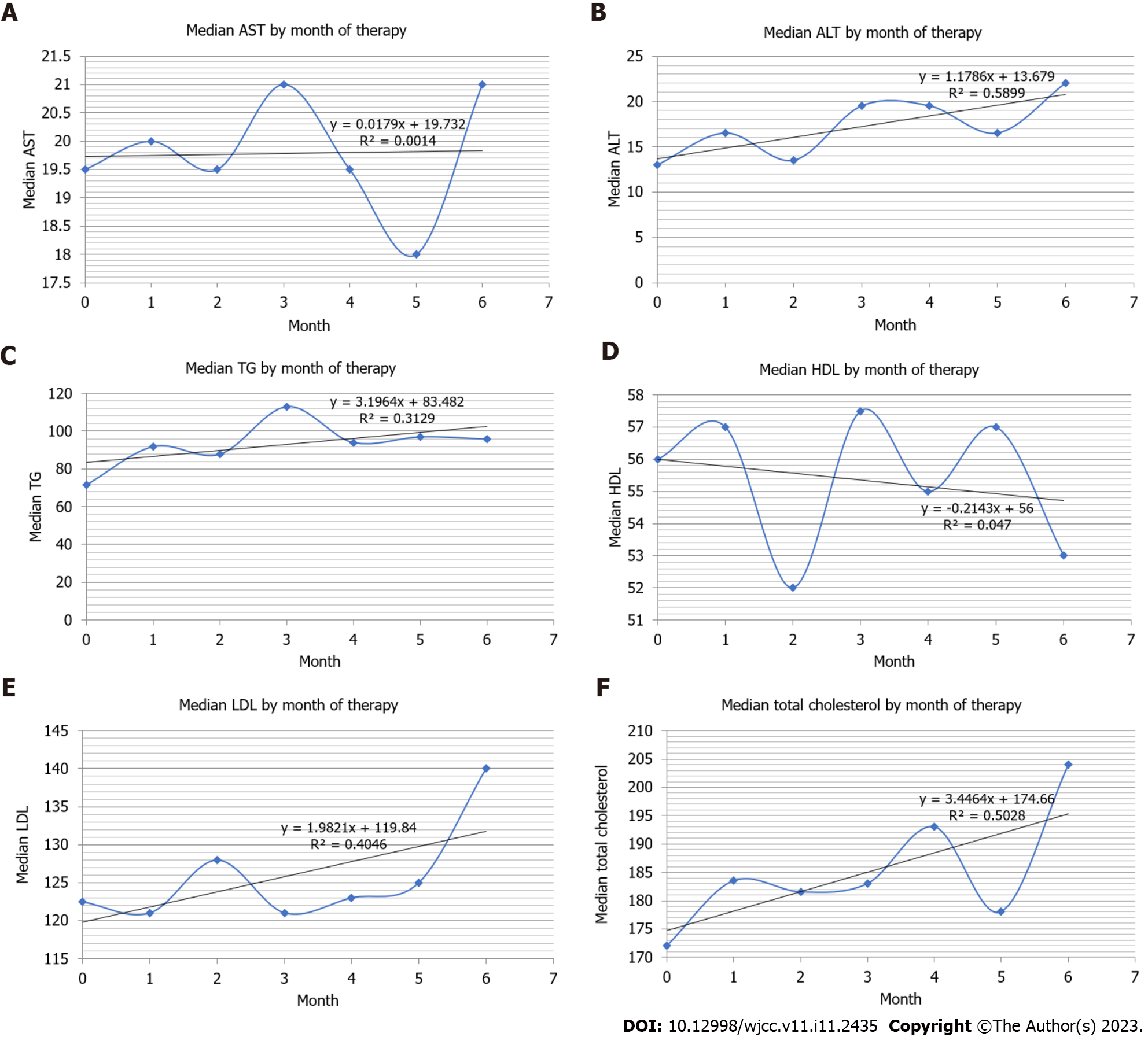
Table 3 and Figure 1 present the monthly changes per blood test in detail.
| Blood test | Variable | 1-2 mo | 2-3 mo | 3-4 mo | 4-5 mo | 5-6 mo |
| AST | Sex | 0.221 | 0.761 | 0.769 | 0.467 | 0.743 |
| Prescription days | 0.721 | 0.341 | 0.584 | 0.777 | 0.020 | |
| Prescription dose | 0.588 | 0.310 | 0.369 | 0.980 | 0.029 | |
| Total | 0.523 | 0.434 | 0.868 | 0.311 | 0.153 | |
| ALT | Sex | 0.296 | 0.362 | 0.174 | 0.912 | 0.927 |
| Prescription days | 0.699 | 0.055 | 0.345 | 0.262 | 0.016 | |
| Prescription dose | 0.948 | 0.081 | 0.527 | 0.314 | 0.021 | |
| Total | 0.580 | 0.261 | 0.689 | 0.709 | 0.126 | |
| TG | Sex | 0.818 | 0.357 | 0.538 | 0.858 | 0.313 |
| Prescription days | 0.801 | 0.681 | 0.823 | 0.718 | 0.008 | |
| Prescription dose | 0.730 | 0.355 | 0.876 | 0.883 | 0.009 | |
| Total | 0.788 | 0.501 | 0.529 | 0.536 | 0.024 | |
| HDL | Sex | 0.552 | 0.999 | 0.747 | 0.498 | 0.190 |
| Prescription days | 0.475 | 0.227 | 0.579 | 0.922 | 0.323 | |
| Prescription dose | 0.632 | 0.190 | 0.528 | 0.950 | 0.083 | |
| Total | 0.521 | 0.232 | 0.716 | 0.611 | 0.018 | |
| LDL | Sex | 0.464 | 0.524 | 0.510 | 0.715 | 0.445 |
| Prescription days | 0.015 | 0.869 | 0.325 | 0.747 | 0.786 | |
| Prescription dose | 0.006 | 0.918 | 0.167 | 0.842 | 0.835 | |
| Total | 0.052 | 0.338 | 0.140 | 0.755 | 0.746 | |
| Total cholesterol | Sex | 0.469 | 0.348 | 0.373 | 0.576 | 0.336 |
| Prescription days | 0.066 | 0.761 | 0.310 | 0.869 | 0.761 | |
| Prescription dose | 0.019 | 0.576 | 0.095 | 0.925 | 0.710 | |
| Total | 0.104 | 0.228 | 0.076 | 0.698 | 0.427 |
AST: The AST level did not change when only AST values were included in the analysis. However, the AST level significantly changed between 5 and 6 mo of isotretinoin use when the prescription period and dose were included in the analysis (Figure 1A).
ALT: Although Figure 1B indicates that the median value of ALT tended to increase over a longer period of use, no significant change in ALT levels was observed when only the blood test values were considered. However, significant changes were observed between 5 and 6 mo of isotretinoin administration when the prescription period and dose were included in the analysis.
TG: Figure 1C presents that the median value of TG tended to increase over a longer period of use; significant changes were observed between 5 and 6 mo of isotretinoin administration. Significant changes in the prescription period and dose were also observed between 5 and 6 mo after including the dependent variable.
HDL: Generally, the median value of HDL in Figure 1D tended to decrease, and a significant change was observed between 5 and 6 mo of isotretinoin administration when only the blood test values were considered. However, this effect disappeared after including the dependent variable.
LDL: Although Figure 1E demonstrates that the median value of LDL tended to increase over a longer period of use, the LDL level did not change when we included only the LDL values in the analysis. However, the LDL level significantly changed between 1 and 2 mo of isotretinoin use when the prescription period and dose were included in the analysis.
Total cholesterol: Although Figure 1F presents that the median value of total cholesterol tends to increase over a longer period of use, the total cholesterol level did not change when we included only the blood test values in the analysis. However, a significant change was observed between 1 and 2 mo of isotretinoin use when the prescription period and dose were included in the analysis.
Oral isotretinoin has revolutionized acne treatment and has become the standard treatment[8]. However, it can cause systemic side effects, including leukopenia, hyperlipidemia, and hepatotoxicity[1-9]. Moreover, most patients require long-term use. Thus, periodic blood tests are necessary to ensure patient safety; however, no consensus on dose and frequency has been established to date[1-6,9].
Since 2006, many studies have argued that only few patients have abnormal laboratory test results[6]. Thus, the frequent monitoring of isotretinoin treatment has little value. For example, a 2016 retrospective study including 515 patients in the United States who received isotretinoin for acne treatment has reported that 2.4% of patients developed leukopenia but they did not require treatment discontinuation, and a clinically non-significant number of patients (1.6%) developed thrombocytopenia[2]. This evidence suggests that routine CBC tests are unnecessary, which has been supported by other authors, such as Barbieriet et al[6]. Furthermore, Hansen et al[2] evaluated patients who received the maximum daily dose of isotretinoin, reporting that liver function and lipid profile monitoring was unnecessary unless the patient had specific risk factors. The 2016 United States and European guidelines also include these recommendations[1]. However, Barbieri et al[6]have reported that many clinicians still perform monthly blood tests, creating a cost burden for patients and the health insurance system.
Here, significant changes in AST, ALT, and TG levels in terms of total prescription period and isotretinoin dose were observed between 5 and 6 mo. Isotretinoin is metabolized in the liver and has an approximately 14 h half-life in the blood[8]. The primary metabolite 4-oxo-isotretinoin has a half-life of almost 28 h[8,10]. Therefore, it would take between 5 and 6 mo to significantly accumulate in the body and affect blood test results. This result is consistent with those of other previously reported studies[11,12]. In addition to liver levels, vitamin A derivatives have the potential to increase lipid levels because they accumulate in fat[13]. As lipid accumulation and elimination take a long time, changes may occur between 5 and 6 mo. Therefore, the contraceptive period of another vitamin A derivative, alitretinoin, is 1-3 years because of this fat accumulation[13]. Therefore, we recommend testing LDL, total cholesterol, liver function, and lipid levels before treatment and 1-2 mo after starting isotretinoin. Subsequently, if no abnormalities are detected, monitoring every 6 mo is sufficient.
Shah et al[3] published a study in 2018 with a similar opinion to that in our study. They also argued that patients should be tested at baseline, 2-3 mo, and a few months after reaching the peak dose; such testing protocol would be cost-effective for patients and the national insurance system[3]. The total cost for patients who underwent baseline and monthly laboratory monitoring for 6 mo was approximately $525, compared with approximately $225 at baseline, after 1-2 mo, and after 6 mo, with estimated savings of $300[3].
In Korea, a liver + lipid panel (excluding direct bilirubin and gamma-glutamyl transferase tests) costs 91650 won, of which 65470 won are covered by insurance. If monthly monitoring is performed for 6 mo (including the baseline exam), the cost to the patient will be 641550 won, of which 458290 won is covered by insurance. However, testing only at the baseline, 1 mo, 2 mo, and 6 mo reduces the general rate to 366600 won, of which 261880 won is covered by insurance, which is a considerable reduction in cost.
Some studies, including those by Hansen et al[2] and Shah et al[3], have discouraged performing liver and lipid tests if the first 3 mo of monitoring indicate normal results. However, significant changes in liver function and lipid test results were observed. Furthermore, the European Medicines Agency, the United States, and other European guidelines recommend performing liver function and lipid tests (at different intervals)[1]. Therefore, minimal tests are necessary. Consequently, we recommend minimal periodic examinations once every 6 mo after reaching the peak isotretinoin dose.
Some limitations of this study are that we could not confirm isotretinoin compliance and that we only included few patients. Furthermore, if blood test abnormalities were detected, the drug may have been immediately stopped, and subsequent progress could not be confirmed.
This study investigated the optimal blood test type and frequency for patients taking isotretinoin for acne. Our results suggest performing a liver and lipid panel before beginning isotretinoin, 1 mo and 2 mo after administration, and every 6 mo thereafter if no abnormalities are detected, to save on costs. However, more frequent monitoring may be necessary if there is a family history of related adverse effects, such as hyperlipidemia, or if the patient has specific risk factors, such as obesity, viral hepatitis, and additional drug or alcohol intake. This article will contribute to Asian-specific isotretinoin laboratory guidelines and further studies.
Isotretinoin is a widely prescribed medication for various dermatological conditions, particularly acne. Regular blood tests are performed to monitor its side effects. However, the optimal tests and their timing remain controversial.
Since 2006, many studies have reported that only a small proportion of patients taking isotretinoin have abnormal laboratory test results. Thus, frequent monitoring of isotretinoin treatment may be unnecessary.
This study aimed to evaluate the most efficient testing regimen for patients with acne taking isotretinoin.
This retrospective study analyzed medical records obtained from Dongguk University Ilsan Hospital’s electronic medical records database. Independent variables (e.g., sex, total prescription date and dose, daily average prescription dose, underlying disease type, and monthly interval per blood test) were analyzed using repeated-measures analysis of variance.
Aspartate aminotransferase, alanine aminotransferase, and triglyceride levels significantly changed between 5 and 6 mo when the total prescription period and dose variables were considered altogether. High-density lipoprotein level also significantly changed between 5 and 6 mo. However, low-density lipoprotein and total cholesterol levels significantly changed between 1 and 2 mo.
Liver and lipid panel should be performed before beginning isotretinoin administration, 1 and 2 mo after administration, and every 6 mo thereafter if no abnormalities are detected to save on costs.
This article will contribute to Asian-specific isotretinoin laboratory guidelines and further studies.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Dermatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Bugaj AM, Poland; Khamdan FA, Bahrain; Kutlubay Z, Turkey; Li CM, China S-Editor: Yan JP L-Editor: A P-Editor: Wu RR
| 1. | Dessinioti C, Zouboulis CC, Bettoli V, Rigopoulos D. Comparison of guidelines and consensus articles on the management of patients with acne with oral isotretinoin. J EurAcad Dermatol Venereol. 2020;34:2229-2240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 2. | Hansen TJ, Lucking S, Miller JJ, Kirby JS, Thiboutot DM, Zaenglein AL. Standardized laboratory monitoring with use of isotretinoin in acne. J Am Acad Dermatol. 2016;75:323-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | Shah R, Kroshinsky D. Re-evaluating the need for routine laboratory monitoring in patients taking isotretinoin: A retrospective analysis. J Am Acad Dermatol. 2021;85:504-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Nazarian RS, Zheng E, Halverstam C, Cohen SR, Wolkoff AW. Prolonged Serum Alanine Aminotransferase Elevation Associated with Isotretinoin Administration. Case Reports Hepatol. 2019;2019:9270827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 5. | Ben-Shoshan D, Gomolin A, Litvinov IV, Netchiporouk E. Time to Change Guidelines for Laboratory Monitoring During Isotretinoin Treatment. J Cutan Med Surg. 2020;24:92-93. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Barbieri JS, Shin DB, Wang S, Margolis DJ, Takeshita J. The clinical utility of laboratory monitoring during isotretinoin therapy for acne and changes to monitoring practices over time. J Am Acad Dermatol. 2020;82:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Lee YH, Scharnitz TP, Muscat J, Chen A, Gupta-Elera G, Kirby JS. Laboratory Monitoring During Isotretinoin Therapy for Acne: A Systematic Review and Meta-analysis. JAMA Dermatol. 2016;152:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 8. | Brzezinski P, Borowska K, Chiriac A, Smigielski J. Adverse effects of isotretinoin: A large, retrospective review. Dermatol Ther. 2017;30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 9. | Öktem A, Hayran Y, Arı E, Yalçın B. Minimize the regular laboratory monitoring during the systemic isotretinoin treatment: data of 704 patients with acne vulgaris. J Dermatolog Treat. 2019;30:813-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Almond-Roesler B, Blume-Peytavi U, Bisson S, Krahn M, Rohloff E, Orfanos CE. Monitoring of isotretinoin therapy by measuring the plasma levels of isotretinoin and 4-oxo-isotretinoin. A useful tool for management of severe acne. Dermatology. 1998;196:176-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Kızılyel O, Metin MS, Elmas ÖF, Çayır Y, Aktaş A. Effects of oral isotretinoin on lipids and liver enzymes in acne patients. Cutis. 2014;94:234-238. [PubMed] |
| 12. | Vieira AS, Beijamini V, Melchiors AC. The effect of isotretinoin on triglycerides and liver aminotransferases. An Bras Dermatol. 2012;87:382-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Jeong JH, Hyun GH, Park YJ, Kwon SW, Lee AY. Clinical Factors Affecting the Serum Retention of a Teratogenic Etretinate after the Acitretin Administration. BiomolTher (Seoul). 2022;30:562-569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |